Low vitamin D (VitD) status is widespread( Reference Kumar, Muntner and Kaskel 1 , Reference Choi, Oh and Choi 2 ) and health concerns related to VitD insufficiency/deficiency are increasing. The VitD receptor is present on most cells of the body; VitD is required not only for bone health but may also play crucial roles in many diseases, including glucose metabolism, CVD, cancer and immune disorders( Reference Bouillon, Carmeliet and Verlinden 3 ).
Low circulating 25-hydroxyvitamin D (25(OH)D) concentrations, an indicator of VitD status, have been linked to type 2 diabetes mellitus (T2DM), metabolic syndrome and other cardiometabolic risk factors in several adult studies( Reference Gagnon, Lu and Magliano 4 – Reference Gagnon, Lu and Magliano 8 ). Few epidemiological surveys have addressed paediatric populations( Reference Reis, von Muhlen and Miller 9 , Reference Ganji, Zhang and Shaikh 10 ). There has been no population-based epidemiological report of VitD status, insulin resistance (IR) and T2DM risk in an Asian paediatric population.
The mechanism by which VitD deficiency and T2DM risk are related is unclear. The theoretical evidence that VitD reduces IR( Reference Maestro, Davila and Carranza 11 – Reference Pittas, Lau and Hu 13 ) and maintains insulin secretion( Reference Pittas, Lau and Hu 13 – Reference Alvarez and Ashraf 16 ) supports the hypothesis that VitD reduces the risk of T2DM. However, VitD deficiency, impaired fasting glucose (IFG) and T2DM share the same risk factors, including African-American, Asian or Hispanic ethnicity, increased adiposity, older age and physical inactivity( Reference Alvarez and Ashraf 16 ). Therefore, adjustment must be made for these confounding factors in analyses of the association between VitD status and glucose homeostasis.
We investigated the relationships between VitD status and markers of IR and/or IFG using data based on the Korea National Health and Nutrition Examination Surveys (KNHANES) 2009–2010, after adjusting for age, sex, physical activity (PA) and adiposity. The adjusted markers of adiposity included BMI Z-score and total body fat measurements made with whole-body dual-energy X-ray absorptiometry (DXA).
Materials and methods
Study population
We used data from the third year (2009) of KNHANES IV and the first year (2010) of KNHANES V, with permission of the Korea Centers for Disease Control and Prevention. The KNHANES is a national survey based on a health interview survey, a nutrition survey and a health examination. KNHANES IV and V have a rolling sample design, with complex, stratified, multistage probability sampling. Among the 264 186 primary sampling units (based on the 2005 National Census Registry), 200 and 192 sampling units were randomly selected, then twenty-three and twenty households were sampled from each unit, yielding 4600 and 3840 households in 2009 and 2010, respectively. In 2009 and 2010, 12 722 and 10 938 individuals were sampled, respectively: 10 078 and 8473, respectively, participated in health interviews and health examinations; and 9397 and 8027, respectively, participated in the nutrition survey. Written informed parental consent and patient assent were obtained before testing. We analysed individuals who participated in KNHANES between 2009 and 2010 because these health examinations included measurements of body fat made with DXA since July 2009. Among the 2584 participants aged 10–19 years, 515 were excluded: those with missing fasting measures and/or those who had fasted for less than 8 h (n 262); those who had a previous history of diabetes (n 1); those whose fasting glucose level exceeded 7 mmol/l (n 2); and those with missing values for 25(OH)D (n 212) and/or fasting insulin or glucose (n 221). Among the 2069 participants, only 1472 were tested with DXA. There were no differences in sex, BMI Z-score, fasting blood glucose and insulin levels between adolescents who had DXA scans and those who did not. The following participants were also excluded: those who did not answer the PA questionnaire (n 4) and/or those whose homeostatic model assessment of β-cell function (HOMA-β) and/or log transformation could not be calculated (n 2). In total, 1466 participants (769 males) were finally included.
Enrolment was conducted in all seasons: spring (March–May, n 206), summer (June–August, n 435), autumn (September–November, n 438) and winter (December–February, n 387). Height, weight, BMI percentile and BMI Z-score were assigned on the basis of the 2007 Korean National Growth Charts( 17 ), and the participants were classified into three groups: lean (n 1158, <85th BMI percentile); overweight (n 189, 85–95th BMI percentile); and obese (n 119, ≥95th BMI percentile). A questionnaire was used to assess the amount and intensity of PA performed. The participants were asked about their levels of PA during a normal week. The definition of ‘regular PA’ was based on the PA guideline provided by the US Department of Health and Human Services( 18 ) and the output indicators of the Korea Youth Risk Behavior Web-based Survey( 19 ). Participants who performed moderate or vigorous PA for at least 60 min/d on 3 d/week, and/or walking activity for at least 60 min/d on 5 d/week, and/or muscular strength activity on more than 3 d/week were assigned to the ‘regular PA’ group. Participants who performed moderate or vigorous PA for at least 180 min/d on 3 d/week were also assigned to the ‘regular PA’ group.
Laboratory measurements
Blood samples were obtained by venepuncture, immediately refrigerated, transported to the central testing institute and analysed within 24 h of transportation. Serum 25(OH)D concentrations were measured with an immunoradiometric assay using a 1470 Wizard gamma-counter (Perkin-Elmer, Turku, Finland) and a 25(OH)D 125I radioimmunoassay kit (DiaSorin, Stillwater, MN, USA). At serum 25(OH)D levels of 21·5, 56·8, 82·5 and 122·5 nmol/l, the inter-assay CV were 11·7 %, 10·5 %, 8·6 % and 12·5 %, respectively, and the intra-assay CV were 9·4 %, 8·2 %, 9·1 % and 11·0 %, respectively. KNHANES participates in the VitD Standardization Program, so the measurement of 25(OH)D was standardized with the recently developed National Institute of Standards and Technology–Ghent University reference procedure( Reference Sempos, Vesper and Phinney 20 ). Insulin levels were measured with a 1470 Wizard gamma-counter and an immunoradiometric assay (Biosource, Fleurus, Belgium). Plasma glucose was measured enzymatically with a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) and commercially available Pureauto S GLU kits (Sekisui, Tokyo, Japan). IR by homeostasis model assessment (HOMA-IR), HOMA-β and the quantitative insulin-sensitivity check index (QUICKI) were calculated as previously described( Reference Matthews, Hosker and Rudenski 21 , Reference Katz, Nambi and Mather 22 ).
Total body fat measurements
Whole-body DXA scans were performed with a QDR Discovery fan beam densitometer (Hologic, Inc., Bedford, MA, USA). The KNHANES data sets included DXA measurements of fat mass (FM) in grams. Body fatness is generally expressed as FM (g) and FM as a percentage of body weight (FM (%); calculated as (FM/total mass) × 100). A more appropriate approach is to normalize body fatness by height( Reference Wells 23 ). FM can be divided by height squared, to produce the fat mass index (FMI; calculated as (body FM excluding the head area)/height2). Until recently, no comprehensive source of body composition reference data for Korean children and adolescents was available. Therefore, we derived Z-scores for FM (g), FM (%) and FMI from centile curves estimated using the LMS method( Reference Cole and Green 24 ).
The DXA FM Z-scores were calculated as follows:
where X is the DXA measurement for each individual, L is the power transformation, M is the median value and S is the CV for each sex–age group centile curve.
Statistical analysis
All analyses were performed considering the KNHANES survey design and the weights used to ensure nationally representative estimates. The statistical software package IBM SPSS Statistics 19·0 was used to accommodate the complex sampling design with stratification, clustering and unequal weighting of the KNHANES sample. Statistical significance was defined as P < 0·05 on two-sided tests.
All continuous variables are expressed as means with their standard errors and categorical variables as numbers and percentages of participants. When the distribution of a continuous variable was skewed, the variable was natural log-transformed for the analysis. The mean 25(OH)D concentrations according to subgroup were compared with an independent-samples t test. Serum 25(OH)D concentrations were categorized into three groups: <37·5 nmol/l (the lowest category, n 553); 37·5 to <50 nmol/l (n 543); and ≥50 nmol/l (the reference group, n 370). The Rao–Scott χ 2 test was used to analyse the associations between the categorical variables and 25(OH) categories. The modifying effects of overweight status on the relationships between the three categories of 25(OH)D and the other dependent variables were evaluated (test for interaction). Tests for linear trends (P for linear trend) across the three categories of serum 25(OH)D were performed. We calculated the age- and sex-adjusted means for fasting glucose, fasting insulin, HOMA-IR, QUICKI and HOMA-β according to the three categories of serum 25(OH)D and analysed these associations with a test for linear trends. Subgroup analyses were then performed to analyse the mean differences in the dependent variables between the reference category (25(OH)D ≥50 nmol/l) and the other two categories.
Multivariate linear regression analyses were performed to assess the associations between the serum 25(OH)D categories and fasting glucose, fasting insulin, HOMA-IR, QUICKI and HOMA-β, after adjustment for potential confounders. Multivariable logistic regression analyses were used to assess the association between the 25(OH)D categories and the risk of IFG, after adjustment for potential confounders. Multivariate-adjusted odds ratios and 95 % confidence intervals for the groups with 25(OH)D <37·5 nmol/l or 37·5 to <50 nmol/l were computed by comparison with the reference category (25(OH)D ≥50 nmol/l). We entered the potential confounders as follows: model 1 included age, sex, PA and BMI Z-score; model 2 included age, sex, PA, FM (g) Z-score; model 3 included age, sex, PA and FM (%) Z-score; and model 4 included age, sex, PA and FMI Z-score.
Results
Table 1 presents the clinical characteristics of the study participants (mean age, 14·3 years). The Z-scores for height, weight and BMI for the total participants were 0·57 (se 0·04), 0·31 (se 0·04) and 0·08 (se 0·04), respectively. The percentages of participants in the overweight and obese groups were 12·9 % and 8·1 %, respectively. The mean level of serum 25(OH)D was 42·0 (se 0·6) nmol/l. The percentages of participants with serum 25(OH)D levels <25 nmol/l (n 104), 25 to <37·5 nmol/l (n 449), 37·5 to <50 nmol/l (n 543), 50 to <75 nmol/l (n 346) and ≥75 nmol/l (n 24) were 7·1 %, 30·6 %, 37·1 %, 23·6 % and 1·6 %, respectively. The fasting glucose and insulin levels were 4·91 (se 0·01) mmol/l and 91·2 (se 1·5) pmol/l, respectively. Among the sixty-one (4·3 %) participants with IFG (5·6 to <7 mmol/l), twenty-nine were overweight or obese.
Table 1 Characteristics of study participants according to sex and 25(OH)D levels according to subgroups; adolescents aged 10–19 years, Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010
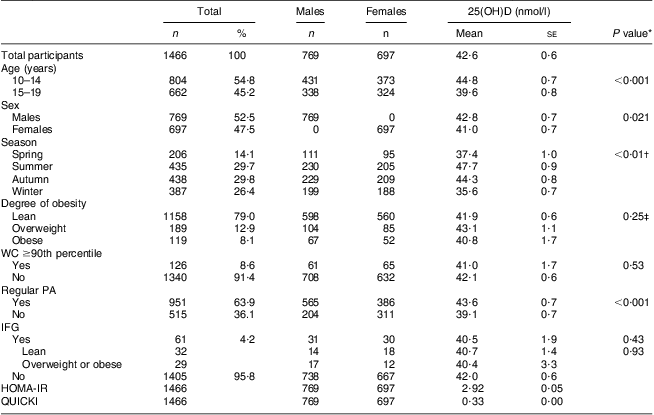
25(OH)D, 25-hydroxyvitamin D; WC, waist circumference; PA, physical activity; IFG, impaired fasting glucose; HOMA-IR, homeostatic model assessment–insulin resistance index; QUICKI, quantitative insulin-sensitivity check index.
*Mean 25(OH)D concentrations according to subgroups were compared with an independent-samples t test.
†Each mean 25(OH)D concentration according to subgroup was compared with the mean for the summer subgroup with an independent-samples t test.
‡A pairwise comparison was performed between all subgroups.
There were significant differences in mean 25(OH)D levels between the subgroups based on age (10–14 v. 15–19 years), sex, season and regular PA, but not based on degree of obesity. In a univariate regression analysis, no significant relationships between the four markers of adiposity and serum 25(OH)D were observed. However, age-, sex-, season- and PA-adjusted regression models showed that serum 25(OH)D was significantly related to Z-scores for FM (g) (P = 0·016), FM (%) (P = 0·023) and FMI (P = 0·035), but not to Z-scores for BMI (P = 0·265).
Table 2 shows the associations between the three categories stratified according to serum 25(OH)D and the participants’ characteristics. Across the 25(OH)D categories, the participants in the highest 25(OH)D group were younger and more physically active than those in the lowest 25(OH)D group. The mean height Z-scores showed a significantly increasing trend (P = 0·033) from the lowest to the highest 25(OH)D category. After adjustment for age and sex, significantly linear downward trends were observed for fasting glucose (P = 0·039), fasting insulin (P < 0·001), HOMA-IR (P < 0·001) and HOMA-β (P = 0·001) and an upward trend for QUICKI (P < 0·001) with increasing 25(OH)D category (Table 2). In the subgroup analysis, the age- and sex-adjusted means of fasting insulin, HOMA-IR and HOMA-β were significantly lower in the reference category (25(OH)D ≥50 nmol/l) than in the other two categories of 25(OH)D. The age- and sex-adjusted mean QUICKI for the reference category was significantly higher than those of the other two categories of 25(OH)D (Table 2).
Table 2 Characteristics of study participants according to categories of serum 25(OH)D concentration; adolescents aged 10–19 years, Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010
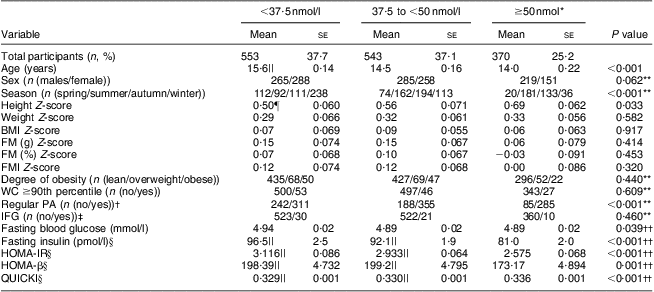
25(OH)D, 25-hydroxyvitamin D; FM, fat mass; WC, waist circumference; PA, physical activity; IFG, impaired fasting glucose; HOMA-IR, homeostatic model assessment–insulin resistance index; HOMA-β, homeostatic model assessment–β-cell function; QUICKI, quantitative insulin-sensitivity check index.
All continuous variables are given as means with their standard errors.
*Reference category.
†Regular PA (n (yes)) means the number of participants who performed moderate or vigorous physical activity for at least 60 min/d on 3 d/week, and/or walking activity for at least 60 min/d on 5 d/week, and/or muscular strength activity on more than 3 d/week, who were assigned to the ‘regular physical activity’ group. Those participants who performed moderate or vigorous physical activity for at least 180 min/day on 3 d/week were also assigned to the ‘regular physical activity’ group.
‡IFG (n (no)) means the number of participants with fasting glucose <5·6 mmol/l.
§The analysis was performed using natural log-transformed values.
||The value was significantly different from that of the reference category (P < 0·001).
¶The value was significantly different from that of the reference category (P=0·027).
**The Rao–Scott χ 2 test was used to analyse the associations between the categorical variables and the three categories of serum 25(OH)D.
††Tests for linear trend (P for linear trend) across the three categories of serum 25(OH)D levels were performed. We calculated the age- and sex-adjusted means for fasting glucose, fasting insulin, HOMA-IR, HOMA-β and QUICKI according to the categories of serum 25(OH)D. Subgroup analyses were then performed to analyse the mean differences in the dependent variables between the reference category (25(OH)D ≥50 nmol/l) and the other two categories.
As further controls for adiposity, we used the BMI Z-scores (model 1) and body fat measurements made with DXA (model 2, FM (g); model 3, FM (%); model 4, FMI). After adjustment for age, sex, PA and markers of adiposity, significantly decreasing trends for fasting insulin (all P < 0·001), HOMA-IR (all P < 0·001) and HOMA-β (P = 0·001 for model 3 and P = 0·002 for the others) and an increasing trend for QUICKI (all P < 0·001) were observed with increasing serum 25(OH)D (Table 3). After adjustment for HOMA-IR, there was no significant inverse relationship between HOMA-β and the 25(OH)D categories. The modifying effects of overweight status on the relationship between the three categories of serum 25(OH)D and markers of IR were significant only for fasting insulin, HOMA-IR and QUICKI (test for interaction: P = 0·046, P = 0·030 and P = 0·034, respectively). In the stratified analyses, 25(OH)D categories were significantly inversely related to fasting insulin and HOMA-IR, and positively related to QUICKI in both lean (all P=0·0 0 1) and overweight or obese participants (all P < 0·001; Table 3).
Table 3 Multivariate linear regression analysis of the associations between serum 25(OH)D category (predictor variable) and fasting glucose, fasting insulin, HOMA-IR, HOMA-β and QUICKI (outcomes); adolescents aged 10–19 years, Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010
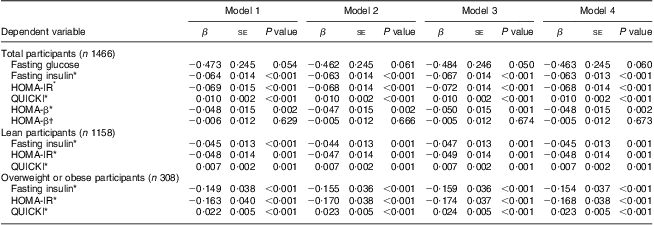
25(OH)D, 25-hydroxyvitamin D; HOMA-IR, homeostatic model assessment–insulin resistance index; HOMA-β, homeostatic model assessment–β-cell function; QUICKI, quantitative insulin-sensitivity check index.
Multivariate linear regression analyses were performed to assess the association between increasing 25(OH)D category (<37·5 nmol/l, 37·5 to <50 nmol/l, ≥50 nmol/l) and the change in fasting glucose, fasting insulin, HOMA-IR, HOMA-β and QUICKI, after adjustment for potential confounders. If there was an interaction between the three categories of serum 25(OH)D and BMI Z-scores (<85th percentile or ≥85th percentile), we split the group into two (lean v. overweight/obese). The modifying effect of overweight status on the relationship between the three categories of serum 25(OH)D and markers of insulin resistance was significant (test for interaction: P=0·046 for fasting insulin, P = 0·030 for HOMA-IR and P = 0·034 for QUICKI). No significant modifying effect of overweight status on the association between the 25(OH)D categories and fasting glucose or HOMA-β was observed. We entered potential confounders as follows: model 1 included age, sex, physical activity (PA) and BMI Z-score; model 2 included age, sex, PA and fat mass (g) Z-score; model 3 included age, sex, PA and fat mass (%) Z-score; and model 4 included age, sex, PA and fat mass index Z-score.
*The analysis was performed using natural log-transformed values for the dependent variables.
†The analysis was performed after additional adjustment of natural log-transformed HOMA-IR.
After adjustment for age, sex, PA and markers of adiposity (models 1–4), the multivariate-adjusted OR for IFG showed a significant decreasing trend with increasing 25(OH)D category (all P for trend < 0·05, Fig. 1). The negative association between 25(OH)D categories and fasting glucose levels was marginally significant (Table 3). In the multivariate logistic regression analysis, the likelihood of participants in the lowest serum 25(OH)D category having IFG was 2·96–3·15 compared with those in the highest 25(OH)D category (all P < 0·05 for models 1–4, Fig. 1). No significant modifying effect of overweight status on the associations between 25(OH)D categories and fasting glucose (or the presence of IFG) or HOMA-β was observed. In addition to body fat measurements (BMI Z-scores (P = 0·001), FM (g) (P < 0·001), FM (%) (P = 0·006) and FMI (P = 0·001)), younger age (P = 0·001) and less PA (P < 0·05) were all significant predictors of IFG.

Figure 1 Multivariate-adjusted odds ratios for impaired fasting glucose (IFG) according to serum 25-hydroxyvitamin D level (![]() , <37·5 nmol/l, n 553;
, <37·5 nmol/l, n 553; ![]() , 37·5 to <50 nmol/l, n 543;
, 37·5 to <50 nmol/l, n 543; ![]() , ≥50 nmol/l, n 370) among adolescents aged 10–19 years, Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Model 1 adjusted for age, sex, physical activity (PA) and BMI Z-score; model 2 for age, sex, PA and fat mass (g) Z-score; model 3 for age, sex, PA and fat mass (%) Z-score; and model 4 for age, sex, PA and fat mass index Z-score
, ≥50 nmol/l, n 370) among adolescents aged 10–19 years, Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Model 1 adjusted for age, sex, physical activity (PA) and BMI Z-score; model 2 for age, sex, PA and fat mass (g) Z-score; model 3 for age, sex, PA and fat mass (%) Z-score; and model 4 for age, sex, PA and fat mass index Z-score
Discussion
The present nationally representative, population-based study showed that three-quarters of Korean adolescents aged 10–19 years were VitD deficient, and IR and the risk of IFG decreased significantly with increasing serum 25(OH)D, independently of age, sex, PA and adiposity. To our knowledge, this is the first nationally representative study suggesting a significant relationship between VitD status and markers of glucose homeostasis, even after adjustment for total body fat measurements (made with DXA), in an Asian paediatric population.
The potential effects of VitD on glucose homeostasis are considered to be mediated by autocrine and paracrine functions involving the transcriptional regulation of genes in pancreatic β-cells, skeletal myocytes and immune cells, improving insulin secretion and sensitivity and reducing inflammation( Reference Pittas, Lau and Hu 13 , Reference Alvarez and Ashraf 16 ). Despite theoretical links between VitD and insulin sensitivity and/or secretion, controversy remains regarding the relationship between 25(OH)D levels and surrogate indices of insulin sensitivity( Reference Gagnon, Lu and Magliano 4 – Reference Kayaniyil, Retnakaran and Harris 7 , Reference Del Gobbo, Song and Dannenbaum 25 , Reference Rajakumar, de las Heras and Lee 26 ) or β-cell function( Reference Kayaniyil, Retnakaran and Harris 7 , Reference Del Gobbo, Song and Dannenbaum 25 , Reference Rajakumar, de las Heras and Lee 26 ) and the risk of glucose intolerance or T2DM( Reference Choi, Kim and Lim 6 , Reference Reis, von Muhlen and Miller 9 , Reference Pacifico, Anania and Osborn 27 ). These discrepancies in the relationships between VitD status and outcomes related to glucose homeostasis in paediatric or adult populations may be attributable to differences in study sample size, participant characteristics (race, ethnicity and degree of obesity), method of measuring insulin sensitivity and/or β-cell function, and the adjusted markers of adiposity used.
Adipose tissue is not only the natural reservoir of liposoluble 25(OH)D, but also a well-known determinant of IR. Traditional measurements, such as BMI, do not measure fat in accurate quantitative terms( Reference Wells 23 ). However, a technique that can divide body weight into FM and fat-free mass (such as DXA) is useful in adjusting for total body adiposity. We first investigated the association between total body fat and serum 25(OH)D and then evaluated whether serum 25(OH)D was related to surrogate indices of insulin sensitivity (HOMA-IR, QUICKI) or insulin secretion (HOMA-β), and the risk of IFG, after adjusting for total body adiposity using DXA.
Racial and ethnic differences in the degree of adiposity and the distribution of body fat are well known( Reference Rajakumar, de las Heras and Chen 28 , Reference Golden, Brown and Cauley 29 ). Asian-American and African-American children have lower BMI and/or total body fat than Caucasian children. Asian-American children have higher visceral adipose tissue( Reference Golden, Brown and Cauley 29 ) and African-American children have lower visceral adipose tissue and more subcutaneous adipose tissue than Caucasian children( Reference Rajakumar, de las Heras and Chen 28 ). Lower levels of 25(OH)D are associated with higher adiposity, measured with DXA, in Caucasian and African-American youth examined concurrently, and VitD deficiency is associated with higher visceral adipose tissue in Caucasians and greater subcutaneous adipose tissue in African-Americans( Reference Rajakumar, de las Heras and Chen 28 ). We demonstrated a significant association between serum 25(OH)D levels and total body fat, measured with DXA, after adjustment for age, sex, season and PA in a Korean paediatric population, although we could not examine the relationship between 25(OH)D status and the distribution of abdominal adipose tissue.
Theoretically, a VitD response element in the human insulin receptor gene promoter might affect insulin receptor expression( Reference Maestro, Davila and Carranza 11 ), and 1,25-dihydroxycholecalciferol (1,25-dihydroxyvitamin D3) directly stimulates its expression and insulin responsiveness for glucose transport( Reference Maestro, Campion and Davila 12 ). VitD also indirectly improves insulin responsiveness by maintaining normal extracellular calcium levels and effective calcium influx through cell membranes( Reference Pittas, Lau and Hu 13 ). Many cross-sectional studies have supported an association between 25(OH)D levels and insulin sensitivity in adults( Reference Gagnon, Lu and Magliano 4 – Reference Choi, Kim and Lim 6 , Reference Gagnon, Lu and Magliano 8 ). Recently, Gagnon et al.( Reference Gagnon, Lu and Magliano 4 ) prospectively demonstrated that lower 25(OH)D levels are associated with increased HOMA-IR and risk of metabolic syndrome in Australian adults. In cross-sectional studies of Chinese( Reference Lu, Yu and Pan 5 ) and Korean adults( Reference Choi, Kim and Lim 6 ), VitD status was inversely related to HOMA-IR. Low 25(OH)D levels are also significantly related to an increased risk of metabolic syndrome in Chinese adults( Reference Lu, Yu and Pan 5 ) and T2DM in Korean adults( Reference Choi, Kim and Lim 6 ). Consistent with studies of adult populations( Reference Gagnon, Lu and Magliano 4 – Reference Choi, Kim and Lim 6 , Reference Gagnon, Lu and Magliano 8 ), cross-sectional nationwide studies of paediatric populations have consistently reported an inverse association between 25(OH)D levels and fasting hyperglycaemia and/or increased HOMA-IR, after adjustment for BMI( Reference Reis, von Muhlen and Miller 9 , Reference Ganji, Zhang and Shaikh 10 ). Our study supports a significant relationship between 25(OH)D levels and HOMA-IR or QUICKI, independent of total body fat. The inverse relationship between 25(OH)D and IR was significant in all participants, not only those who were overweight or obese.
VitD receptors( Reference Johnson, Grande and Roche 14 ) and 1α-hydroxylase( Reference Bland, Markovic and Hills 15 ) are present in pancreatic β-cells and calcium plays an essential role in β-cell insulin secretion( Reference Pittas, Lau and Hu 13 , Reference Alvarez and Ashraf 16 ). Therefore, VitD may directly or indirectly affect insulin secretion. Contrary to expectation, HOMA-β showed an increasing trend with decreased serum 25(OH)D, which was not significant after adjustment for HOMA-IR. Therefore, the failure of β-cell mass compensation for decreased insulin sensitivity related to VitD deficiency and/or other risk factors may be associated with the development of fasting hyperglycaemia or IFG.
The risk of diabetes associated with IR and β-cell compensation varies with race/ethnicity. Compared with Caucasians, total adiposity is lower, but the risk of incident diabetes is 18% higher in Asian-Americans( Reference Golden, Brown and Cauley 29 ), which may be linked to their greater abdominal obesity, smaller muscle mass and lower β-cell insulin secretion in the context of IR( Reference Golden, Brown and Cauley 29 , Reference Chan, Malik and Jia 30 ). Unexpectedly, about half of the participants with IFG in our study were lean. The lean participants with IFG may have had a ‘metabolically obese, but normal-weight’ phenotype (lean individuals with a disproportionate amount of fat stored within the abdomen) and/or a family history of T2DM. Unfortunately, the KNHANES data did not include body fat topography measurements and/or complete answers to the family history questionnaire for the paediatric population. Although there is insufficient evidence to recommend consistent thresholds of serum 25(OH)D in relation to non-calcaemic metabolic benefits, numerous epidemiological studies have suggested that 25(OH)D >75 nmol/l may confer health benefits by reducing the risk of T2DM, CVD, etc.( Reference Holick, Binkley and Bischoff-Ferrari 31 ). Fewer than 2 % of our participants were VitD sufficient (>75 nmol/l), irrespective of their overweight status. VitD insufficiency and/or deficiency may be related to hepatic IR, and the β-cell mass cannot fully compensate or overcome IR in Korean adolescents.
The study was limited by a lack of information about the participants’ pubertal status and family history of T2DM, levels of parathyroid hormone and/or several adipocytokines, and visceral fat measurements. We used HOMA-IR and QUICKI, reflecting only hepatic insulin sensitivity rather than in vivo whole-body insulin sensitivity, which encompasses both hepatic and peripheral tissues, measured using hyperinsulinaemic–euglycaemic and hyperglycaemic clamps. Rajakumar et al.( Reference Rajakumar, de las Heras and Lee 26 ) recently reported that 25(OH)D levels are not independently related to in vivo insulin sensitivity or β-cell function, after adjustment for total body fat or visceral adipose tissue, in healthy black and white youth aged 8–18 years without dysglycaemia. Our study is the first to show that a low VitD status is significantly related to hepatic IR and IFG, independently of total body fat, in Korean adolescents. Further studies are required to investigate whether the association between 25(OH)D status and the parameters of glucose homeostasis is significant in Korean adolescents after adjustment for visceral adiposity, which determines metabolic obesity in Asian populations.
Unexpectedly, the mean height Z-score showed an increasing trend with increasing 25(OH)D. Serum 25(OH)D levels were positively related to final adult height in ninety young women( Reference Kremer, Campbell and Reinhardt 32 ). Adolescent girls with VitD deficiency showed a significant reduction in height( Reference Hatun, Islam and Cizmecioglu 33 ). Because of its essential role in skeletal development and bone health, VitD may have a beneficial effect on linear growth and final height.
Conclusion
Low VitD status was significantly associated with hepatic IR and IFG, independently of total body fat, in Korean adolescents. It remains to be determined whether VitD optimization improves insulin sensitivity and reverses IFG in VitD-deficient adolescents, irrespective of changes in their total or visceral adiposity.
Acknowledgements
Sources of funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. There was no funder's contribution to the study design, conduct of the study, analysis of samples or data, interpretation of findings or the preparation of this manuscript. Conflicts of interest: There are no conflicts of interest for any author. Ethics: The study complied with the recommendations of the Declaration of Helsinki. The institutional review board of the Korea Centers for Disease Control and Prevention approved the study protocol. Authors’ contributions: S.J.C., Y.A.L., M.J.K., S.W.Y. and C.H.S. were responsible for the conception and design of the study. S.J.C., Y.A.L., H.S.H. and H.J.K. contributed to preparation of the data set. S.J.C., Y.A.L. and H.S.H. performed the statistical analysis. S.J.C. and Y.A.L. drafted the manuscript. All authors played a role in interpretation of the results and approved the final manuscript.






