Introduction
Dementia is a chronic condition caused by progressive changes in the brain, with no effective cure. The brain changes lead to loss of memory and other cognitive functions, and patients may experience distressing neuropsychiatric symptoms (NPS).
As much of our efforts aim at alleviating the symptoms of dementia, measures of quality of life (QoL) are being increasingly used as outcome scores in clinical practice and research (Thorgrimsen et al., Reference Thorgrimsen2003).
In recent years, research on QoL in dementia has evolved considerably with the development and use of disease-specific QoL assessments (Brod et al., Reference Brod, Stewart, Sands and Walton1999; Logsdon et al., Reference Logsdon, Gibbons, McCurry and Teri2002; Smith et al., Reference Smith2005). A 2009 summary of studies showed a consistent association between QoL and depression in dementia, while clinical and sociodemographic characteristics were associated only weakly or not at all with QoL (Banerjee et al., Reference Banerjee2009).
Lately, a number of studies have been conducted to uncover QoL changes over time, QoL predictors, and agreement between patient and proxy ratings of QoL in persons with dementia. Results indicate that patient-rated QoL remains fairly stable over time and the course of the disease, while proxy ratings of patient QoL are lower and decline over time and across disease stages (Missotten et al., Reference Missotten2007; Tatsumi et al., Reference Tatsumi2009; Bosboom et al., Reference Bosboom, Alfonso, Eaton and Almeida2012, Reference Bosboom, Alfonso and Almeida2013; Huang et al., Reference Huang2015). However, most previous studies are based on small sample sizes up to about 100 participants. Many studies lack controls, and only a few studies have a longitudinal design with longer observation periods than 12 months.
In this study, we aimed at investigating longitudinal changes in patient- and proxy-rated QoL and determining the differences in QoL between persons with and without dementia. Furthermore, we wanted to explore the factors that were associated with changes in QoL during the observation period.
Methods
This was a longitudinal study of a subsample from the CONSIC-study that followed 1,000 home-dwelling individuals with three assessments over a period of 36 months. The sample was recruited from 19 municipalities, both rural and urban, in five counties in eastern part of Norway. To be considered for participation the candidates had to be 70 years and over receiving some kind domiciliary care and having a next of kin who saw them at least once a week. After a random selection was made, 1,795 eligible candidates were contacted, resulting in a final sample of 1,000 people (Wergeland et al., Reference Wergeland, Selbaek, Hogset, Soderhamn and Kirkevold2014). In the CONSIC-study, QoL was not measured at baseline (BL) but only at an 18-month follow-up (FU), and at a 36-month FU. We included all 412 individuals for whom both QoL assessments were completed. A flowchart of the participants is presented in Figure 1.
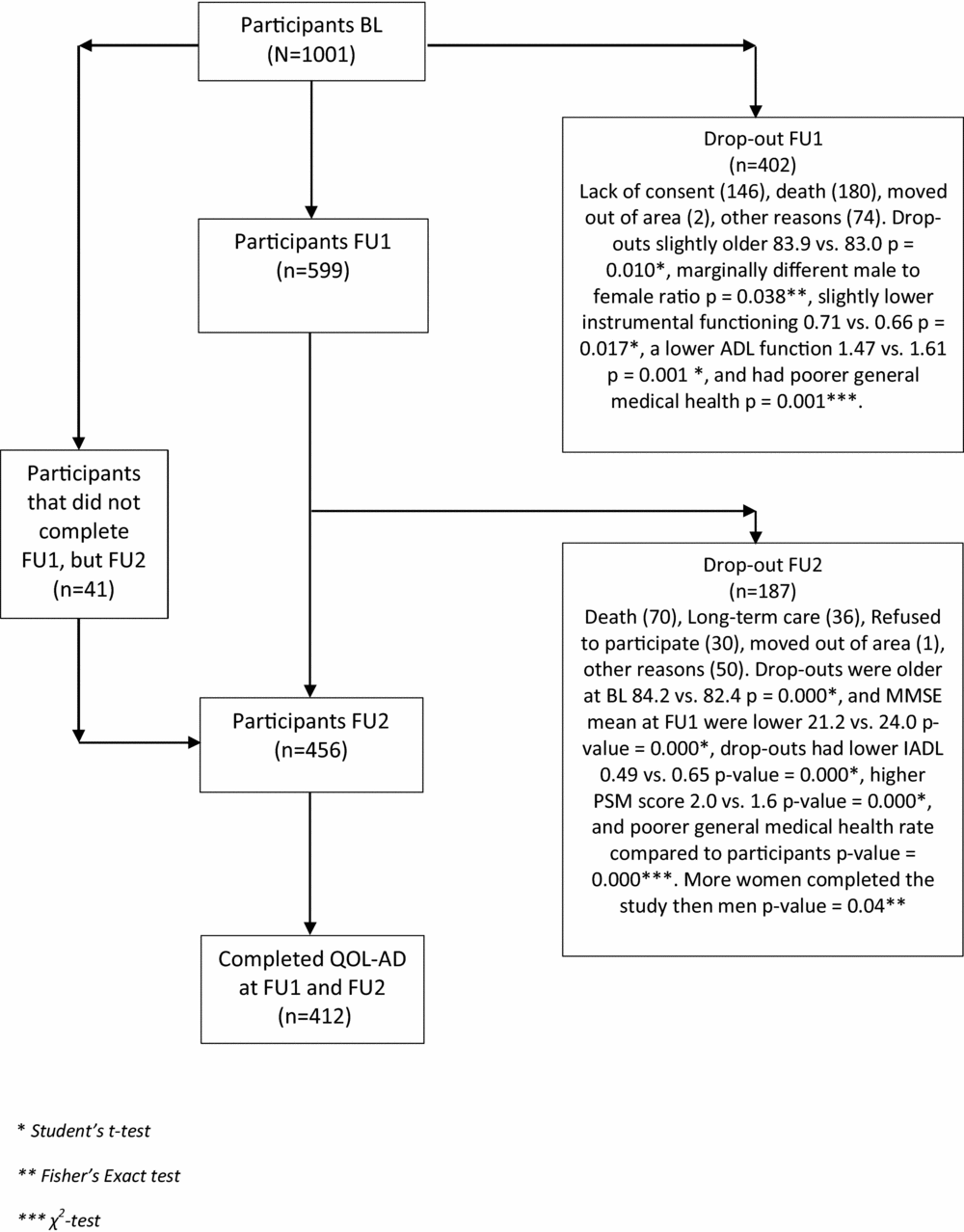
Figure 1. Flow-chart of participant inclusion and drop-out through the study.
Data were collected in the participants’ homes and interviews were conducted separately for participants and their proxies. Trained healthcare workers collected data, and participants were examined between January 2009 and August 2012. For more details regarding the data collection process, see Wergeland et al. (Reference Wergeland, Selbaek, Hogset, Soderhamn and Kirkevold2014).
Besides demographic data and cohabitation status, the following clinical data were obtained:
Cognitive impairment: Participants were classified as no cognitive impairment, mild cognitive impairment (MCI) according to the Winblad criteria (Winblad et al., Reference Winblad2004), or dementia according to the International Statistical Classification of Diseases and Related Health Problems (ICD-10) criteria (World Health Organization, 1992). The classification was done independently by two experts (GS and SB), based on collected information about cognitive function, activities of daily living (ADL) function, and NPS. If the two experts did not reach consensus, a third expert was consulted. For the analyses in the present study, patients without cognitive impairment and patients with MCI were merged into the category “no dementia.”
Quality of Life in Alzheimer's Disease (QoL-AD): It is a dementia-specific instrument assessing QoL. The QoL-AD contains 13 items covering physical health, energy, mood, living situation, memory, family, marriage, friends, self as a whole, ability to do chores around the house, ability to do things for fun, money, and life as a whole (Logsdon et al., Reference Logsdon, Gibbons, McCurry and Teri2002). Each item is rated from 1 (poor) to 4 (excellent), resulting in a sum score ranging from 13 to 52. The QoL-AD scale is widely used to assess QoL in patients with dementia. It is recommended because of good psychometric properties in varied cultural settings (Logsdon et al., Reference Logsdon, Gibbons, McCurry and Teri2002; Revell et al., Reference Revell, Caskie, Willis and Schaie2009; Gomez-Gallego et al., Reference Gomez-Gallego, Gomez-Garcia and Ato-Garcia2014), and has performed well on validity and reliability tests (Logsdon et al., Reference Logsdon, Gibbons, McCurry and Teri2002; Thorgrimsen et al., Reference Thorgrimsen2003; Gomez-Gallego et al., Reference Gomez-Gallego, Gomez-Garcia and Ato-Garcia2014). The QoL-AD was administered separately to the participants and their proxies. The participants evaluated their own QoL, while the proxies were asked to evaluate the QoL of the participants based on how they believed the participants would evaluate their own QoL. If not more than three items were missing, values were imputed by determining the empirical distribution for each item in the scale and drawing a random number from that distribution for each missing value. To assess dimensions of QoL-AD, we included three subscales previously identified by Revell et al. (Reference Revell, Caskie, Willis and Schaie2009). The dimensions include physical well-being containing the items such as physical health, energy, ability to do chores, and ability to do things for fun; social well-being containing the items such as living situation, family, marriage, friends, and money; psychological well-being containing the items such as mood, memory, self, and life as a whole.
General Medical Health Rating (GMHR): It is a four-category, reliable, and valid global bedside assessment tool staging the severity of physical health (Lyketsos et al., Reference Lyketsos1999). The score is based on an overall assessment by the caregiver.
Physical Self-Maintenance Scale (PSMS): It is a scale consisting of six items to evaluate functional status in ADL (Lawton and Brody, Reference Lawton and Brody1969). Each item is scored from “1” (independent) to “5” (totally dependent), and a mean score is calculated based on the total score divided by six.
Instrumental activities of daily living (IADL): It includes eight items (Lawton and Brody, Reference Lawton and Brody1969). Each item is scored “0” (dependent) or “1” (independent), and a mean score is calculated based on the total score divided by eight. For women, all eight items were included in the sum score, while we excluded the items “food preparation,” “housekeeping,” and “laundry” for men, as these items were not applicable for many male participants in this study (Lawton and Brody, Reference Lawton and Brody1969; Wattmo et al., Reference Wattmo, Paulsson, Minthon and Londos2013). We calculated a sum score and divided it by the number of items evaluated, thus obtaining a score ranging from 0 = completely dependent to 1 = completely independent in terms of IADL.
Mini-Mental State Examination (MMSE): It is a screening tool that measures cognitive impairment (Folstein ‘et al., 1975). The maximum score is 30 points.
Clinical Dementia Rating-Sum of Boxes score (CDR-SOB): It is obtained by applying the six-item CDR scale and then summing each of the domain box scores so as to end up with a total score ranging from 0 to 18. The higher the score, the more severe the dementia (O'Bryant et al., Reference O'Bryant2008).
Neuropsychiatric Inventory (NPI): It assesses NPS (Cummings et al., Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein1994). The scale considers 12 types of NPS. The presence of symptoms and their frequency and intensity are assessed based on an interview with the closest carer. A higher score denotes more severe NPS. Three sub-syndromes of NPI were identified (NPI-Agitation, NPI-Psychosis, and NPI-Affective) based on a principal component analysis with direct oblimin rotation. For details, see Ydstebo et al. (Reference Ydstebo, Bergh, Selbaek, Benth, Luras and Vossius2015).
Cornell Scale for Depression in Dementia (CSDD): It is a 19-item dementia-specific depression screening tool. Each item is scored zero (absent), one (mild), two (severe), or unable to evaluate, and the total score (0–38) is calculated by adding the item scores (Alexopoulos et al., Reference Alexopoulos, Abrams, Young and Shamoian1988).
Statistical analysis
Demographic factors and clinical symptoms were described by means and standard deviations (SD). Categorical variables are described as frequencies and percentages. The group differences were analyzed by Student's t-test (with no equal variance assumption) for continuous variables and by χ2 or Fisher's exact test for categorical variables. The distribution of continuous variables was assessed by inspecting the histograms.
By means of an exploratory approach, group-based trajectory models using censored normal mixture were estimated to identify potential distinct homogeneous subgroups of participants, following similar profiles from BL to FU in patient- and proxy-rated QoL-AD. The aim was to describe the longitudinal change within each subgroup as well as to assess the differences among the groups. According to this approach, the groups are identified in a post-hoc matter, where the group belonging is determined based on individual profiles. Several statistical criteria were applied in the process. Akaike's Information Criterion (AIC) and Bayesian Information Criterion (BIC) were used to ascertain the best-fitting models, where smaller values of AIC and BIC denote better fit. Other criteria were reasonable sample sizes in each group, non-overlapping 95% confidence intervals (CI), and average within-group probability larger than 0.7. The group-based trajectory models were estimated using plugin STATA command TRAJ (Jones and Nagin, Reference Jones and Nagin2013).
The agreement between groups of patients and their proxies was assessed by kappa statistic applying guidelines for interpretation suggested by Cicchetti (Reference Cicchetti1994), where a statistic below 0.40 is considered to be of poor clinical significance, while 0.40–0.59 is fair, 0.60–0.74 is good, and 0.75–1.00 indicates excellent clinical significance.
Bivariate and multiple nominal regression models were estimated to identify potential characteristics associated with group membership. The interaction terms between all variables and the dichotomous variable dementia were included into the multiple-regression models. Models were further reduced by AIC. Significant interactions imply that there are differences between persons with and without dementia regarding associations between group membership and clinical and/or demographic characteristics.
The results for QoL ratings were presented as odds ratios (OR) with the corresponding 95% CI and p values. ORs were calculated separately for persons with and without dementia for variables included into interaction terms. However, as the analyses generated vast amounts of information, main findings for the subdimensions of QoL-AD are only reported in text. The Statistical Package for Social Science (SPSS) version 23 for Windows and STATA version 14 were used for the data analysis.
Ethical considerations
The study was approved by the regional ethics committee (registration number 2010/119). All participants gave informed written consent.
Results
Study population
A total of 412 participants were included, 254 (61.7%) persons without dementia and 158 (38.3%) with dementia at BL. The demographic and clinical characteristics of the study population are presented in Table 1. Between BL and FU, 103 participants were admitted to permanent nursing home stay, of whom 92 (89%) had dementia at BL.
Table 1. Demographic and clinical variables for all participants, and comparisons of participants with and without dementia at BL
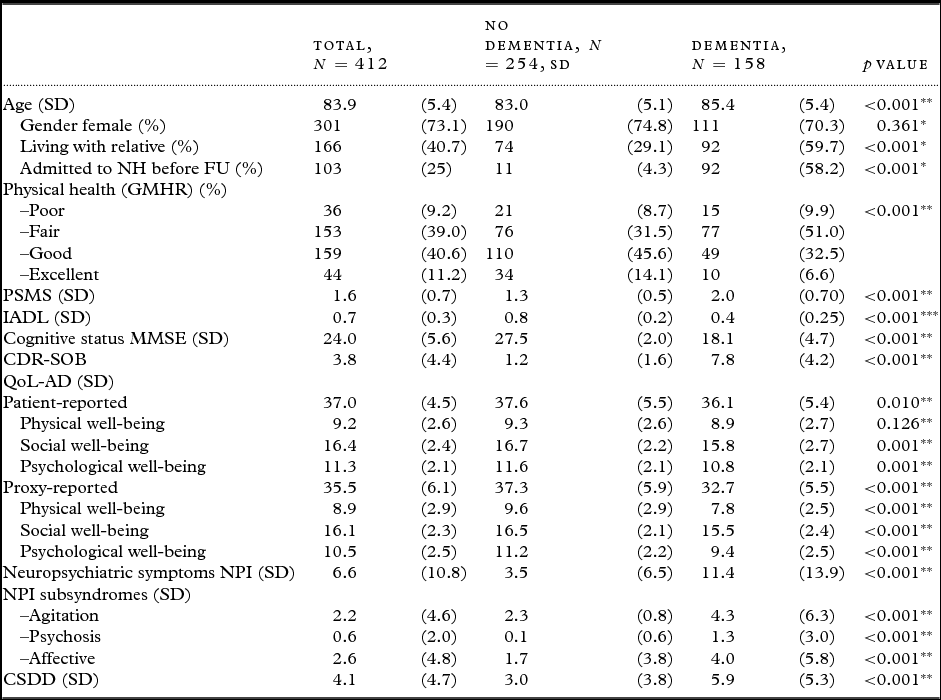
SD = Standard deviation; NH = nursing home; FU = follow-up; GMHR = General Medical Health Rating; PSMS = Physical Self-Maintenance Scale; IADL = Instrumental activity of daily living; MMSE = Mini-Mental State Examination; CDR-SOB = Clinical Dementia Rating-Sum of Boxes; QoL-AD = Quality of Life in Alzheimer's Disease; NPI = Neuropsychiatric Inventory; NPI-Agitation = agitation/aggression, euphoria, disinhibition, aberrant motor behavior, and irritability, NPI-Psychosis = delusions and hallucinations, NPI-Affective = depression, anxiety, and apathy; CSDD = Cornell Scale for Depression in Dementia.
*Fisher's exact test, **Student's t-test (equal variances not assumed), ***χ2-test.
Three trajectory groups based on the patients’ QoL scores at BL and the changes in QoL over time (G1 n = 80, G2 n = 249, G3 n = 83) and three trajectory groups based on the proxies’ QoL scores at BL and the changes in QoL over time (G1 n = 165, G2 n = 199, G3 n = 48) were identified, each following a distinct trajectory (Figure 2). The BL QoL mean scores for the patients were G1 = 31.1 (SD = 9.8), G2 = 38.2 (SD = 12.5), G3 = 44.3 (SD = 9.6), and for the proxies; G1 = 30.6 (SD = 12.2), G2 = 38.5 (SD = 13.5), G3 = 43.0 (SD = 10.3). For both patient- and proxy-rated QoL, G1 represents the participants with the lowest QoL score at BL. The QoL score was significantly different between the three groups, as judged by non-overlapping 95% CI, both for patients and proxies. The average probabilities for within-group membership were all above 0.80. The QoL remained stable in patient-rated G1 and G3, while there was a statistically significant reduction in the QoL scored by the patients in G2 by a mean of 1.04 points (p = 0.008) (Figure 2). Changes in proxy-rated QoL were not significant in any of the three groups. The agreement between groups of patients and proxies was poor with a κ of 0.22.
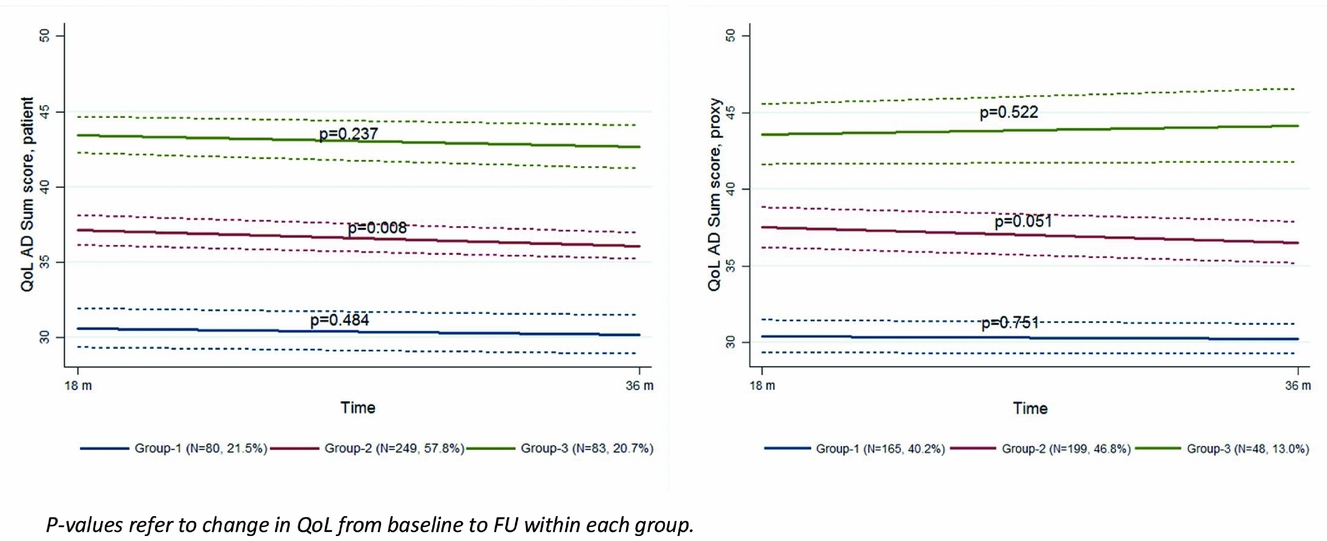
Figure 2. Trajectories for 18-months change in patient- and proxy-rated QoL-AD. p values refer to change in QoL from baseline to follow-up within each group.
Table 2 presents descriptive statistics for the patients in each trajectory group. Bivariate analyses for patient-rated QoL, as presented in Table 3, show that the chances of belonging to G1 versus G3 were higher for persons with a dementia diagnosis, more cognitive impairment (MMSE), more severe dementia (CDR-SOB), lower instrumental functioning (IADL), and more affective symptoms (NPI-Affective). The chances of belonging to G1 versus G2 and G1 versus G3 were higher for persons with poor/fair physical health (GMHR), lower functional ADL (PSMS), and more depressive symptoms (CSDD).
Table 2. Baseline data in the different trajectory groups (cases with at least one missing covariate excluded). G1 = group with lowest QoL
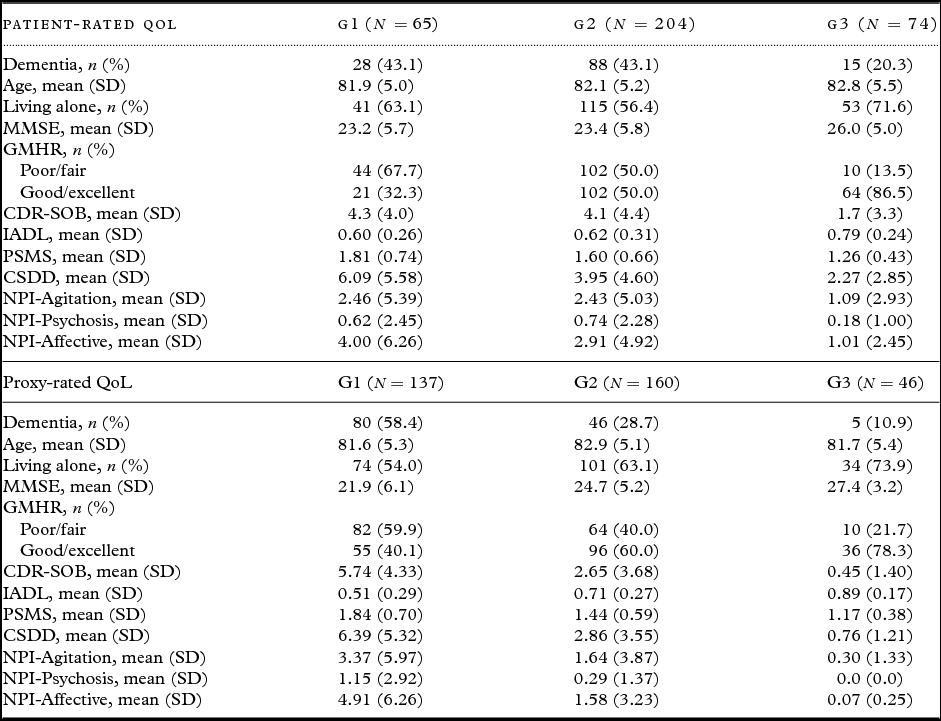
QoL = Quality of life; SD = standard deviation; MMSE = Mini-Mental State Examination; GMHR = General Medical Health Rating; CDR-SOB = Clinical Dementia Rating-Sum of Boxes; IADL = Instrumental activity of daily living; PSMS = Physical Self-Maintenance Scale; CSDD = Cornell Scale for Depression in Dementia; NPI = Neuropsychiatric Inventory; NPI-Agitation = agitation/aggression, euphoria, disinhibition, aberrant motor behavior, and irritability; NPI-Psychosis = delusions and hallucinations; NPI-Affective = depression, anxiety, and apathy.
Table 3. Results from bivariate and multiple nominal regression of trajectories for patient- and proxy-rated QoL-AD. Multiple models were reduced by AIC, odds ratios are presented for persons with and without dementia for variables which were a part of interaction with dementia diagnosis
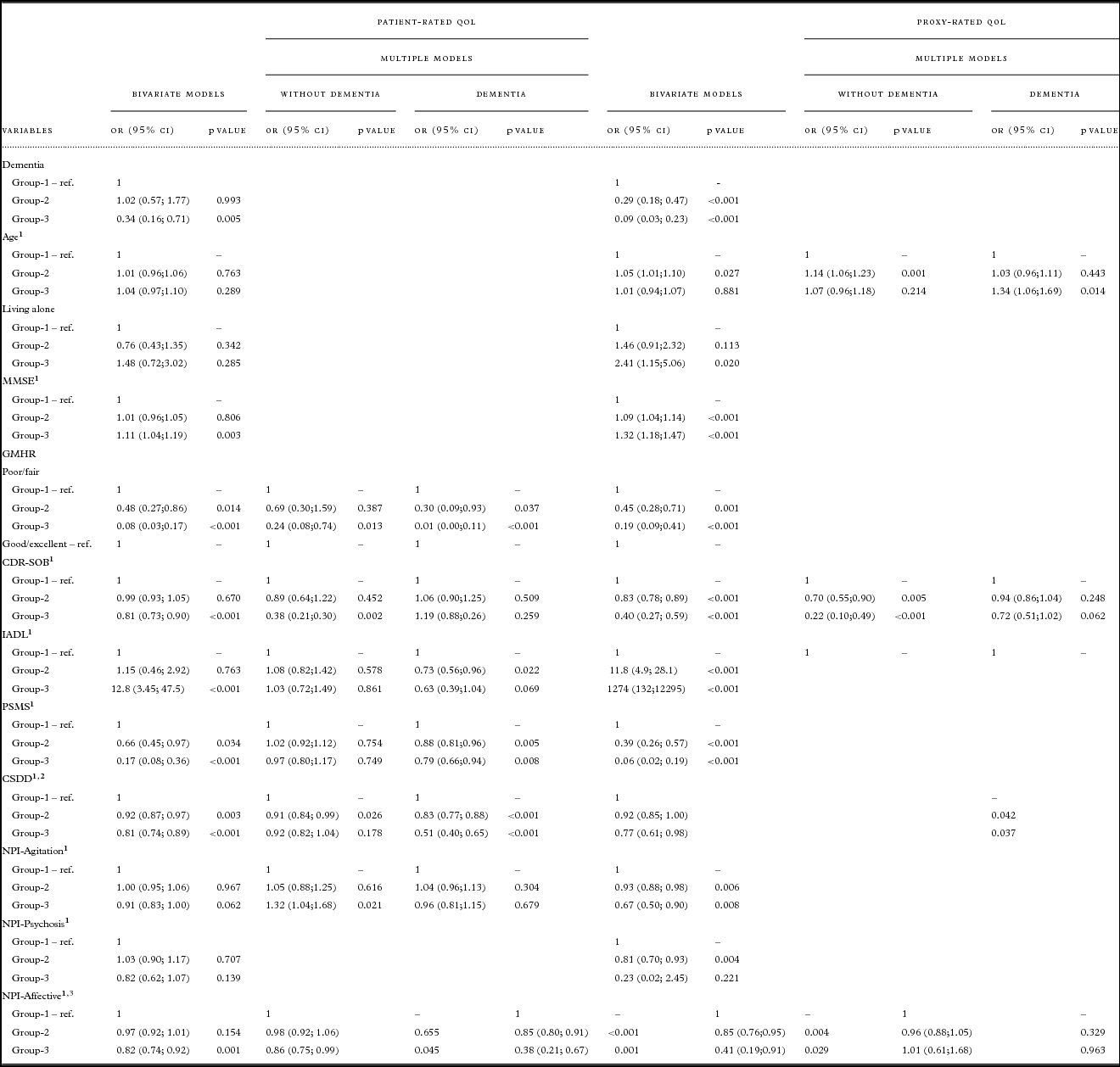
QoL = Quality of life; OR = odds ratio; CI = confidence interval; Coeff. = coefficient; SE = standard error; ref = reference value; MMSE = Mini-Mental State Examination; GMHR = General Medical Health Rating; CDR-SOB = Clinical Dementia Rating-Sum of Boxes; IADL = instrumental activity of daily living; PSMS = Physical Self-Maintenance Scale; CSDD = Cornell Scale for Depression in Dementia; NPI = Neuropsychiatric Inventory NPI-Agitation = agitation/aggression, euphoria, disinhibition, aberrant motor behavior, and irritability; NPI-Psychosis = delusions and hallucinations; NPI-Affective = depression, anxiety, and apathy.
1 Odds for one-unit change.
2 No interaction between dementia diagnosis and CSDD present, i.e. odds are the same for those without and with dementia.
3 No interaction between dementia diagnosis and NPI-Affective among patients present, i.e. odds are the same for those without and with dementia.
Bivariate analyses for proxy-rated QoL (Table 3) show that the chances of belonging to G1 versus G2 and G1 versus G3 were higher for persons with a dementia diagnosis, more cognitive impairment (MMSE), poor/fair physical health (GMHR), more severe dementia (CDR-SOB), lower instrumental functioning (IADL), lower functional ADL (PSMS), more depressive symptoms (CSDD), more agitation (NPI-Agitation), and more affective symptoms (NPI-Affective), while the chances of belonging to G1 versus G2 were higher for persons with lower age and more psychosis (NPI-Psychosis), while the chances of belonging to G1 versus G3 were lower for persons living alone.
Nominal regression analysis in patient-rated QoL
Multivariate analyses presented in Table 3 show that more depressive symptoms (CSDD) were associated with the higher chances of belonging to G1 versus G2, while more affective symptoms (NPI-Affective) were associated with higher chances of belonging to G1 versus G3, independently of dementia diagnosis.
Persons with dementia and poor/fair physical health (GMHR) and lower ADL functioning (PSMS) were more likely to belong to G1 versus G2 and G1 versus G3. Lower instrumental functioning (IADL) for persons with dementia increased the chances of belonging to G1 versus G2.
Among persons without dementia the chances of belonging to G1 versus G3 increased with poor/fair physical health (GMHR), a higher score on the CDR-SOB scale, and a lower score on NPI-Agitation.
Nominal regression analysis in proxy-rated QoL
As presented in Table 3, more symptoms of depression (CSDD) were associated with higher chances of belonging to G1 versus G2 and G1 versus G3, independently of dementia diagnosis.
For persons with dementia, higher age was associated with lower chances of belonging to G1 versus G3. For persons without dementia, higher age was associated with lower chances for belonging to G1 versus G2. In addition, a higher score on the CDR-SOB scale as well as more affective symptoms (NPI-Affective) were associated with higher chances of belonging to G1 versus G2 and G1 versus G3.
Discussion
This study assessed longitudinal changes in QoL in persons with and without dementia and explored the factors that were associated with changes in QoL. We found three different BL levels and trajectories of QoL in both patient- and proxy-rated QoL.
The changes in QoL scores during the 18-month observation period were, however, small and mostly non-significant.
The type of QoL-trajectory group membership was associated with the severity of the symptoms of depression, dementia severity, physical health, physical and instrumental functioning, NPI-Agitation, and age, with small variations between patient- and proxy-rated QoL-AD. However, we observed that the agreement between patient- and proxy-rated QoL-AD was poor, implying that patients and proxies assess QoL differently.
While former studies have demonstrated significant reductions in proxy-rated QoL for patients with dementia over an 18–24-month period (Lyketsos et al., Reference Lyketsos, Gonzales-Salvador, Chin, Baker, Black and Rabins2003; Tatsumi et al., Reference Tatsumi2009; Bosboom et al., Reference Bosboom, Alfonso and Almeida2013; Bosboom and Almeida, Reference Bosboom and Almeida2016; Conde-Sala et al., Reference Conde-Sala2016a), this was not found in our study, neither for the cohort as a whole nor for the respective trajectory groups.
We saw a statistically significant QoL reduction in only one of the patient-rated trajectory groups. The total decline in QoL was, however, small and has probably only minor clinical implications.
The lack of changes to QoL could be explained by the inclusion of a larger and more heterogenic population in our study with a broader variety in age and cognitive and functional limitations than previous studies. Considering the highly subjective nature of QoL assessments (Ready and Ott, Reference Ready and Ott2003) there is also a possibility that the individuals general positive or negative life perceptions has a stronger influence than dementia on their QoL evaluation. In total, the lack of QoL changes supports a former proposition that BL QoL is more strongly associated to later QoL than to QoL changes (Conde-Sala et al., Reference Conde-Sala, Turro-Garriga, Portellano-Ortiz, Vinas-Diez, Gascon-Bayarri and Rene-Ramirez2016b).
The agreement between patient ratings and their proxy ratings regarding QoL trajectory affiliation showed a low kappa score of 0.22 (Cicchetti, Reference Cicchetti1994). Such discrepancies between patient self-ratings and proxy ratings have been reported in several previous publications (Logsdon et al., Reference Logsdon, Gibbons, McCurry and Teri2002; Tatsumi et al., Reference Tatsumi2009; Thorgrimsen et al., Reference Thorgrimsen2003; Andrieu et al., Reference Andrieu2016). Nonetheless, Bosboom et al. found the agreement to be reasonably high, though proxy ratings were systematically lower than patient self-ratings (Bosboom et al., Reference Bosboom, Alfonso, Eaton and Almeida2012).
The discrepancy we found might also be – as suggested by others – a result of proxy bias from higher carer burden (Andrieu et al., Reference Andrieu2016) or carers’ depression (Logsdon et al., Reference Logsdon, Gibbons, McCurry and Teri2002), causing proxies to project their own QoL onto the participants. Unfortunately, this study design does not comprise these factors.
Another confounding factor could be that the participants’ lack of insight causes an overestimation of their own QoL (Conde-Sala et al., Reference Conde-Sala2016a). There is also a possibility that patients undergo a process of adaption to their disability and thus perceive their QoL as higher than their proxies do (Banerjee et al., Reference Banerjee2009).
Characteristics associated with QoL
More severe depressive symptoms were associated with lower QoL in both patient- and proxy-rated QoL in our study, independent of dementia status. Also, more affective symptoms covering the items depression, anxiety, and apathy in the NPI were associated with lower QoL in patient ratings independent of dementia status and in proxy ratings for persons without dementia. This relation has been described in several previous studies confirming that depressive symptoms are strongly associated with QoL (Banerjee et al., Reference Banerjee2009; Tatsumi et al., Reference Tatsumi2009; Bosboom et al., Reference Bosboom, Alfonso, Eaton and Almeida2012, Reference Bosboom, Alfonso and Almeida2013; Heggie et al., Reference Heggie2012; Andrieu et al., Reference Andrieu2016; Conde-Sala et al., Reference Conde-Sala, Turro-Garriga, Portellano-Ortiz, Vinas-Diez, Gascon-Bayarri and Rene-Ramirez2016b). We should therefore stress the importance of addressing depression as part of our care services.
Furthermore, being in the two lowest categories of physical health (GMHR) considerably reduced the chance of belonging to the higher QoL trajectories, independent of dementia status for patient-rated QoL, indicating that physical health affects QoL regardless of cognitive functioning. Contrary to our findings, a previous study (Huang et al., Reference Huang2015) could not find any association with QoL and comorbidity.
Low ADL functioning (PSMS) and low instrumental functioning (IADL) were associated with lower patient-rated QoL in persons with dementia. The association between functional ability and QoL has more often been described with proxy ratings (Banerjee et al., Reference Banerjee2009; Tatsumi et al., Reference Tatsumi2009). However, in more recent studies, associations between limitations in IADL and low patient-rated QoL (Andrieu et al., Reference Andrieu2016), and between poorer functional ability (ADL) and low patient-rated QoL (Conde-Sala et al., Reference Conde-Sala, Turro-Garriga, Portellano-Ortiz, Vinas-Diez, Gascon-Bayarri and Rene-Ramirez2016b) (Heggie et al., Reference Heggie2012), could be found.
Higher age was associated with higher proxy-rated QoL independent of dementia status. This association has been reported in a previous study, where it was interpreted as representing various levels of expectation and social support with age (Banerjee et al., Reference Banerjee2006).
In contrast to previous studies (Conde-Sala et al., Reference Conde-Sala2014; Huang et al., Reference Huang2015), we found no association between dementia severity and patient-rated QoL. As suggested previously (Bosboom et al., Reference Bosboom, Alfonso, Eaton and Almeida2012; Andrieu et al., Reference Andrieu2016), persons with dementia gradually lose insight as the disease progresses; thus, a link between dementia severity and QoL in this group is less likely with patient ratings. Proxies, to the contrary, are more likely to evaluate the patient's QoL from a disease and disability perspective rather than from an individual perspective (Bosboom et al., Reference Bosboom, Alfonso, Eaton and Almeida2012; Andrieu et al., Reference Andrieu2016). However, in our study, more cognitive impairment (CDR-SOB) was associated with a lower QoL in proxy and patient-rated QoL in participants without dementia.
We also found that higher NPI-Agitation scores were associated with higher patient-rated QoL in persons without dementia. We do not fully comprehend this finding in our population but suggest that this could indicate that the NPI may not be an appropriate assessment for persons without cognitive impairments, or that this result illustrates the difference in opinions between proxy and patient ratings, as NPI was also rated by proxy.
Limitations and strengths of the study
As QoL was first evaluated 18 months after the inclusion of patients in the original CONSIC-study, there is a possibility of selection bias towards participants with better health outcomes.
Approximately, 100 participants were admitted to nursing homes between BL and FU observation, resulting in a more heterogenic study population at FU. Data on relevant domains associated with QoL changes in dementia, such as carer burden and carers’ depression, could have added more depth to the analysis if they had been included in the data collection. The observation period was probably too short to catch changes in QoL over time in such a heterogenic population.
We used the QoL-AD to assess QoL in all participants, although it has only been validated for use in persons with dementia. However, only one of 13 items in the questionnaire refers to memory impairment. We therefore consider the results as sufficiently reliable to compare QoL in persons with and without dementia.
The strengths of the study were the large cohort of 412 persons that included persons with dementia as well as persons without dementia. A further advantage was the data collection organized by experts in the field. It provided a rich characterization of clinical parameters with standard outcome measures. Another plus was the QoL assessment taken from both the patients’ and the proxies’ points of view, with a scale well adapted for use in longitudinal studies (Selwood et al., Reference Selwood, Thorgrimsen and Orrell2005).
Conclusion
In this study, we found that, despite significant changes in clinical parameters, patient- and proxy-rated QoL in an elderly population did not change substantially over a period of 18 months.
We confirmed findings from previous studies that patients and proxies evaluate the patients’ QoL differently. QoL is an important aspect of person-centered care, but obviously QoL was determined not only by the clinical aspects we examined, especially in patients with dementia. Thus, we need to focus on the patients’ personalities, comprising their history and culture as well as their beliefs, values, family relations, and individual perceptions of QoL.
The foremost factors associated with lower QoL in our study sample were more severe symptoms of depression, NPI-Affective symptoms, and poorer physical health for the whole population, while low functional abilities were only associated with low QoL for persons with dementia. Efforts aiming at preventing low or decreasing QoL at any stage of dementia should, therefore, target these factors as well as keeping a person-centered approach open to the individual perceptions of QoL.
Conflicts of interest
None.
Description of authors’ roles
A. E. Ydstebø designed the study and wrote the paper. S. Bergh organized the data collection and supervised the development of the paper. G. Selbæk organized the data collection and supervised the development of the paper. J. Šaltytė Benth was responsible for the statistical design of the study and for carrying out the statistical analysis. K. Brønnick supervised the development of the paper. C. Vossius supervised the design of the study and the development of the paper.
Acknowledgments
The authors would like to thank Dr. Knut Engedal for his contributions as an expert consultant in the early phase of the study.







