Introduction
Measles is an acute, highly infectious human disease caused by a virus of the family Paramyxovirus, genus Morbillivirus [Reference Rota1]. Since 2000, global mortality attributed to measles has decreased by an impressive 79%, from an estimated 546 800 deaths to 114 000 in 2014. During this period, worldwide annual reported measles incidence declined 73%, from 146 to 40 cases per million population [Reference Perry2]. Despite this progress, outbreaks have continued in some regions. In 2010, 98% of the 139 300 estimated global measles deaths occurred in Africa and Asia. Significant measles outbreaks continued to occur in sub-Saharan Africa in 2009–2011 [Reference Simons3, 4], and World Health Organization (WHO) recently reported an increase of 33% of the number of measles cases in the African region from 2014 to 2015 [Reference Patel5].
Population displacement may be a factor in this increase in measles cases, particularly in nations that experienced humanitarian disasters that disrupted public health services and displaced significant numbers of people [Reference Simons3]. There were an estimated 15.4 million refugees globally in 2010 [6]. Crowded living conditions, low vaccination coverage and malnutrition in refugee camps can increase the likelihood and severity of measles outbreaks [Reference Toole7–Reference Kouadio10].
Cameroon has experienced numerous measles epidemics, typically occurring in two distinct patterns [Reference Cummings11]. The three northern regions (Far North, North and Adamawa) experience major yearly epidemics; the seven southern regions have historically experienced major epidemics every 3 years. Recently, this pattern has changed, yearly epidemics now occur in the southern regions as well, despite improved vaccination coverage (from 74% in 2009 to 83% in 2013) [12]. Cameroon's measles vaccination coverage rate placed it among the 72 countries that did not attain ⩾90% immunisation coverage with MCV (measles-containing vaccines) [12]. Despite efforts to improve vaccine coverage, the number of reported measles cases has increased in Cameroon from 1869 cases in 2013, to 2176 in 2015, to 2815 in 2016 [13].
Genetic characterisation of wild-type measles virus (MeV) combined with standard epidemiological methods is an essential component of laboratory-based surveillance of measles. Such characterisation is performed throughout the world by members of the WHO Global Measles and Rubella Laboratory Network [Reference Mulders14]. Genetic and antigenic characterisation of wild-type MeV has identified eight clades (A–H) subdivided into 24 genotypes (A, B1–B3, C1 and C2, D1–D11, E, F, G1–G3, and H1–H2) [Reference Rota, Featherstone and Bellini15–17]. The genotyping of MeV is based on the 450-nt sequence encoding the C-terminal 150 amino acids of the nucleoprotein (N) gene(N-450) [18].
MeV was first isolated in Cameroon in 1983; these strains belonged to genotype B1 [Reference Rima19, Reference Rota20]. MeV strains found in Cameroon in 2001 were genotype B3 [Reference Kouomou21] more recently, Demanou and collaborators showed that the circulating MeV strains in Cameroon between 2010 and 2011 were also the B3 genotypes [Reference Demanou22]
On December 10, 2012, the Central African Republic (CAR) experienced a serious political crisis [Reference Baptiste23] that led to displacement of a significant portion of the population as refugees into East Cameroon. There are no current reports of circulating MeV genotypes from East Cameroon. This report describes the measles genotypes detected of measles cases that occurred among refugees from the CAR during an epidemic in East Cameroon in 2014. Knowledge of currently circulating MeV genotypes in Cameroon will help in monitoring the success of the measles elimination programme and will contribute to the evaluation of the effectiveness of vaccination campaigns.
Methods
Urine samples were collected from suspected measles cases among children under 15 years of age at the Garoua-Boulai and Gado-Badzerie refugee camps (Fig. 1), using the guidelines from the WHO Regional Office for Africa (AFRO) [24]. A suspected measles case was defined as any person in whom a clinician suspected measles, or any person with fever, maculopapular rash, and coryza, conjunctivitis or cough. All samples were sent to the Mother-Child Center, Chantal Biya Foundation Virology Laboratory, Yaoundé, for analyses.
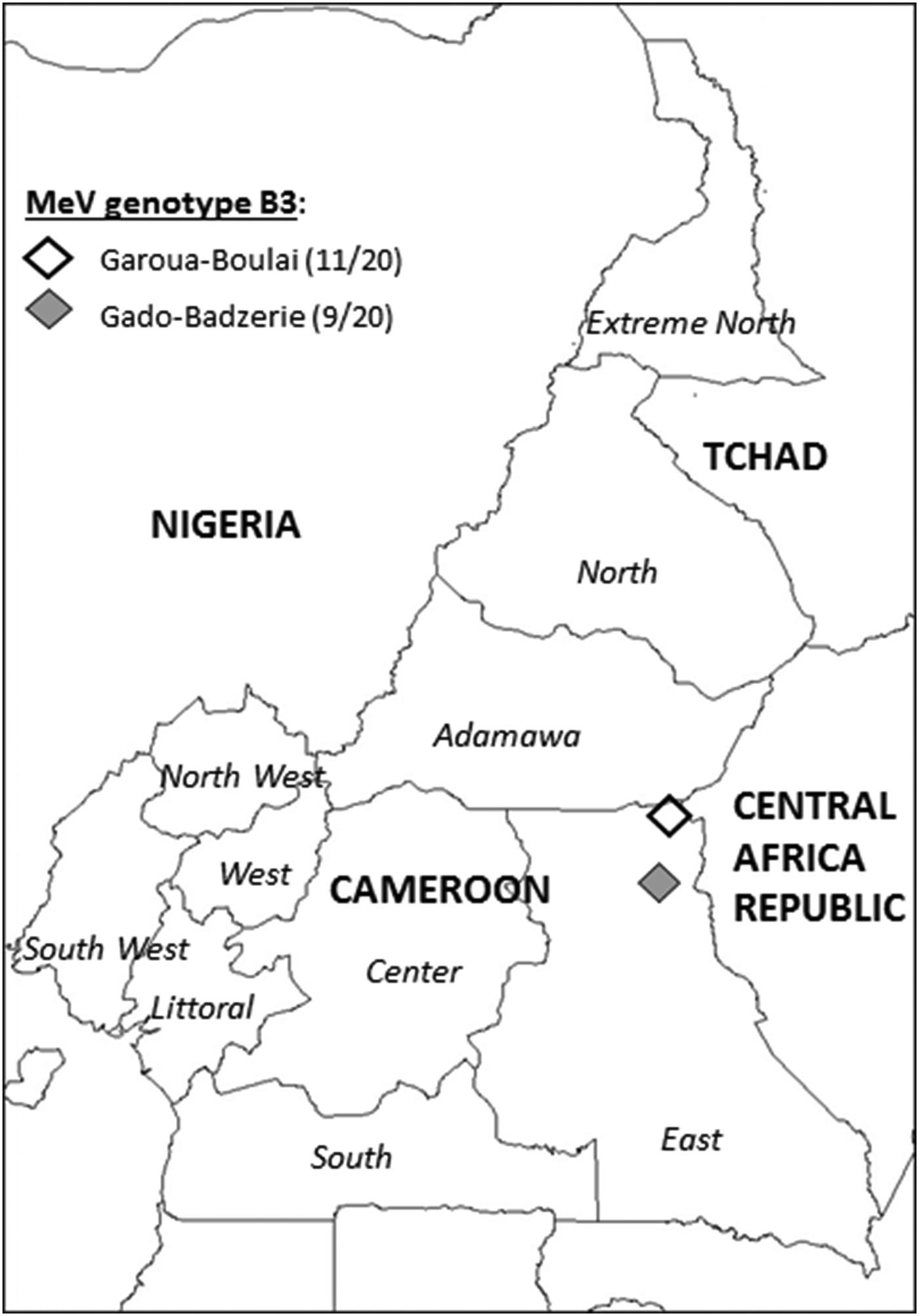
Fig. 1. Map of Cameroon showing location of collection sites of Garoua-Boulai and Gado-Badzerie in East region .The number of MeV strains sequenced in this study is indicated in brackets for each location. MeV genotype B3 was found in both location.
Viral RNA was extracted directly from these samples using the commercial QIAmp Viral RNA Mini Kit (Qiagen Inc., Valencia, CA, USA), as per the manufacturer's instructions. Detection of MeV RNA was performed by real-time reverse transcription polymerase chain reaction (RT–PCR) using the SuperScript III Platinum OneStep qRT–PCR Kit (Invitrogen, Carlsbad, CA, USA) to detect a 75 bp nucleotide fragment of the N gene as previously described [Reference Hummel25]. RNA samples that were positive by real-time PCR were used as template in an RT–PCR assay to amplify 634 bp of the MeV N containing N-450. The RT–PCR assay was carried out using the Qiagen one-step RT–PCR kit (Qiagen Inc., Valencia, CA, USA) with the previously described forward primer MeV216 (5′-TGG AGC TAT GCC ATG GGA GT-3′) and reverse primers MeV214 (5′-TAA CAA TGA TGG AGG GTA GG-3′) [Reference Bankamp26].
The nucleotide sequence of N-450 was obtained from the amplicons using the Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) and a 3130 DNA sequencer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions.
Sequence data were analysed using the Clustal W program for multiple alignments and Mega version 6.06 [Reference Tamura27]. Genotypes were assigned to Cameroon MeV strains by comparing them to the WHO MeV reference strains and drawing a phylogenetic tree using the neighbour-joining method. Bootstrap values were obtained through 1000 resampling of datasets. MeV sequences previously found in Cameroon [Reference Kouomou21, Reference Demanou22] and the CAR [Reference Tricou28] were also added to this analysis.
Results
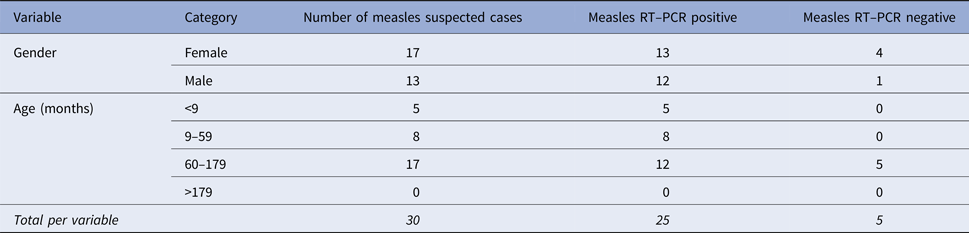
Urine samples were collected from 30 suspected measles cases. The mean age of the participants was 98 months (range 6–179 months); most were female (17/30, 56.7%). During the outbreak, most of measles positive cases were found among children aged 60–179 months (17/30, 56.7% of suspected cases) (Table 1).
Table 1. Gender and age distribution of suspected measles cases in refugees from the Central African Republic in East Cameroon, 2014
MeV RNA was detected in 83% (25/30) of the suspected cases by real-time RT–PCR including eight of eight children aged 9–59 months, and five of five samples from children <9 months. Of the measles cases that were confirmed by real-time RT–PCR, 52.6% were found in the Garoua-Boulai refugee camp and the remaining 47.4% were found in the Gado-Badzerie refugee camp.
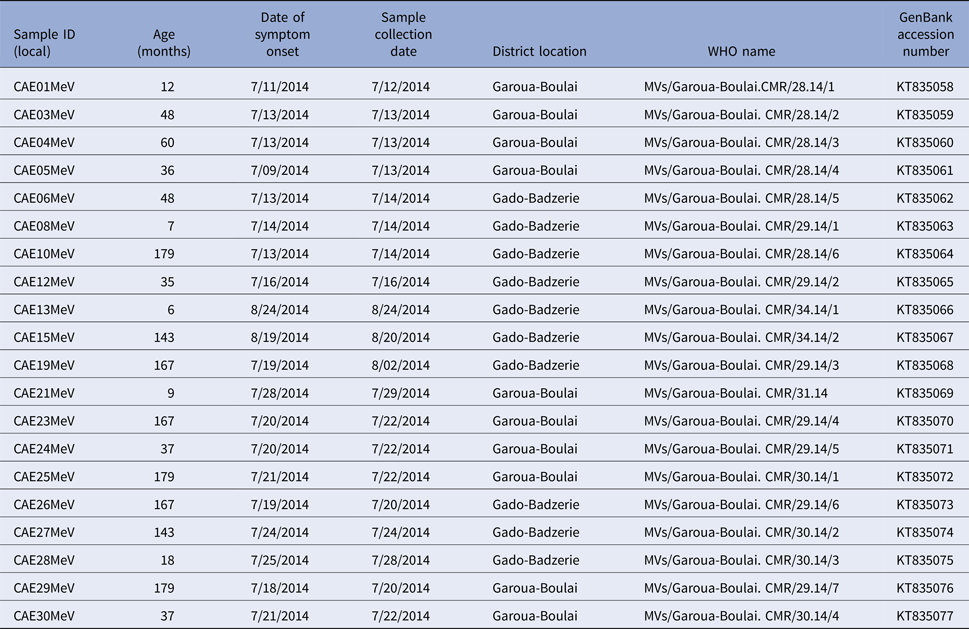
Among the 25 samples that were positive for measles with real-time RT–PCR, MeV sequences could be obtained from 20 (80%). Five different MeV sequences were found during this epidemic, however the MeV strains characterisation showed that they were all genotype B3 and closely related to the reference strain, MVi/Ibadan.NGE/0.97/1. The sequences from Cameroun clustered with other genotype B3 strains from Africa (Cameroon, Benin, Nigeria and Zimbabwe); Europe (particularly strains from UK); and Australia (Fig. 2). Comparison of the sequences obtained in this study with available sequences on the Measles Nucleotide surveillance (MeaNS) database [17] and GenBank showed no exact match with any of the sequences in the databases. The sequences from Cameroon were deposited in the MeaNS database and GenBank and the strains were named according to the WHO measles nomenclature [18]. The strain name and GenBank accession numbers (KT835058–KT835077) are shown in Table 2.
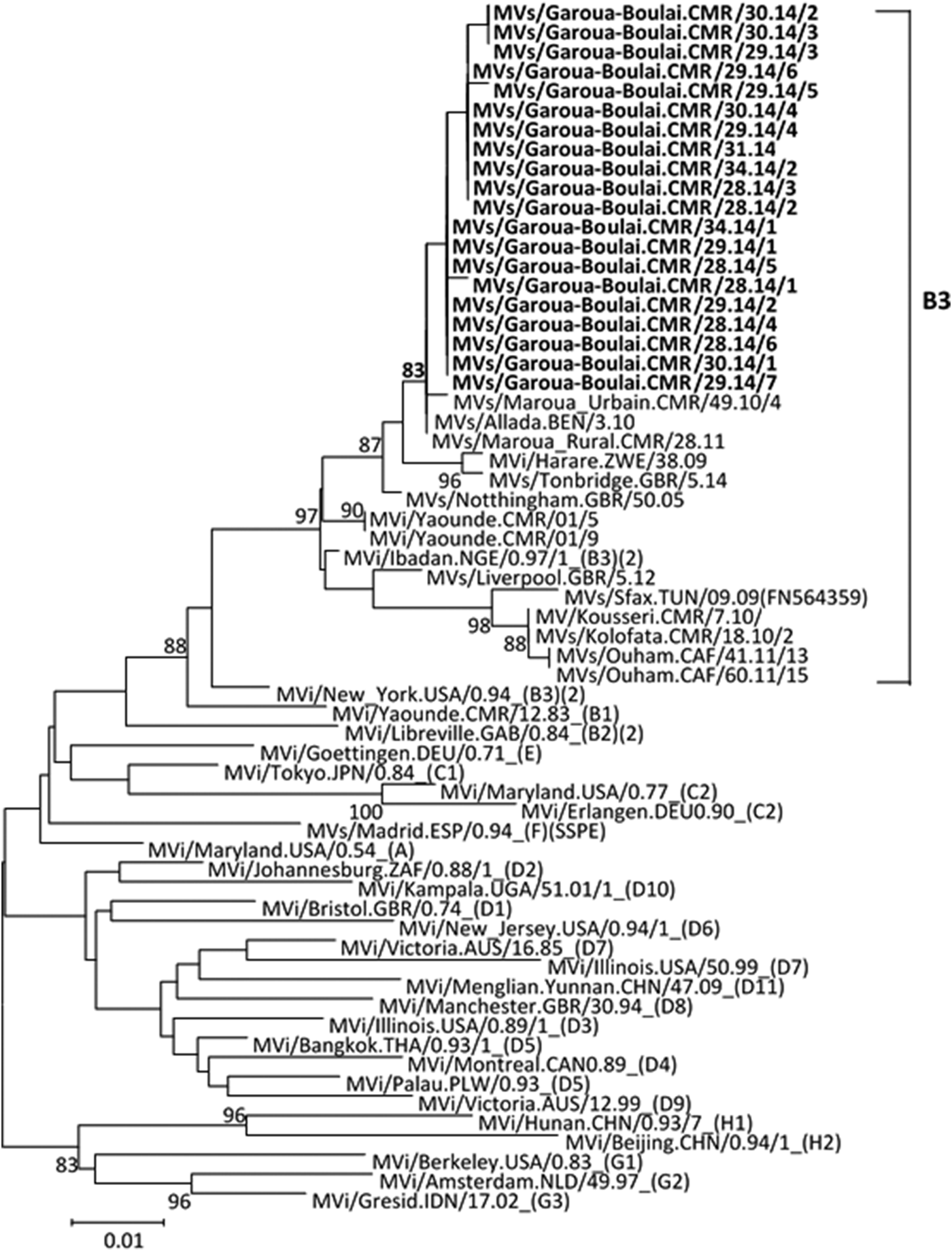
Fig. 2. Phylogenetic analysis of Cameroonian measles strains (in bold) based on N-450 sequences. WHO reference sequences are indicated by genotype in parenthesis on the taxon label. The GenBank number of N-450 sequences from related strains in genotype B3 (not bold) are indicated on the taxon label. Bootstrap values (>80%) are indicated.
Table 2. Strains of measles identified in refugees from the Central African Republic in East Cameroon with GenBank accession numbers
Discussion
This study sought to determine the circulating measles genotypes in a series of measles cases among refugees from the CAR during a measles outbreak in East Cameroon in 2014. The age distribution among confirmed cases showed that 52% of patients were <5 years of age; a similar proportion to the 51% found in Africa by Goodson and colleagues in 2011 [Reference Goodson29] and greater than 31.8% that observed by Wairagkar et al. in 2011 in India [Reference Wairagkar30]. Therefore, the results from this study are consistent with the results from other studies showing that the incidence of measles infection remains high among children <5 years of age in African countries despite efforts to improve immunisation coverage during the recent years. Most African countries have not yet achieved the goal of 90% of measles immunisation coverage as recommended in the WHO and UNICEF Global Immunization Vision and Strategy for 2006–2015 [31].
The 20 sequences from this study clustered with the reference sequences for the genotype B3 reference strain MVi/Ibadan.NGE/0.97/1. Genotype B3 is the endemic genotype in most of the African continent, with the exception of the North African countries in the Eastern Mediterranean Region [Reference Patel5, 17]. MeV genotype B3 strains that are more closely related to reference strain MVi/New York.USA/0.94, which appears to be more limited to Western Africa [Reference Mulders32], were not detected in this study in contrast to the earlier findings [Reference Demanou22] .In addition, the genotype B3 reference strain MVs/Sfax.TUN/09.09 was absent, contrary to what was observed in the Far North region of Cameroon [Reference Demanou22]. MeVs from clade B are endemic in sub-Saharan Africa [Reference Haddad-Boubaker33], and B3 genotype has been detected in several countries, including Ghana, Gambia, Nigeria, Libya and Tunisia, elsewhere in Europe (France and Germany) and in the USA [Reference Riddell, Rota and Rota34, 35]. Since 2014, genotype B3 has become widely distributed globally [17].
Phylogenetic analysis showed that the MeV strains found in this study were closer to MeV strains circulating in the northern part of Cameroon in 2010–2011 (MVs/Maroua.Urbain.CMR/49.10/4 and MVs/Maroua.Rural.CMR/28.110) [Reference Demanou22] than to MeV strains circulating in CAR in 2011 (MVs/Ouham.CAF/41.11/13-JG669934) [Reference Tricou28]. This genotype B3 strain was previously detected in the Northern, Central, and Western regions of Cameroon [Reference Kouomou21, Reference Demanou22] and CAR [Reference Tricou28, Reference Gouandjika-Vasilache36]. However, there is no information available on previously circulating measles strains in the East region of Cameroon, and therefore it is not possible to determine if the strains found in this study were already circulating in the region or if they were imported from CAR during the migration caused by the crisis.
Crisis situations are common driving factors for epidemic disease outbreaks such as measles, as they are often accompanied by systemic breakdowns of healthcare, mass population migration, and widespread homelessness. It is common to find refugees living in overcrowded camps, and this facilitates the transmission of communicable diseases, particularly with the frequent presence of malnutrition and poor hygiene.
The MeV sequences found in this study belonged to genotype B3, but no identical sequences have been reported before in available databases (MeaNS and GenBank). This study highlights the importance of enhancing virologic surveillance of measles in the CAR and Cameroon.
Acknowledgements
The authors gratefully thank the Cameroon Ministry of Public Health for its commitment to measles and rubella elimination; the WHO country office for supporting the national measles/rubella surveillance program in Cameroon; the United Nations High Commissioner for Refugees (UNHCR) in Cameroon for taking care of the refugees in East Cameroon; and all health care workers at the Garoua-Boulai health district in the East region of Cameroon.
This study was supported by the Ministry of Public Health, Cameroon; the World Health Organization (WHO); and the U.S. Centers for Disease Control and Prevention (CDC).
Declaration of interest
The authors declare no conflicts of interest.
Ethical considerations
The Cameroon Measles/Rubella surveillance is a national programme approved by the Ministry of Public Health and supported by WHO/AFRO as part of the global goal to control and eliminate measles and rubella. Patient information and specimen collection respected the procedures stipulated in the WHO/AFRO measles/rubella surveillance protocol.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.






