INTRODUCTION
Q fever is a zoonotic disease caused by Coxiella burnetii which is primarily transmitted to humans by inhalation of infected aerosols [Reference Maurin and Raoult1]. Cattle, sheep and goats are the most common reservoirs and the main source of human infection [Reference McQuiston, Childs and Thompson2, Reference Parker, Barralet and Bell3]. Animals shed the organism in birth products, urine, faeces and milk and as C. burnetii is a very hardy organism, it can remain in the environment for weeks to months [Reference McQuiston, Childs and Thompson2]. Illness has been associated with contact with contaminated straw, dust, wool, clothing and raw milk. Q fever has a variable clinical presentation in humans, ranging from asymptomatic infection to a self-limiting febrile illness through to severe pneumonia and hepatitis, and chronic infection [Reference Raoult, Marrie and Mege4].
In Australia, disease notification rates of Q fever are highest in the eastern states of Queensland and New South Wales (NSW) with most notifications occurring in working-age males [Reference Yohannes5]. In the past, Q fever notifications have been strongly associated with the number and level of activity of abattoirs and number of livestock properties in the area [Reference Garner6]. Some of the earliest recorded outbreaks in Australia were in urban abattoirs in Queensland and South Australia (SA) [Reference McKelvie7–Reference Beech, Duxbury and Warner9]. Abattoir outbreaks have continued to occur and mainly implicate cattle and, less commonly, sheep and feral goats [Reference McKelvie7, Reference Buckley10–Reference Sam and Van12]. The majority of Q fever cases have been linked to occupational exposure; however, there have also been a number of cases where a source could not be identified [Reference Chong13]. A number of serological and epidemiological surveys undertaken in Queensland and NSW have highlighted the high prevalence of exposure in people working with livestock and living in rural communities [Reference Taylor, Hunter and Tan14–Reference Islam17].
A nationally funded Q Fever Management Programme ran from 2001 to 2004, initially targeting abattoir workers and shearers and expanded to include graziers and dairy farmers (although these groups were required to meet the cost of screening and medical visits) [Reference Gidding18]. Since its implementation there has been a decline in notifications of Q fever nationally. However surveillance inconsistencies between states and territories make it difficult to assess whether this decline is occurring in the target vaccination group [19].
In SA, there is a legal obligation for medical officers and laboratories to report Q fever to the Communicable Disease Control Branch (CDCB) as it is a notifiable disease under Section 30 of the Public and Environmental Health Act (1987).
In December 2004, the CDCB was informed of eight cases of acute Q fever by the local public health laboratory. Cases were clustered in the mid-north of the state. Prior to December, 17 cases of Q fever had been notified in SA in 2004, compared with a 5-year average of 15 cases. The average annual number of cases for the mid-north region of SA is between 0 and 5 cases [20]. An investigation was undertaken to characterize the outbreak, generate and test hypotheses as well as identify an appropriate public health intervention to minimize further cases. This report outlines the findings from the outbreak investigation and epidemiological study.
METHODS
Study design
A case-series investigation was conducted via telephone interviews by two principal investigators using a semi-structured hypothesis-generating questionnaire. The questionnaire elicited information on illness, occupation, travel, vaccination and exposure to a range of known risk factors. Cases were identified by both laboratory and medical referrals and active case-finding through local General Practitioners (GPs) and hospitals in the area.
A case-control questionnaire was designed incorporating risk factors identified from the hypothesis-generating questionnaires including attendance at the local saleyard, shearing, slaughtering and transporting sheep. Furthermore, any person who reported attending a saleyard was asked additional questions regarding activities undertaken at the saleyard and likely duration of exposure at the saleyard. A case was defined as a person notified to the CDCB with seroconversion, or high titre of IgM antibody, to Phase II C. burnetii antigen and a clinically compatible illness. Controls were selected from a database, operated by Department of Health, SA [21] that contains a representative sample of South Australian households. This survey method has previously been found to be reliable in measuring health behaviours [Reference Starr22, Reference Kirk23]. The study population was defined as the general population living in the mid-north of the state in 2004. Three controls were matched to each case by 5-year age groups, sex and postcode or closest postcode. At the time of the interview, controls were asked whether they would provide a blood sample to test their Q fever immunity status.
Data were entered into Epi Info v. 6 and analysed in Epi Info v. 6 (Epi Info, CDC, USA), Microsoft Office Excel 2003 (Microsoft Corp., USA) and Stata v. 11 (StataCorp., USA). Univariate analysis of matched cases and controls was performed by calculating Mantel–Hanszel odds ratios using the mhodds command. ‘Attendance at saleyard in previous 2 months’ is a summary variable consisting of the four other variables of attendances on a particular date at the saleyard and was excluded from further analysis. The remaining variables with a P value <0·1 in the univariate analysis were included in the model generated using exact conditional logistic regression. An exhaustive stepwise process was used to evaluate whether variables, or all combinations of variables, that did not remain significant in the initial model could be effect modifiers.
Environmental investigation
Environmental samples of soil and dust from the saleyard and an adjoining truck wash area were collected by Primary Industries and Resources South Australia (PIRSA) on 14 December 2004. The environmental samples underwent nucleic acid testing (NAT) at the Australian Rickettsial Reference Laboratory based in Geelong, Victoria. Details on weather conditions were obtained from the Bureau of Meteorology using data measured at their closest weather station located 15 km north of the saleyard. Information about the sale day was gathered from stock and station agents who organized the sheep sale. This included an approximate number of sheep at the saleyard, their origin, presence of lambing, sheep deaths and how the sale day was conducted. General information on livestock in the mid-north region was provided by PIRSA.
Laboratory investigation
The laboratory diagnosis of Q fever infection was confirmed at the Institute of Medical and Veterinary Sciences (IMVS) located in Adelaide using immunofluorescence. Clinical specimens sent to other laboratories were forwarded to IMVS for confirmation.
RESULTS
Descriptive epidemiology
Twenty-five cases with dates of onset of illness between 6 November 2004 and 7 January 2005 were identified (Fig. 1). Of these, 23 had attended a local sheep saleyard with 22 attending the saleyard on the same day, 21 October 2004 (Fig. 2). The demographic and clinical details of cases are outlined in Table 1. Most cases were working-age males from the same regional area as the saleyard. The only case not residing in the mid-north of SA was a resident of Adelaide who had visited the same town as the saleyard earlier in the month (2 October 2004) and reported not visiting the saleyard area. The only female case was pregnant and her pregnancy proceeded without complications.
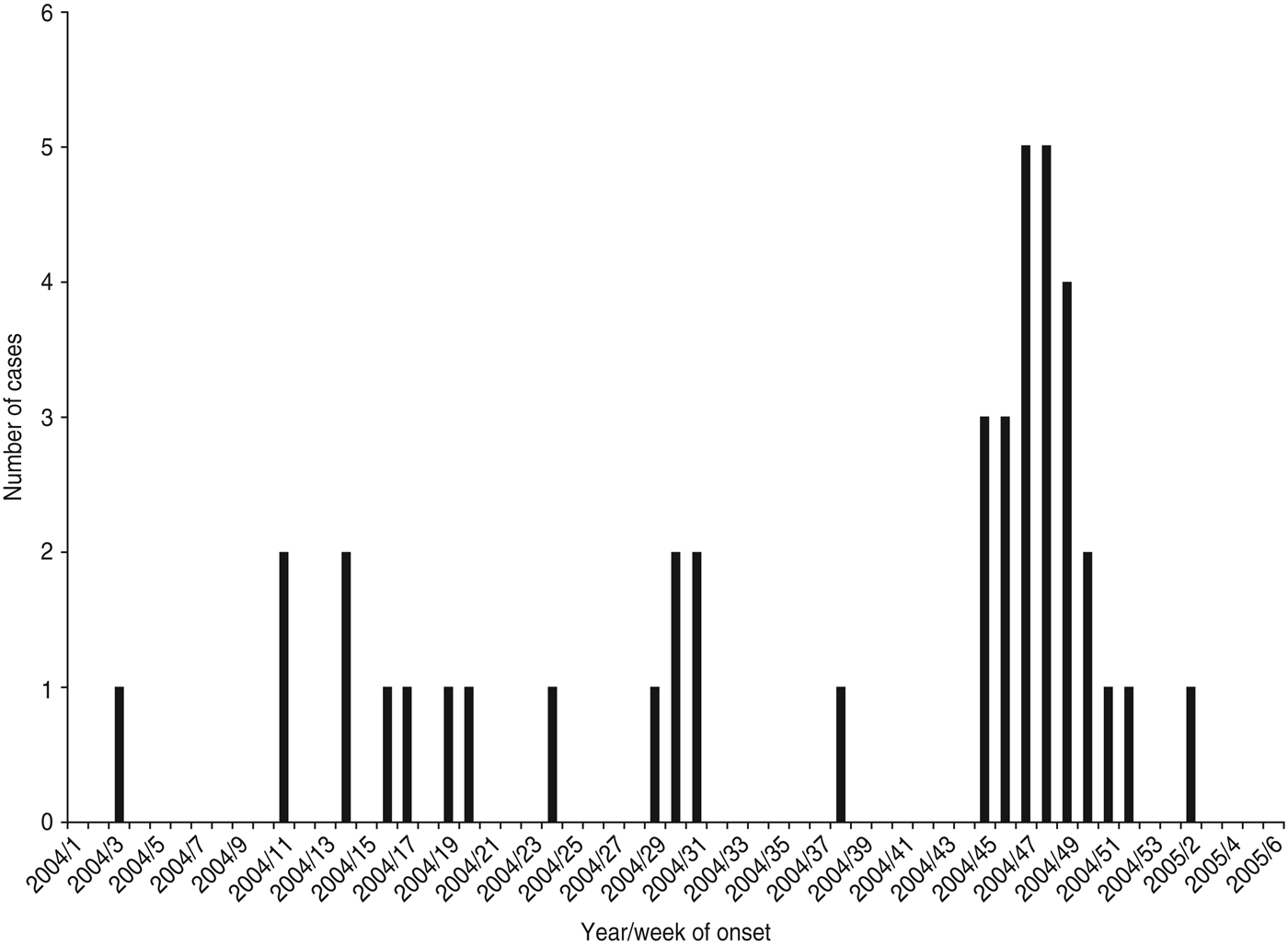
Fig. 1. Cases of Q fever by year and week of onset of symptoms, January 2004–February 2005, South Australia.
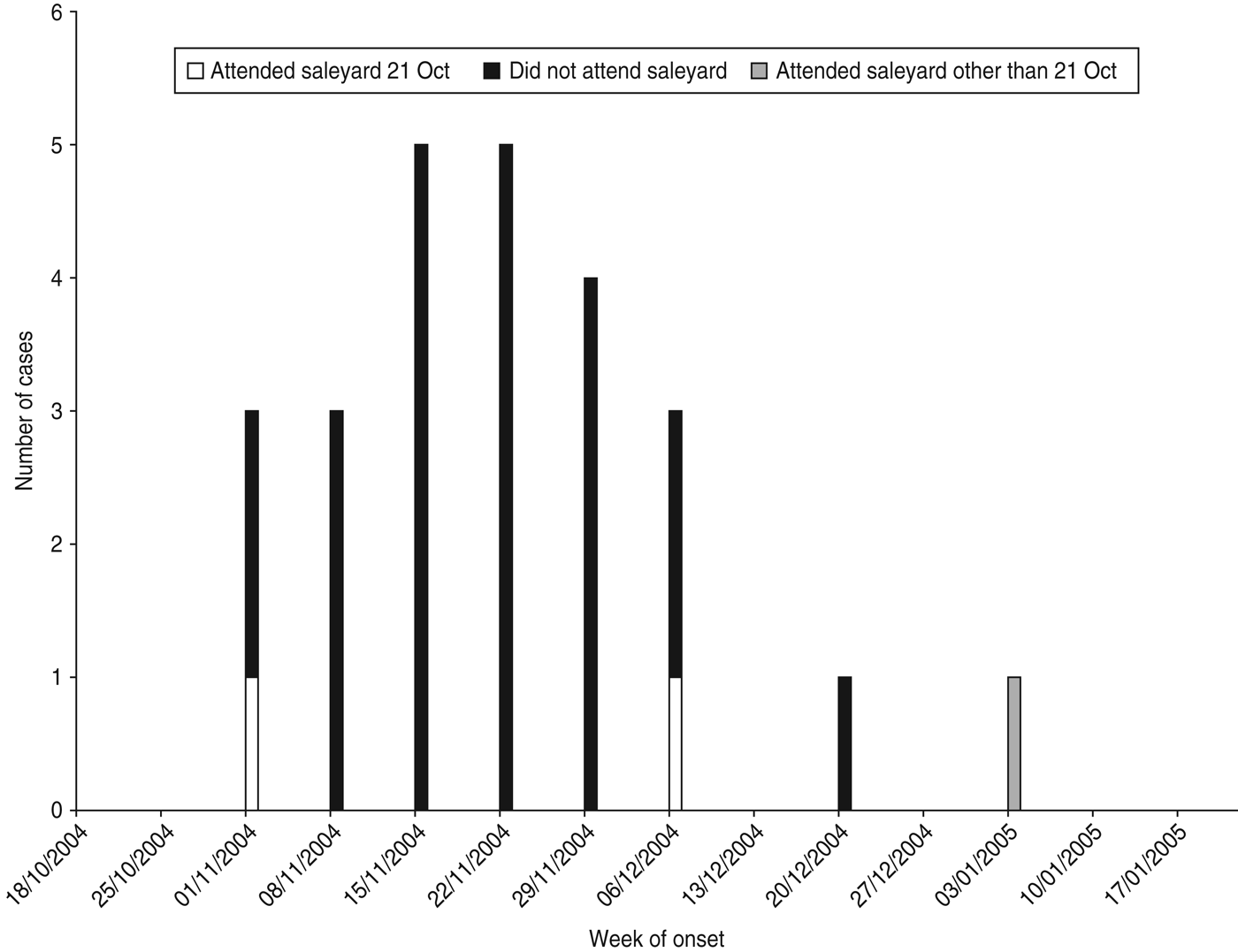
Fig. 2. Cases of Q fever by week of onset of symptoms and attendance at saleyard, November 2004–January 2005, South Australia.
Table 1. Descriptive epidemiology of Q fever cases notified November 2004–January 2005, South Australia
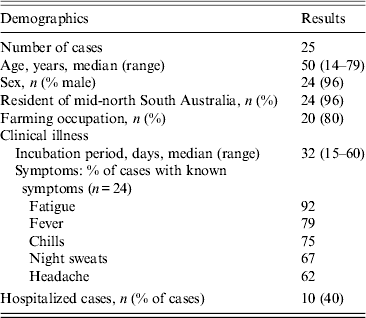
About 200–250 people were reported to have attended the saleyard on 21 October 2004 giving an estimated attack rate of 9–11% for this outbreak. The median time cases attended the saleyard was 2·5 h (range 40 min to 11 h).
Analytical epidemiology: case-control study results
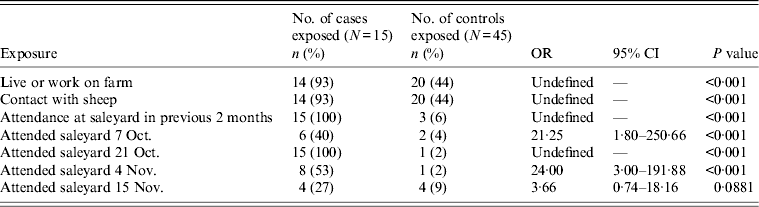
The hypothesis tested in the case-control study was that attendance at the saleyard on 21 October 2004 was associated with contracting Q fever. The case-control study used the first 15 confirmed cases from the case-series and 45 controls matched on age (within 5 years), sex and postcode. A higher proportion of cases (93%) than controls (33%) reported working with sheep in the study.
Table 2. Matched univariate analysis of exposure variables in case-control study
OR, Odds ratio; CI, confidence interval.
The results of the matched univariate analysis indicated the likelihood of contracting Q fever was greatest in those people who attended the saleyard on the 21 October 2004 [odds ratio (OR) undefined, P < 0·01] and declined to statistically non-significant levels [OR 3·66, 95% confidence interval (CI) 0·74–18·16, P = 0·0881] by mid-November (Table 2). After adjusting for other variables in a matched exact logistic regression model, only attending the saleyard on 21 October 2004 remained significantly associated with illness (OR 15·28, 95% CI 1·69–∞, P = 0·014).
Environmental
The implicated saleyard is the main sheep market for the mid-north region of SA and only sheep were reported in the vicinity of the saleyard. Over 20000 sheep attended the sale day (21 October 2004), some arriving up to a day or two prior. The sheep were mainly from the mid-north region of SA. One mob of sheep had been transported from western NSW and another from central Victoria. These NSW and Victorian regions reported no change in Q fever notifications around this time.
The weather conditions during the time of the sale on 21 October 2004 included a south to south-westerly wind at a speed of 18–28 km/h. Many of the cases reported extremely dusty conditions on the day including having to wash the dust from their eyes and faces prior to leaving the saleyard.
The saleyard was located on the same road as a smaller independent saleyard that conducted sheep sales on different days and a truck wash used to wash out the livestock transport carriers. No cases reported attending this adjacent saleyard or the truck wash.
Laboratory
The soil and dust samples collected from the saleyard and surrounding areas were negative for C. burnetii. Serum samples of persons investigated for the outbreak confirmed 21 persons with evidence of seroconversion to Phase II C. burnetii antigen tested in paired sera and a further four persons with single high IgM antibody to Phase II C. burnetii antigen of 1:1280. All 25 persons had a clinically compatible illness and no history of recent Q fever vaccination. In addition, 17 (67%) persons with positive serology tested positive via NAT. The use of NAT for diagnosis in this outbreak investigation has been reported elsewhere [Reference Turra24].
Public health action
There was considerable pressure from members of the local community to cease operation of the saleyard, particularly as there were further sale days planned during the time of the outbreak investigation. A decision was made against closing down the saleyard based on the strong descriptive and supportive univariate analytical epidemiology suggesting a peak of risk on one day and no evidence to suggest that exposure was continuing. The action undertaken by CDCB included issuing a public health notice at the saleyard informing the public of an increase in Q fever cases and recommending persons get vaccinated in accordance with Australian guidelines. In addition, the local Environment Health Officer ensured that dust control measures at the saleyard were improved. Notifications of Q fever cases returned to expected endemic numbers in the months following.
DISCUSSION
This outbreak was one of the largest Q fever outbreaks reported in Australia not linked to an abattoir. Findings of the investigation suggest a point-source exposure caused by the introduction of infected sheep into the local saleyard on, or just prior to, the sale day on 21 October 2004. Contaminated soil and dust was then subsequently blown across the area of the saleyard on the day of the sale as reported by cases.
The descriptive and analytical epidemiology strongly supports this account although some cases may have alternate explanations. One case with a date of onset 60 days following their attendance at the sale was also a sheep farmer and therefore had opportunities to be exposed outside the saleyard. However, this case may provide an example of longer incubation periods from smaller infective doses [Reference Tigertt and Benenson25]. In fact the 32-day median incubation period for all cases that attended the saleyard on 21 October is also outside the ‘usual’ incubation period reported for Q fever and perhaps reflects the infective dose size in this outbreak. One of the cases who did not attend the saleyard at all appeared to have a likely explanation as his workplace was only ½ kilometre away from the saleyard and he regularly cycled past. However the wind direction recorded on that day does not support windborne spread of the organism to his workplace and the case reported not cycling past on that day. This case regularly visited farms as part of his job and may therefore have been exposed elsewhere. The other case who did not attend the saleyard is unlikely to be explained by other risk factors for acquiring Q fever, as they reported no occupational exposure to animals. An alternate explanation is that their exposure occurred while at the racecourse (in the same town as the saleyard) where sheep commonly graze. With spring lambing occurring between September and November, it is likely there were newly lambed ewes in the area by early October when the case spent the day at the racecourse. However, this reasoning means there must have been newly infected sheep in the area and more non-occupationally exposed cases would be expected from the local town or visiting the town.
The other explanation is that these cases, with a long incubation period or who did not attend the saleyard, could be considered part of background Q fever activity. However, given the cases were located in close proximity (in time and place) to an outbreak, this explanation is less likely. Moreover, the expected number of Q fever cases from the mid-north region of SA during this time had already been reached [20].
There are a number of limitations to the case-control study. The decision to stop the case-control study at 15 cases was based on the need to combine both the analytical and descriptive epidemiology evidence to inform public health action. However, the small sample size certainly influenced the number of undefined results in the analysis and the modelling within the multivariate analysis. Another limitation of the case-control study is that, although we asked whether controls were prepared to provide a blood sample during the telephone interview, due to time and resource limitations, the serology testing was not completed and therefore the immune status of the controls was not established. This would confirm whether the controls were non-immune to Q fever and therefore had the same opportunity to have been infected as the cases, reducing the potential for misclassification.
Ideally controls should have been chosen only from others just as likely to attend the saleyard or be in its vicinity. The requirement for speed during an outbreak and the limitations of the control bank, given the sparseness of the population in the region, prevented this refinement. However, we controlled for the effect of attending the saleyard during other sale days in October and November. When these variables were included in the analysis they did not affect the adjusted results.
This outbreak may also support the role of animal husbandry practices in Q fever outbreaks. Spring lambing occurs in this region between September and November. PIRSA reported it was possible recently lambed ewes could have been present at the 21 October sale day, providing a reservoir of C. burnetii and contaminating the soil and dust of the saleyard. In other countries it has been suggested that changes in sheep husbandry, particularly lambing during warmer and drier seasons may be potential explanations for recent seasonal shift and increase in Q fever outbreaks during this time [Reference Hellenbrand, Breuer and Petersen26].
The role of wind in the transmission of C. burnetii is well established in previous outbreaks. The findings of an investigation into a large outbreak of Q fever in mainly metropolitan residents of the West Midlands of Great Britain strongly suggested windborne spread from a rural area up to 18 km away. The weather conditions on one day during the likely period of exposure included southerly 125 km/h winds from a farming area where lambing and calving was occurring [Reference Hawker27]. One area in France bordered by high-density sheep farming has commonly seen higher than expected Q fever cases approximately a month following peak lambing season. This timing coincides with an incubation period for Q fever and increased frequency of the mistral wind from the sheep-farming area [Reference Tissot-Dupont28, Reference Tissot-Dupont29]. There are further documented Q fever outbreaks where airborne transmission probably had a role, particularly involving sheep reservoirs but also in the massive, goat-associated outbreak in The Netherlands, where living within 5 km of an infected farm was the most important risk [Reference Carrieri30, Reference van der Hoek31]. It would appear reasonable to expect that the weather conditions on the 21 October at the saleyard (18–28 km/h winds) could effectively distribute dust around the immediate saleyard area. While the negative results of the environmental tests may reflect the limitation of sampling, they are consistent with a risk of infection limited to a single sale day and not persisting beyond that.
Previous outbreaks have also been associated with close proximity to sheep pens. Nearly 300 cases were linked to a farmers’ market in Germany where only a small number of sheep were exhibited including a newly lambed ewe which was found to be positive [Reference Porten32]. Although we do not have proof of infected sheep at the saleyard on the sale day, the point-source appearance of the outbreak demonstrates this to be the necessary link.
This outbreak had a lower attack rate than other Q fever outbreaks reported. The outbreak linked to a farmers’ market in Germany had an estimated attack rate of 20% compared to about 10% in this outbreak [Reference Porten32]. This finding may reflect either less contact between persons and infected sheep at the saleyard compared to the farmers’ market or a smaller infectious dose. A result of note from this outbreak is that an exposure time as short as 40 min resulted in infection.
An important feature of this outbreak was that most of the cases occurred in the target group for vaccination. The majority of farmers in this outbreak were aware of the nationally funded Q Fever Management Programme; however, vaccine uptake was poor. Reasons for this ranged from not getting around to making the appointment with the doctor, to not considering themselves at risk. It demonstrates that awareness of the risk of Q fever has not infiltrated all risk groups targeted for vaccination. This result has also been found in a survey of Q fever cases in the neighbouring state of NSW [Reference Massey, Irwin and Durrheim33]. An important outcome of this outbreak was an increase in uptake of Q fever vaccination in this region of SA as reported by local GPs. The increased uptake was reported as community-wide including uptake in the farming population and was accompanied by training of additional Q fever vaccination providers in the region to assist in providing a sustainable service to this occupational group.
Another interesting feature of this outbreak is that cases were predominantly men who have worked with sheep in this region for many years. It provides evidence that Q fever has not been endemic in sheep flocks in the mid-north of SA. Many serological surveys of occupational groups overseas have indicated that farmers have shown a proportion already positive for Q fever, 27% in English farmers [Reference Thomas34] and 24% in sheep farmers in Sweden [Reference Macellaro, Akesson and Norlander35]. This outbreak supports the findings from Queensland that farmers are an important, often overlooked and harder to reach, occupational group at high risk of acquiring Q fever infection in Australia [Reference Boland and Parker15].
CONCLUSION
This outbreak represents one of the largest non-abattoir associated outbreaks of Q fever reported to date in Australia with a considerable attack rate. The likely source was infected sheep introduced to the saleyard area on or just prior to 21 October 2004, contaminating dust in the saleyard; this dust was subsequently spread across the area of the saleyard by windy conditions reported on the day. This explanation is backed by strong descriptive and analytical epidemiology supporting peak exposure on one particular day. The occurrence of this outbreak may also have been influenced by animal husbandry practices such as spring lambing. This outbreak has also highlighted an unvaccinated group of farmers at risk of Q fever. The specific barriers to vaccination of this risk group need to be addressed to prevent further outbreaks occurring in similar settings.
ACKNOWLEDGEMENTS
The authors acknowledge members of the Disease Surveillance and Investigation Unit of the Communicable Disease Control Branch, Department of Health, South Australia for their assistance with the outbreak investigation. Ming Qiao and Mark Turra from IMVS provided interpretation of serology and NAT results. John Weaver and Ryan Garnett from PIRSA provided input on animal health. At the time of this outbreak investigation, B. A. O'Connor was undertaking the Master of Applied Epidemiology Program at the Australian National University which was funded by the Australian Government Department of Health and Ageing.
DECLARATION OF INTEREST
None.







