INTRODUCTION
The hepatitis E virus (HEV) epidemic is increasing throughout the world. HEV infects both humans and animals, and there is direct evidence for zoonotic transmission of HEV. Human HEV cases have been attributed to consumption of uncooked or undercooked pork or deer meat infected with HEV strains that share high genetic similarity [Reference Masuda1, Reference Tei2]. Zoonotic transmission has also been suggested by indirect evidence, such as high genetic similarity between human and swine HEV strains isolated in the same areas by phylogenetic analysis [Reference Bouquet3, Reference Wang4]. In addition, animal models have demonstrated that swine HEV can successfully infect non-human primates and that human HEV can infect swine [Reference Arankalle, Chobe and Chadha5, Reference Williams6].
A dozen HEV subtypes (1b, 1c, 3a, 3b, 4a, 4b, 4c, 4d, 4e, 4g, 4h, 4i) have been reported in China [Reference Liu7]. Of these, seven subtypes (3b, 4a, 4b, 4d, 4g, 4h, 4i) have been identified in both humans and swine. Although genotype (GT)1 and GT4 HEV strains have been isolated in most regions of China (GT3 HEV has mainly been isolated in eastern China), HEV genotypes and subtypes have varying geographical distributions. For both humans and swine, the predominant subtype is 4a in northern China [Reference Zhao8, Reference Li9], whereas the predominant subtypes are 4a and 4g in the northeast [Reference Zhu10, Reference Yu11], 4a and 4i in the east [Reference Zhang12, Reference Dong13], and 4b in the southwest [Reference Ji14, Reference Li15]. Moreover, some subtypes may be prevalent in humans but uncommon in swine or vice versa [Reference Fu16].
HEV is transmitted through the oral–fecal route and is detectable at 4°C for as long as 70 days [Reference Schielke17]. A cell culture system infected with heated HEV faecal suspensions suggested that the virus was inactivated at 56°C in as short a time as 15 min [Reference Emerson, Arankalle and Purcell18], but successful infection of pigs with HEV-positive pig liver homogenates revealed that HEV cannot be effectively inactivated with incubation at 56°C for 1 h [Reference Feagins19]. The survival and infectiousness of HEV in the environment may be related to seasonal temperature changes. Thus, we hypothesized that HEV epidemics in humans and swine exhibit a seasonal pattern. We conducted a study to describe seasonal patterns of swine HEV prevalence and geographical distribution of HEV subtypes in eastern and southwestern China.
METHODS
Sample collection
From January 2008 to December 2011, we collected 100 swine bile specimens every 2 months from slaughterhouses in the cities of Anqing in eastern China and Nanning in southwestern China (Table 1). The pigs were aged 4–6 months, awaiting slaughter and later marketing. The total number of specimens was 4200, including 2400 from Anqing and 1800 from Nanning. These two slaughterhouses are the largest in these two cities, and the pigs were from local farms.
Table 1. Detection rates of hepatitis E virus (HEV) RNA in swine in two Chinese cities (%)

* Trend test for annual detection rates of HEV RNA: Anqing, 2008–2011 (χ 2 = 4·38, P = 0·036); Nanning, 2009–2011 (χ 2 = 0·82, P > 0·05).
Specimen processing and HEV RNA preparation
The swine bile specimens collected from the slaughterhouses at the two study sites, were shipped to our laboratory in Shanghai where they were stored at −80°C. HEV RNA was extracted from 100 μl swine bile with TRIzol Reagent (Invitrogen Corporation, USA) in accordance with the manufacturer's instructions, and subsequently diluted in 10 μl DEPC-treated autoclaved distilled water.
Reverse transcription–polymerase chain reaction (RT–PCR)
HEV RNA was tested with RT–PCR. For the reverse transcription assays, specimens were incubated at 4°C for 40 min in a 20 μl reaction volume as follows: 1 U avian myeloblastosis virus (AMV) reverse transcriptase, 10 U ribonuclease inhibitor, 1 μl dNTP (10 mm each), 4 μl 5 × RT buffer (TaKaRa Biotechnology Co., China), 12 pmol external anti-sense primers and 5 μl extracted RNA solution. A two-step PCR (nested PCR) was then conducted with synthesized cDNA. In order to identify the phylogenetic relationship of HEV cases, an 821 nucleotide (nt) fragment within the RNA-dependent RNA polymerase domain (RdRp) in ORF1 [corresponding to 3961–4781 nt of the prototype Burmese HEV strain (GenBank accession no. M73218)] was amplified. PCR thermal profiles were applied according to methods described by Tanaka et al. [Reference Tanaka20]. Duplicate assays were performed for positive results to avoid contamination.
Phylogenetic analysis
Positive PCR products were purified from the agarose gel for sequencing (GeneCore Biotechnologies, China) and were sequenced in both directions to resolve possible ambiguous nucleotides. Genetic distances between pairwise sequences were calculated using the Kimura two-parameter method with MEGA v. 5.05 software (www.megasoftware.net). The nucleotide sequences were subsequently compared for their similarity to known human and animal full-length HEV strains available in the GenBank database to determine genotypes and subtypes. The neighbour-joining method was employed to construct a phylogenetic tree with bootstrap tests of 1000 replications (values >70% to generate a consensus tree) and 19 GT4 HEV full-length strains available in GenBank were included as references. The HEV sequences detected and completely sequenced by our study have been deposited in the GenBank database under accession nos. JX064501, JX064502, and JX893377–JX893487.
Statistical analysis
The χ 2 trend test was used to assess the change of prevalence in HEV RNA during the study years in each city. All P values were two-sided. Results were considered statistically significant at the 0·05 level. All analyses were performed using SAS v. 8.02 for Windows (SAS Institute Inc., USA).
RESULTS
Seasonal patterns of HEV RNA prevalence in swine
From January 2008 to December 2011, 3·83% (92/2400) of swine bile specimens in Anqing were positive for HEV RNA. Between January 2009 and December 2011, 2·61% (47/1800) of specimens collected in Nanning were positive. The trend test for change in the annual prevalence of HEV RNA was significant for Anqing (χ 2 = 4·38, P = 0·036), but not for Nanning (χ 2 = 0·82, P > 0·05) (Table 1).
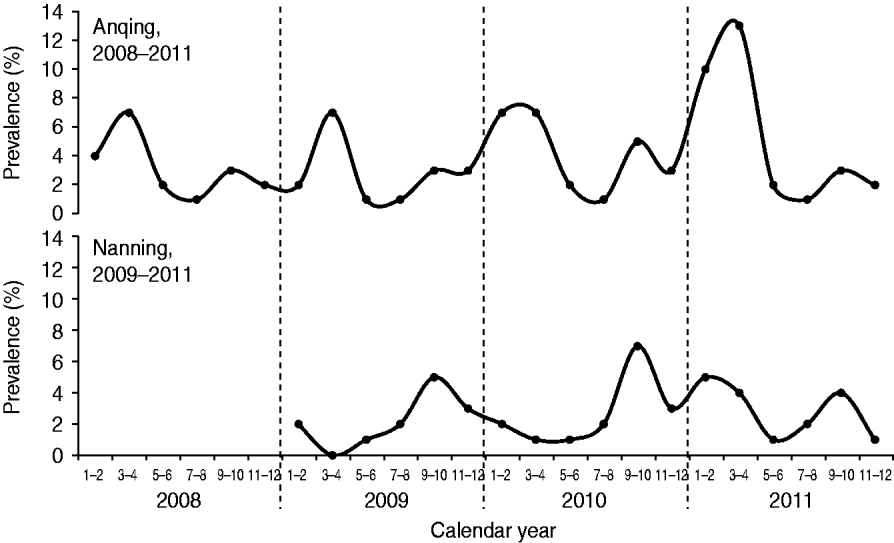
The prevalence of HEV RNA in swine in Anqing had two peaks each year: a major peak during March–April and a minor peak during September–October (Fig. 1). The cases detected during these two periods accounted for 36·96% (34/92, March–April) and 15·22% (14/92, September–October) of all cases in the four study years (2008–2011). The prevalence was lowest each year during July–August. The number of detected cases during this period accounted for 4·35% (4/92) of all cases in the four study years. In Nanning, there was also a seasonal pattern with a peak during September–October in each of the three study years (2009–2011) that accounted for 34·04% (16/47) of all cases detected during the study. There was a dip in Nanning during March–April for 2009 and 2010, although there was an additional unexpected peak during January–April 2011, which delayed the lowest prevalence to May–June that year.

Fig. 1. Seasonal changes in the detection of hepatitis E virus RNA in swine in two Chinese cities.
HEV subtypes in swine
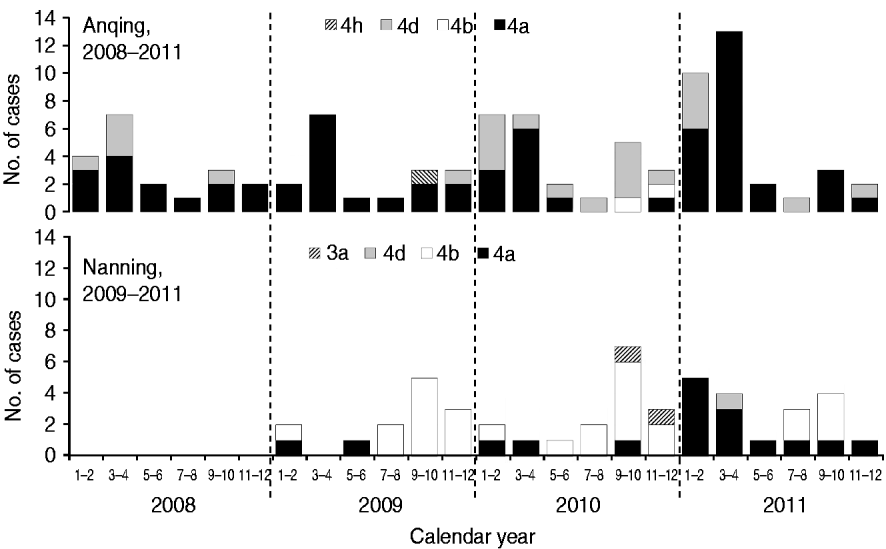
Five subtypes of HEV were detected in the two study cities. However, the dominant subtypes were different; 4a and 4d were dominant in Anqing and 4b and 4a were dominant in Nanning (Fig. 2). Specifically, the distribution of subtypes were: 4a (70·65%, 65/92), 4d (26·09%, 24/92), 4b (2·17%, 2/92), 4 h (1·09%, 1/92) in Anqing; and 4b (57·45%, 27/47), 4a (36·17%, 17/47), 3a (4·26, 2/47), 4d (2·13%, 1/47) in Nanning.

Fig. 2. Hepatitis E virus subtypes in swine in two Chinese cities.
Based on combined data of HEV subtypes in the two cities, the majority of subtype 4a cases (76·83%, 63/82) were detected in the first half of the year, although this subtype was detected throughout the year, while most subtype 4b cases (89·66%, 26/29) were concentrated in the second half of the year. The unexpected peak from January to April 2011 was mainly caused by subtype 4a, which is uncommon in the history of the HEV epidemic in Nanning.
Phylogenetic analysis
In our study, a total of 113 fragments of 821 nt RdRp were completely sequenced, including two of subtype 3a and 111 of subtypes 4a (n = 72), 4b (n = 16), 4d (n = 22), and 4 h (n = 1). Phylogenetic analysis of all 111 sequences of GT4 HEV showed that they shared moderate genetic similarity of 86·15–87·76%, and the average similarity within each subtype was 90·65–91·85% (4a), 94·35–95·24% (4b), and 93·87–94·74% (4d).
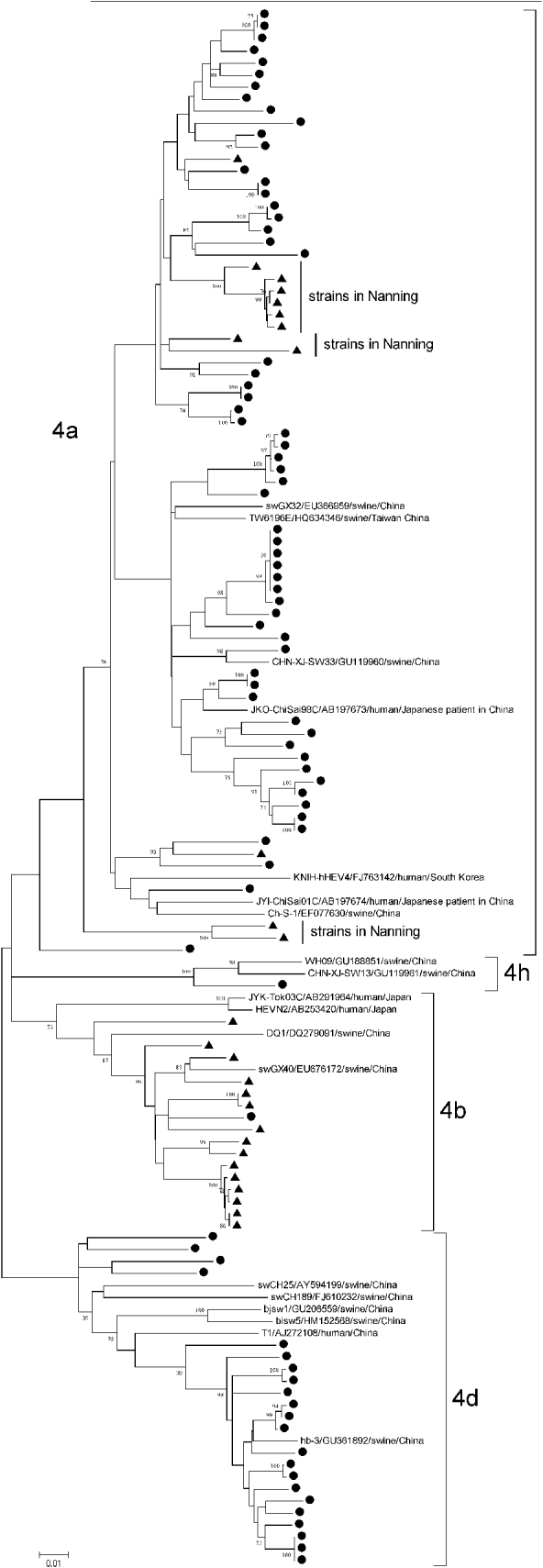
Subtype 4a strains came from both Anqing (60/72) and Nanning (12/72), with an average genetic identity between Anqing strains and Nanning strains of 89·91–91·20%, whereas subtype 4b came mainly from Nanning (15/16) and subtype 4d came mainly from Anqing (22/22). Of 12 subtype 4a strains from Nanning, 10 were clustered together in three clades and away from those from Anqing, as shown in Figure 3. The differences in the geographical distribution of the subtypes may explain why the genetic similarity of subtype 4a was lower than that of subtypes 4b and 4d.

Fig. 3. Phylogenetic tree produced by the neighbour-joining method with an 821 nt fragment in ORF1. ● Indicates GT4 hepatitis E virus (HEV) strains isolated from Anqing city; ▲ indicates those strains from Nanning city. Three clades of subtype 4a strains isolated in Nanning are indicated. Nineteen GT4 HEV full-length sequences available in GenBank were included as reference, with strain name, accession number, host and country. Bootstrap values of >70% are indicated.
DISCUSSION
Our study showed differences in seasonal patterns of the swine HEV epidemic in two Chinese cities. The main peak of the epidemic was during March–April in Anqing, whereas in Nanning the peak occurred during September–October. Anqing is located in eastern China and had average temperatures of 21·4°C during September–October and 12·9°C during March–April for the years 2008–2010 [21]. Nanning is located in southwestern China and had average temperatures of 25·3°C during Septembe–October and 20·3°C during March–April for the years 2008–2010 [21]. The temperature during September–October is similar between the two cities, while the temperature during March–April is much lower in Anqing than it is in Nanning. Considering the water-borne feature of HEV, we also compared the rainfall in the two cities for 2008–2011. The average rainfall during March–April, July–August, and September–October was 88·0 mm, 209·1 mm, and 58·5 mm in Anqing, respectively; whereas in Nanning, it was 58·6 mm, 229·0 mm, and 86·2 mm, respectively [21]. There was great fluctuation in the rainfall between years in each city, which showed no significant relationship with prevalence. Therefore, temperature and rainfall do not appear to be causes of the HEV seasonal patterns; our data indicate that HEV subtypes might be the true cause. For both cities, our study found that subtype 4a was the major contributor to the March–April peak, whereas subtype 4b was the major contributor to the September–October peak. The exception to this seasonal pattern in Nanning in 2011 may also be due to the fact that there was an unexpected increase in subtype 4a from January to April. The seasonal fluctuations of detected cases were further confounded by subtype 4d, and to a lesser extent, by subtype 3a.
In this study, we collected bile specimens from every pig in the slaughterhouses at the two study sites for examination of HEV RNA to avoid mutual contamination which often occurs when testing faecal specimens. Mixing of multiple swine faecal samples collected in pig farms often causes repeatedly identical results from the same pig but can be analysed as different ones. We amplified an 821 nt fragment to determine the phylogenetic relationship, which proved to be effective in detecting both GT4 and GT3 HEV strains [Reference Tanaka20]. In addition, we have previously reported higher detection rates of HEV RNA in swine bile specimens in Anqing city, with rates of 5·0% (20/400) with a 150 nt fragment in ORF2 [Reference Xia22] and 7·0% (7/100) with a 507 nt fragment in ORF1 [Reference Lu23]. The difference between the detection rates was believed to be attributable to the nucleotide fragment amplified and sample size.
The seasonal pattern of HEV prevalence in humans and swine is always a disputed issue. So far, HEV GTs1–3 have been tested for thermal stability in in vitro experiments and some differences have been observed in these three genotypes. GT2 HEV survived higher temperatures (∼95°C) compared to GT1 and GT3 (56–60°C) [Reference Schielke17, Reference Emerson, Arankalle and Purcell18]. However, GT3 HEV was not inactivated in pig livers even after incubation at 56°C for 1 h [Reference Feagins19]. Thus, the seasonal pattern of HEV prevalence, if proven, may be attributable to factors other than temperature, such as the medium in which it is found (water, food, etc.).
To the best of our knowledge, there is no literature on seasonal patterns of swine HEV. However, previous studies in humans have reported inconsistent seasonal patterns for HEV infection. For example, the prevalent season occurred in winter and spring (GT4 was predominant in the human study population, China) [Reference Zhang24], spring and summer (GT3, England) [Reference Dalton25], summer (GT1, Pakistan and Nepal) [Reference Saeedi26, Reference Shrestha27], autumn (GT3 and GT4, Japan) [Reference Inoue28], or even there was no seasonal pattern (GT3, France) [Reference Mansuy29]. This variation may also be attributable to HEV genotypes and their epidemic patterns.
Although there is a seasonal pattern, it is thought that HEV is prevalent in swine herds year round. Given that the present results were obtained from the bile specimens of slaughtered pigs, which were delivered directly to pork retailers and could come into contact with the general population through trading, a direct risk of transmission of swine HEV infection to humans exists throughout the year. Pork is the main meat consumed by Chinese people. Thus, consumption behaviour may enhance the transmission of HEV from swine to humans. Phylogenetic analysis also suggests that the strains of subtype 4a, which was common in both cities in our study, are highly genetically similar, suggesting that HEV strains in different areas of China are temporally and spatially related.
In conclusion, we observed seasonal changes in HEV prevalence in swine in different regions of China, and this variation may be attributable to the geographical distribution of different HEV genotypes and subtypes. The seasonal and geographical patterns of swine HEV have significant public health implications for preventing the spread of the disease from swine to humans. Special attention should be given to the hygiene of pork production and the cooking methods used for pork foods. Moreover, further epidemiological surveys in humans in the same geographical areas are warranted in order to evaluate potentially similar seasonal patterns in human HEV infection.
ACKNOWLEDGEMENTS
We thank Dr Xiao-Ou Shu for her valuable comments, and thank Ms. Bethanie Rammer and Mrs Jacqueline Stern for editing this manuscript. This work was supported by grants from the National Natural Science Foundation of China (Y.H.L., grant 81001264), (Y.J.Z., grant 30872158); and in part, by a grant from the Fogarty International Center of the National Institutes of Health (X.O.S., grant D43TW008313).
DECLARATION OF INTEREST
None.