Introduction
Eastern equine encephalitis (EEE) neuro-invasive disease can be severe, with symptoms including seizures, paralysis, coma and death [Reference Sherwood1]. EEE has a mortality rate of 42% [2]. In the USA, the first recognised human cases occurred in the State of Massachusetts, in 1938 [Reference Komar and Spielman3]. From 1964 to 2010, cases occurred in 17 States, and from 2009 to 2018, 72 cases occurred in 21 States [4]. The causative microorganism is the EEE virus. No specific antiviral treatment has been available [4]. A vaccine has been developed but has not been approved by the US Food and Drug Administration for general human use [4]. There is a potential risk of EEE in the European Union via importation of live animals carrying the virus, from North America [Reference Durand5]. A vaccine to prevent EEE has been commercially available for livestock.
The mosquito species Culiseta melanura (Coquillett) is a vector that maintains a life cycle of the virus, through birds [Reference Hayes6, Reference Hayes7]. In New York State, in this study area, this species of mosquito breeds in a habitat of wooded wetlands [Reference Morris8]. Such habitats are scattered throughout and have been mapped [Reference Howard9]. The two largest are 16 and 20 km2.
In 1959, occasional testing of mosquitoes for arboviruses, including the EEE virus, began on Long Island in New York State [Reference Jamnback, Berg and Whitney10], in the northeastern USA. The first reported case of EEE in a human in New York State [Reference Morris11] occurred in Oswego County in 1971, and within 1 week, surveillance for the EEE virus in mosquitoes began in Oswego County, and has continued in varying numbers of Counties annually until the present [Reference Srihongse12–Reference Oliver14]. From 1971 to 2012, there were six cases diagnosed in humans in New York State [Reference Sherwood1, Reference Oliver15].
Occurrences of human cases [Reference Sherwood1, Reference Howard9, Reference Morris11, Reference Howard13, Reference Oliver15] along with the presence of mosquito species having the virus [Reference Morris8, Reference Howard13, Reference Oliver14, Reference Morris, Zimmerman and Edman16] have had a notable intermittency. Various mosquito species have been suggested as the transmitters of the virus to humans, or causative vectors [Reference Morris8, Reference Morris, Zimmerman and Edman16–Reference Armstrong and Andreadis23]. The objective of this work was to look for associations between cases in humans and the virus in mosquito species, towards identifying mosquito species that transmit the virus to humans.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed [Reference Moher24]. Data on human cases of EEE and on mosquitoes with the EEE virus were obtained from a US National Library of Medicine database, using search words: Eastern equine encephalitis, Eastern equine encephalitis virus, human, mosquito and New York State (http://www.ncbi.nlm.nih.gov/pubmed/). These data were originally obtained by the members of this Department of Health. One of the authors has been present since the beginning of data collection.
Data
The study area consisted of the four contiguous counties of Madison (1710 km2), Oneida (3140 km2), Onondaga (2020 km2) and Oswego (3398 km2), located in a central geographic region of New York State having a centre point at a coordinate of 43.2° north and 75.8° west. Cases of EEE in humans and horses have been documented within a 3600 km2 area in these counties [Reference Oliver15]. The study area has two wooded wetlands of 1600 hectares (16 km2) and 2000 hectares (20 km2) [Reference Morris8]. The environment, including geography, vegetation and animals, of this area has been described [Reference Morris8].
Cases
Cases of EEE in humans in New York State were obtained from published literature. For the study period 1971–2012, published articles with human cases covered the years: 1966–1977 [Reference Deibel, Srihongse and Woodall25], 1971 [Reference Morris11], 1970–1992 [Reference Howard13], 1971–2012 [Reference Sherwood1, Reference Oliver15], 1972–1974 [Reference Morris26], 1976 [Reference Srihongse27], 1978–1985 [Reference Howard9], 1990–1991 [Reference Howard28] and 1992–2012 [Reference Sherwood1, Reference Oliver15]. Data included dates of onset of cases in humans [Reference Sherwood1, Reference Howard9, Reference Oliver15]. Dates of transmission were obtained from published cases or estimated based on an average of the incubation time period [1, 29]. The first five cases were located within an area that extended approximately 23 km north to south and 30 km east to west, all within the counties of Onondaga and Oswego. The distance between case 1 during 1971 and the western edge of a wooded wetland 16 km2 in Oswego County named ‘Toad Harbor’ was approximately 3 km [Reference Howard9]. The distance between case 2 during 1983 and the eastern edge of a wooded wetland 20 km2 in Onondaga County named ‘Cicero’ was approximately 0.8 km [Reference Howard9]. Cases 3–6 [Reference Oliver15] were located approximately 6, 8, 3 and 29 km, respectively, from the closest large wooded wetland.
Mosquitoes
Detection of the EEE virus in mosquitoes in New York State was obtained from published literature. For the study period 1971–2012, published articles of surveillance for the EEE virus in mosquitoes covered the years: 1971 [Reference Morris11], 1970–1992 [Reference Howard13], 1972–1974 [Reference Morris26], 1976 [Reference Srihongse27], 1976–1977 [Reference Morris and Srihongse30], 1978–1985 [Reference Howard9], 1984–1991 [Reference Howard28], 1993–2012 [Reference Oliver14] and 1994 [Reference Howard31]. Data included dates of collections of mosquitoes with the virus. A majority of the trapping sites were within an area of 2600 km2 which has had human and other vertebrate cases. Some trapping sites were located at the perimeters of the two largest wooded wetlands [Reference Howard28]. Among all mosquito poolings having the EEE virus, from 1971 to 2012, 80% were collected within 2 km of a wooded wetland [Reference Howard9]. The population and vector potential of a mosquito species, Cs. melanura, in these wetlands have been characterised in detail [Reference Morris, Zimmerman and Edman16]. In these 42 years, there have been 11 species of mosquitoes in which the EEE virus was detected [Reference Oliver14]. For this study, we did not include Culex salinarius Coquillett and Psorophora ferox (Humboldt). Standard nomenclature for mosquito genus and species was used [Reference Means32–Reference Wilkerson34]. The standard nomenclature of Aedes canadensis (Theobald) was used, in preference to a recently proposed renaming to Ochlerotatus canadensis (Theobald) [Reference Reinert35].
Statistical analysis
The hypothesis that the presence of an infected species of mosquito was associated with the presence of a case in a human was evaluated using Fisher's exact test [Reference Fisher36]. This test was applied to 2 × 2 tables of the numbers of years with and without the EEE virus in each species of mosquito and the numbers of years with and without EEE in humans, using data in Table 1. A one-sided P value was calculated.
Table 1. Human cases of Eastern equine encephalitis disease and poolings of mosquitoes testing positive for the Eastern equine encephalitis virus, in the four counties of Madison, Oneida, Onondaga and Oswego, in Central New York State, 1971–2012a

a An oversight in tabulating resulted in the change from ‘not tested’ to ‘tested’ for mosquito species Cx. pipiens-restuans, An. quadrimaculatus, An. punctipennis in years 2007–2011, in comparison with the analogous Table 3 in reference [Reference Oliver14].
b Positive integer in this column denotes the number of human cases of EEE disease reported.
c ‘0’ in this column denotes no human case of EEE disease was reported.
d Positive integer in this column denotes the number of poolings of this mosquito species in which the EEE virus was found. Poolings were groupings of from 10 to 100 mosquitoes. The total number of poolings in which the virus was detected was 532.
e ‘0’ in this column denotes this mosquito species was found in collections and tested and the virus was not detected.
f Culex pipiens and Culex restuans were on occasion submitted as Culex pipiens-restuans group.
g ‘n’ denotes this mosquito species was not tested for the EEE virus.
An alternative hypothesis tested was that the proportion of years with human cases would be higher when the virus was present in a species of mosquito than the corresponding proportion of years with human cases when the virus was absent in a species of mosquito. Relative risk was used to quantify the strength of the association between years in which a human case was present with the presence of the virus in the mosquito species. Relative risk was defined as the probability of a human case occurring in a year in which the virus was present for the listed species divided by the probability of a human case occurring in a year in which the virus was absent for the listed species.
Statistical Analysis Software (SAS) was used (Cary, North Carolina, USA, http://www.sas.com).
Results
From 1971 to 2012, there were six human cases of EEE and eight mosquito species for analysis (Table 1).
A statistical association, between the years with or without human cases and the years with or without the presence of the mosquito species with the virus, was found for Ae. canadensis (P = 0.005, Fisher's exact test), Coquillettidia perturbans (Walker) (P = 0.03) and Cs. melanura (P = 0.03) (Table 2). There was insufficient evidence to establish a statistical association between the number of years with or without human cases and the number of years with or without the presence of the virus in mosquito species Culiseta morsitans (Theobald) (P = 0.05), Aedes vexans (Meigen) (P = 0.40), Culex pipiens-restuans complex of Culex pipiens Linnaeus and Culex restuans Theobald (P = 0.33), Anopheles quadrimaculatus Say (P = 1.00) or Anopheles punctipennis (Say) (P = 1.00, Fisher's exact test) (Table 2).
Table 2. Estimated relative risk of the presence of human cases of Eastern equine encephalitis as a function of the presence or absence of mosquitoes testing positive for the Eastern equine encephalitis virus, in the four counties of Madison, Oneida, Onondaga and Oswego, in Central New York State, 1971–2012a
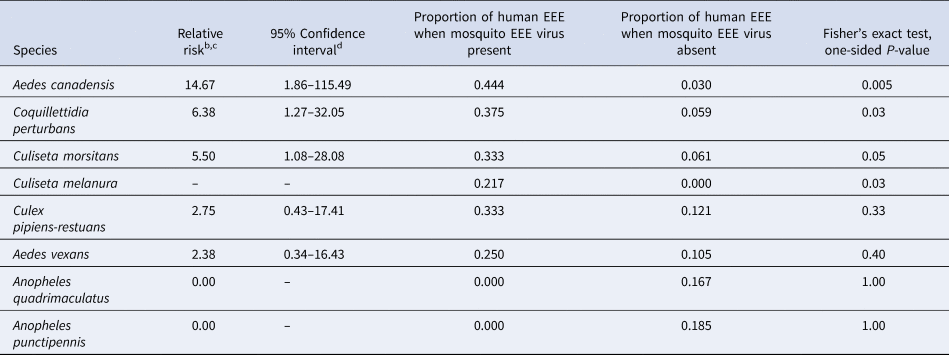
a Using data in Table 1.
b Relative risk is defined as the probability of a human EEE case occurring in a year in which the EEE virus was present for the listed species divided by the probability of a human EEE case occurring in a year in which the EEE virus was absent for the listed species.
c Relative risk cannot be computed if the denominator is 0 (see Cs. melanura) and a confidence interval for relative risk cannot be computed if the proportion of human EEE when mosquito EEE virus present is 0.
d Confidence intervals that include a relative risk of 1 would not be statistically significant (α = 0.05) which is the case for the last four species listed in the table.
The estimated relative risk of a human case in a year in which the virus was detected vs. not detected, in a specific mosquito species, was 14.67 for Ae. canadensis, 6.38 for Cq. perturbans and 5.50 for Cs. morsitans. There was no year during which a case was found in a human when the virus was absent in Cs. melanura (Table 2).
Temporal data
Dates, either documented or estimated, of virus transmission to human cases and dates, documented, of onset of symptoms in human cases were noted (Table 3). The dates of first and last detections of each mosquito species having the virus, and the number of days between these dates, were noted (Table 3), for context.
Table 3. Dates and time periods of detections of mosquito species having the Eastern equine encephalitis virus and occurrences of human cases, in the four counties of Madison, Oneida, Onondaga and Oswego, in Central New York State, 1971–2012
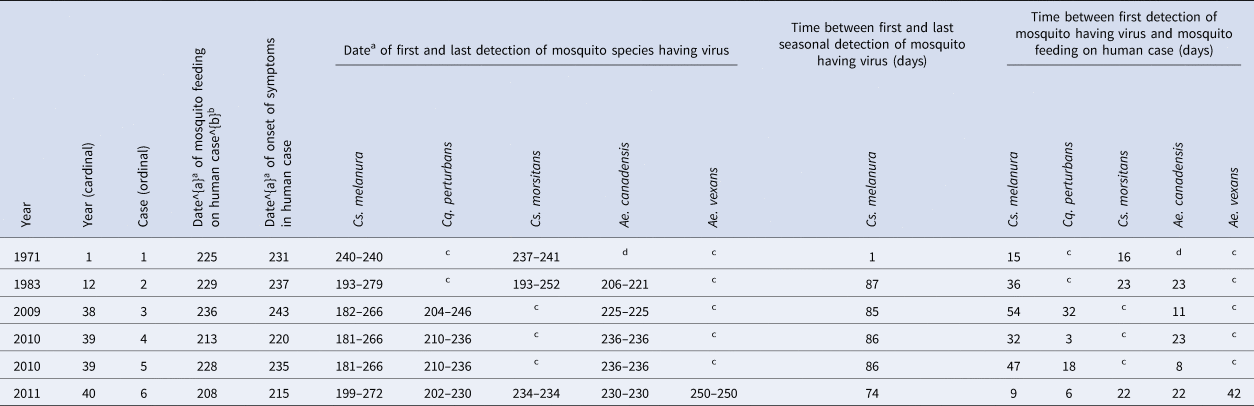
a Julian date of the year (from 1 to 365).
b Based on incubation time periods documented for: 1971 case 1 of 5 days, and for 1983 case 2 of 8 days and for cases 3–6 estimated based on the average of (8 + 5)/2 = 7 days [Reference Sherwood1, Reference Sherwood and Oliver29].
c This mosquito species was found in collections and tested and the virus was not detected.
d This mosquito species was not found in collections.
Spatial data
The cases of EEE occurred in two counties, Onondaga and Oswego. No cases occurred in the counties of Madison or Oneida. Mosquitoes with the virus were found in four counties, Madison, Oneida, Onondaga and Oswego. Of all mosquito poolings with the virus, approximately 91% were collected in the two counties of Onondaga and Oswego, from 1971 to 2012. The range of distances was 0.6–41.7 km, from human cases to trap sites with mosquitoes having the virus. Data on the distances between human cases and species of mosquitoes with the virus are shown in Table 4.
Table 4. Spatial relation between human cases of Eastern equine encephalitis and mosquito species testing positive for the Eastern equine encephalitis virus, in the four counties of Madison, Oneida, Onondaga and Oswego, in Central New York State, 1971–2012a,b
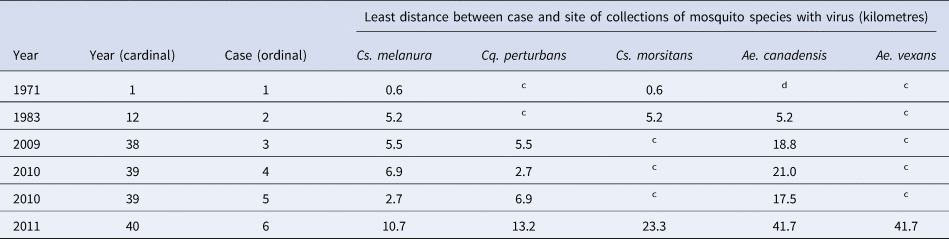
a Among all mosquito poolings having the EEE virus, 80% were collected within approximately 2 km of a wooded wetland.
b A map of the study site has been published [Reference Howard9, Reference Morris11].
c This mosquito species was found in collections and tested and the virus was not detected.
d This mosquito species was not found in collections.
Discussion
Any of these species of mosquitoes having the virus has the potential to transmit the virus to a human [Reference Nasci and Edman19, Reference Moncayo and Edman20, Reference Hachiya37]. In any human case, the actual mosquito that transmits the virus may depend on circumstances that include habitat, weather, flight of a mosquito, time of day or night and location of the host. In this study area, habitat and time period, the hypothesis that Ae. canadensis, Cq. perturbans and Cs. melanura transmitted the EEE virus to humans is supported by these statistical results. These species have been suspected as vectors [Reference Nasci and Edman19, Reference Moncayo and Edman20, Reference Hachiya37]. It would be expected that species in which human blood has been detected would be the most likely candidates to transmit the virus.
The statistical association observed between cases in humans and the presence of Ae. canadensis with the virus is consistent with the previous results that human blood has been detected in wild caught Ae. canadensis [Reference Magnarelli18, Reference Nasci and Edman19, Reference Apperson21]. Ae. canadensis hosts include mammals [Reference Magnarelli18, Reference Turell38]. Ae. canadensis feeds daytime [Reference Means32, Reference Turell38] and evening and early morning [Reference Means32].
Similarly, the statistical association here between EEE in humans and the presence of Cq. perturbans infected with the EEE virus is consistent with the finding that human blood has been detected in field-collected Cq. perturbans [Reference Nasci and Edman19] and that Cq. perturbans feeds on birds [Reference Edman17], on mammals [Reference Edman17] and on humans [Reference Means32]. Cq. perturbans feeds daytime [Reference Means33] and night-time [Reference Means33, Reference Turell38].
Field-collected Cs. melanura have been found to contain blood from humans [Reference Apperson21], mammals [Reference Nasci and Edman19, Reference Apperson21, Reference Molaei22] and birds [Reference Magnarelli18, Reference Nasci and Edman19, Reference Apperson21, Reference Molaei22, Reference Turell38]. A mix of mammalian and bird blood has been detected in Cs. melanura [Reference Nasci and Edman19, Reference Molaei22]. The presence of both mammalian and bird blood in Cs. melanura is of epidemiological importance. It has been observed that Cs. melanura feeds more often on birds prior to mid-July [Reference Nasci and Edman19] and feeds more often on mammals after mid-July, through to September [Reference Nasci and Edman19]. This is consistent with a life cycle of the virus in birds maintained by Cs. melanura [Reference Hayes6, Reference Hayes7, Reference Howard9, Reference Nasci and Edman19, Reference Hassan39]. There were no human cases in any year without Cs. melanura having the virus (Tables 1 and 2). Cases in humans have been infrequent in the presence of Cs. melanura containing the virus [Reference Hachiya37, Reference Edman, Timperi and Werner40], despite Cs. melanura containing higher virus titres – on the order of 106 plaque forming units per pooling of up to 50 mosquitoes [Reference Armstrong and Andreadis23]. In New York State, from 1971 to 2012, there were 5 years when there were human cases, and Cs. melanura with the virus were present, during those 5 years (Table 1); a similar feature was observed in a study of 26 years of data in Massachusetts, where Cs. melanura with the virus were present in 9 of 9 years with human cases [Reference Hachiya37]. There were 18 years when Cs. melanura with the virus was present, but during those 18 years, there were no human cases (Table 1); a similar feature was observed in that same study in Massachusetts, where Cs. melanura with the virus were present in 12 of 17 years without human cases [Reference Hachiya37]. In that same study in Massachusetts, there were 376 detections of the EEE virus in poolings of mosquitoes, of which 371 were Cs. melanura, four were Cq. perturbans and one was Ae. canadensis [Reference Hachiya37]; a similar feature was observed in New York State, where 563 detections of the EEE virus in poolings of mosquitoes, of which 461 poolings were Cs. melanura, 26 were Cq. perturbans and 14 were Ae. canadensis. During each of 4 years having human cases, 1983, 2009, 2010 and 2011, there were from 9 to 11 weeks during which Cs. melanura with the virus was present, but during these weeks, there were no additional human cases (Table 3). The persistent presence of Cs. melanura with the virus, from week to week, during the transmission season, has been shown in Massachusetts [Reference Edman, Timperi and Werner40], similar to New York State.
This present analysis did not find a statistically significant association between human cases and the presence of Cs. morsitans with the virus. In a previous study, human blood was not detected in Cs. morsitans collected in the area of this present study [Reference Molaei22]. Cs. morsitans with the virus has been present in some years with cases [Reference Howard9, Reference Howard13], but not in other years [Reference Oliver14, Reference Oliver15]. Cs. morsitans most often feeds on birds [Reference Nasci and Edman19, Reference Molaei22], but will take blood from mammals [Reference Nasci and Edman19, Reference Molaei22].
The results for Ae. vexans are anomalous. Human blood has been detected in field-collected Ae. vexans [Reference Magnarelli18, Reference Apperson21], and Ae. vexans is attracted to humans [Reference Means32] and mammals [Reference Edman17, Reference Magnarelli18, Reference Means32, Reference Turell38], but there was no statistically significant association, here in this study, between human cases and the presence of Ae. vexans with the virus. This may have been due to the virus having been found at relatively low titres [Reference Nasci and Mitchell41], on the order of 101 plaque forming units per pooling of up to 50 mosquitoes [Reference Armstrong and Andreadis23]. EEE virus has not been detected in Ae. vexans saliva as shown by the absence of cytopathic effect on baby hamster kidney cell cultures [Reference Vaidyanathan42].
The statistical results for Cx. pipiens-restuans complex of Cx. pipiens and Cx. restuans, An. quadrimaculatus and An. punctipennis may be because these three species had 6, 17 and 14 years, respectively, during which these species were not tested for the virus (Table 1). The inability to detect a statistically significant association for any of these three species could be due to the lack of statistical power. It is possible that none of these species has an association with the occurrence of human EEE. The host preferences of Cx. pipiens and Cx. restuans are for birds [Reference Magnarelli18, Reference Apperson21, Reference Turell38] although human blood has been detected in field-collected Cx. pipiens and Cx. restuans [Reference Apperson21]. Human blood has been detected in field-collected An. quadrimaculatus [Reference Apperson21]. An. quadrimaculatus saliva has been found to contain the EEE virus after feeding on viremic chicks, as saliva produced a cytopathic effect on baby hamster kidney cell cultures [Reference Vaidyanathan42]. An. punctipennis saliva did not have the virus after feeding on viremic chicks [Reference Vaidyanathan42]. Human blood has been detected in An. punctipennis [Reference Apperson21].
There was a spatial relationship between human cases and mosquitoes with the virus. All cases were located in only two counties, and 91% (483 of 532) of mosquito poolings with the EEE virus were collected in those two counties. Flight distance maximums, for Cs. melanura of 9.1 km and for Cs. morsitans of 9.8 km, have been determined [Reference Howard, White and Muller43]. A flight distance maximum for Ae. vexans of 48 km was determined [Reference Burkot and DeFoliart44]. Flight distances for Cq. perturbans and Ae. canadensis have been determined to be 5 and 2 km, respectively [Reference Turell38]. The longest distance between any human case and either of the two largest Cs. melanura breeding habitats, the two named wooded wetlands, was 29 km. Thus, distances between human cases and mosquito traps were within ranges published.
Limitations of this study
This study has the limitation of being dependent on reporting of cases by doctors, hospitals, commercial laboratories, agency laboratories and departments of health of counties and cities [45, 46]. During the period from 1971 to 2012, there were or may have been changes in medical practice, clinical diagnosis, laboratory testing, insurance, finance, public health law, code, rules and regulations, definitions of cases, requirements for reporting, employee interpretations and agency feasance [45–Reference Timen49]. A delay or oversight in recognition, testing, tabulating or reporting may result in a subsequent addition or subtraction of an occasional human case or mosquito detection of the virus.
Sampling error can occur, because the sample sizes customarily allowed or accepted and published by laboratories have been 10–100 individual mosquitoes. If the prevalence of the virus in a population of a species of mosquito was one in 1 000 or one in 10 000, then nine of 10 samples, referred to here as poolings or pools, may have no virus detected. In such instances, the results may lead to a conclusion that virus was not present in that population, when in actuality it was present at a level below the limit of detection in that circumstance.
This study applies to one region of New York State and is not necessarily generalisable to other geographic areas of the state or country. The laboratory that tested for the virus in mosquitoes set limits to the number of poolings of mosquitoes that each county could send for testing. Each county made the decision on what species of mosquitoes to send for testing, based in part on the species of viruses of concern for public health or vector control. Therefore, not all species of mosquitoes could be sent to the laboratory for testing. The absence of a statistically significant association between the presence of cases in humans and the virus in Cx. pipiens-restuans, Ae. vexans, An. quadrimaculatus or An. punctipennis should not be misinterpreted to imply that these species could not transmit the virus to humans in other circumstances.
Practical application
This information may be useful for public health officials deciding whether or not to initiate methods to suppress vectors, for individuals using personal protection to reduce exposure to mosquitoes and for clinicians to include EEE in the differential diagnosis of encephalitis.
Acknowledgement
David C. Brittain of the State of New York, Department of Health, Director of the Central New York Regional Office, for reading the manuscript and suggestions. P. Bryon Backenson of the State of New York, Department of Health, Division of Epidemiology, Bureau of Communicable Disease Control, Head of the Vector Surveillance Unit, and Head of the Communicable Disease Investigations Unit, for institutional support. William Snyder and Rebecca Hargrave of the State University of New York, School of Agriculture Business and Technology, Division of Environmental and Renewable Resources, for institutional support. The observations, reasonings or inferences presented here are not to be construed as official findings, determinations or policies of the Department of Health of the State of New York.
Financial support
This work was supported by full-time salaries as staff members of the respective Departments. No other funding was used.
Conflict of interest
The authors James A. Sherwood, Stephen V. Stehman, John J. Howard and JoAnne Oliver each declare no conflict of interest. No author gave or received anything tangible or intangible, in connection with this work.
Disclaimer
Any observation, inference or deduction of the scientific work presented here is not to be construed as an official finding, determination or policy of the Department of Health of the State of New York.






