The composition and possible health effects of human gut micro-organisms have been the focus of renewed interest since the development of metagenomic techniques enabling the identification and characterisation of intestinal bacteria that cannot be cultured. In addition, the discovery of differences in gut microbial composition between lean and obese individuals( Reference Diamant, Blaak and de Vos 1 ) and people with and without type 2 diabetes( Reference Wu, Ma and Han 2 , Reference Larsen, Vogensen and van den Berg 3 ) has highlighted the potential role played by the colonic microflora and their fermentation products in the pathogenesis of host metabolic health and disease.
Although the number and diversity of bacterial species within an individual's gastrointestinal tract remain relatively constant throughout life, it is possible to stimulate the proliferation of specific micro-organisms known to have beneficial health effects by manipulating the host diet. Prebiotics are defined as non-digestible plant-derived carbohydrates that act as a fermentation substrate within the colon, stimulating the preferential growth and activity of a limited number of microbial species that confer health benefits on the host( Reference Gibson, Probert and Loo 4 ). Carbohydrates with an established prebiotic effect include inulin-type fructans (inulin, oligofructose and fructo-oligosaccharides) and galactans (galacto-oligosaccharides)( Reference Roberfroid, Gibson and Hoyles 5 ), known to promote the proliferation of beneficial lactic acid-producing species such as bifidobacteria and lactobacilli( Reference Ramirez-Farias, Slezak and Fuller 6 ).
Gut bacteria play an important role in the development of the host immune system( Reference Chung and Kasper 7 ) and modulation of inflammatory processes( Reference Ding, Chi and Scull 8 ), extraction of energy from the host diet( Reference Backhed, Manchester and Semenkovich 9 ), fermentation of dietary fibres to produce SCFA( Reference Havenaar 10 ), alteration of human glucose and fatty acid metabolism( Reference Cani, Neyrinck and Fava 11 ), regulation of intestinal permeability( Reference Cani, Possemiers and Van de Wiele 12 ), production of vitamins( Reference Hill 13 ) and promotion of mineral absorption by the host( Reference Scholz-Ahrens, Ade and Marten 14 ). They may also be involved in the modification of the secretion of gut hormones to enhance satiety and improve gastrointestinal function( Reference Musso, Gambino and Cassader 15 ). Dietary prebiotic supplements capable of favourably altering the composition of the intestinal microflora might represent a potential therapeutic strategy for the prevention and treatment of metabolic abnormalities widespread in modern society.
The present review aimed to examine the current evidence supporting dietary prebiotic supplementation in adults on biochemical parameters associated with the development of metabolic abnormalities such as obesity, glucose intolerance, dyslipidaemia, non-alcoholic fatty liver disease and low-grade chronic inflammation.
Methods
A computer search of databases such as MEDLINE, CINAHL, Embase, Current Contents, PubMed, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews and AMED was undertaken for the period between 1 January 2000 and 30 September 2013. Databases were not searched before 2000, to exclude studies utilising non-molecular, culture-dependent techniques for the characterisation of intestinal bacteria. Reference lists of all identified studies were hand-searched for relevant trials. The following search terms were used: (1) (prebiotic* OR fructan* OR oligofructose OR inulin OR fructooligosaccharide* OR galactooligosaccharide*) and (gut OR obes* OR diabet* OR lipid* OR hepat* OR immune* OR metaboli*); (2) limit 1 to year = ‘2000–2003’; (3) limit 2 to humans. Trials were included if they were published in English and involved human participants aged ≥ 18 years and at least one group of participants were randomised to receive a dietary prebiotic intervention. For the purposes of the present review, a prebiotic intervention was defined as one that contained inulin, oligofructose, fructo-oligosaccharides or galacto-oligosaccharides. Additional plant-derived carbohydrates such as arabinoxylan and β-glucan were excluded from the search, as, although demonstrated to have prebiotic effects( Reference Neyrinck and Delzenne 16 , Reference Cloetens, Ulmius and Johansson-Persson 17 ), these compounds require further research before being formally classified as prebiotics.
Dietary prebiotic intervention studies of less than 24 h duration were excluded from the present review, as the growth of colonic microflora is unlikely to be affected in this brief time period( Reference Kootte, Vrieze and Holleman 18 ). Nutritional intervention studies involving the administration of probiotics (beneficial live micro-organisms) or synbiotics (a combination of pre- and probiotics) were also excluded. Trials involving prebiotic supplementation in people with disease conditions such as HIV and inflammatory bowel disease were considered to be outside the scope of the present review and were therefore excluded. The methodological quality of all the included trials was assessed by two authors independently using the Heyland Methodological Quality Score( Reference Heyland, Novak and Drover 19 ) (Table 1). This checklist rates primary research based on the use of allocation concealment during randomisation, intention-to-treat analysis, double-blinding, patient selection with minimal risk of bias, comparability of intervention and control groups at baseline, 100 % participant follow-up, clearly described treatment protocol and well-defined outcome measurements. Trials scoring ≥ 8 out of a possible 14 points were considered to be of high methodological quality. Disagreements between authors in assigning methodological quality scores were resolved by discussion until consensus was reached.
Table 1 Heyland Methodological Quality Score( Reference Heyland, Novak and Drover 19 )

The Heyland Methodological Quality Score for individual studies is based on nine quality criteria. The maximum possible score is 14 with studies scoring ≥ 8 considered to be of high methodological quality.
Trials measuring similar outcomes were subjected to a random-effects model meta-analysis using RevMan 5.1 (The Cochrane Collaboration, Copenhagen 2011). Treatment effects and 95 % CI were calculated using the Hedges (adjusted-g) standardised mean difference (SMD), to enable the comparison of effect sizes between trials using different outcome measures. SMD values of 0·2, 0·5 and 0·8 were considered to represent small, moderate and large effect sizes, respectively( Reference White and Thomas 20 ). Limited numbers of studies investigating comparable outcomes, small sample sizes and heterogeneity among trial subjects, disease conditions, prebiotic supplements, intervention duration and outcome measures limited the majority of data synthesis to a narrative analysis.
Results
Description of the selected trials
A total of 1130 citations were originally identified at the time of the initial database search and were selected to be included in the review based on the predefined inclusion criteria (Fig. 1). In the present review, twenty-nine articles reporting on twenty-six randomised controlled trials involving 831 participants were ultimately included( Reference Bunout, Hirsch and Pia de la Maza 21 – Reference Whelan, Efthymiou and Judd 49 ). The characteristics of the included trials are outlined in Table 2. Of the twenty-six trials included in the present review, thirteen trials included only healthy participants, five trials included only overweight or obese participants, one trial included only overweight participants with the metabolic syndrome, two trials included only participants with type 2 diabetes, two trials included only participants with hypercholesterolaemia, one trial included only participants with non-alcoholic steatohepatitis (NASH), one trial included only participants with gastro-oesophageal reflux disease and one trial included only elderly participants diagnosed with mild malnutrition or at risk of becoming malnourished. The duration of intervention ranged from 2 d to 28 weeks and the participants were aged 19–99 years. A variety of post-intervention outcome measures were reported including self-reported hunger and satiety ratings, total body weight, BMI, waist circumference, energy intake, gastric emptying times, concentrations of appetite-regulating hormones (ghrelin, cholecystokinin, peptide YY and glucagon-like peptide (GLP)-1), concentrations of lipids (total cholesterol, LDL, HDL, TAG, Lp(a) and NEFA), indicators of glucose homeostasis (glucose, insulin, glucagon, homeostasis model assessment for insulin resistance (HOMA-IR), HbA1c and fructosamine), inflammatory markers (TNF-α, C-reactive protein and IL), indices of immune function (natural killer cell activity and T-cell activation), and parameters associated with oxidative stress (total radical-trapping antioxidant parameter (TRAP), photosensitive chemiluminescence, total antioxidant capacity, superoxide dismutase and malondialdehyde) and liver function (aspartate aminotransferase). All the trials were of high methodological quality as assessed by the Heyland Methodological Quality Score. Methodological strengths of the trials included double-blinding utilised in the majority of the studies and random allocation of participants to intervention and control groups or treatment sequence. Methodological limitations of most of the trials included small sample sizes and short study duration. Some cross-over studies did not have a washout period or did not stipulate the duration of their washout period.

Fig. 1 Flow chart showing the progression of trials through each stage of the selection process. RCT, randomised controlled trial. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
Table 2 Summary of published human intervention randomised controlled trials examining the relationship between dietary prebiotic intake and metabolic health

RCT, randomised controlled trial; HMQS, Heyland Methodological Quality Score, where trials scoring ≥ 8 out of 14 points are considered to be of high methodological quality; FOS, fructo-oligosaccharide; GLP, glucagon-like peptide; NASH, non-alcoholic steatohepatitis; AST, aspartate aminotransferase; DM, diabetes mellitus; CRP, C-reactive protein; TAC, total antioxidant capacity; SOD, superoxide dismutase; HOMA-IR, homeostasis model assessment for insulin resistance; ALA, α-linolenic acid; OGTT, oral glucose tolerance test; Lp(a), lipoprotein (a); XOS, xylo-oligosaccharide; NK, natural killer; CD, cluster of differentiation; TRAP, total radical-trapping antioxidant parameter; PCL, photosensitive chemiluminescence; ICAM-1, intracellular adhesion molecule-1; GOS, galacto-oligosaccharide; TC, total cholesterol; ↓ , significantly lower than that in the comparison diet group after intervention; ↑ , significantly higher than that in the comparison diet group after intervention; ↔ , no significant difference between the prebiotic-supplemented diet and control diet groups after intervention; HLA-DR, human leucocyte antigen-D-related.
Outcomes associated with body weight
Of the five trials investigating the effect of dietary prebiotic supplementation on self-reported quantitative ratings of satiety, three demonstrated improvements in subjective satiety measurements in healthy participants consuming prebiotics in comparison with controls( Reference Cani, Joly and Horsmans 22 , Reference Cani, Lecourt and Dewulf 23 , Reference Whelan, Efthymiou and Judd 49 ). After the meta-analysis (n 52), the pooled SMD for satiety was − 0·57 (95 % CI − 1·13, − 0·01; P< 0·05), indicating a statistically significant effect favouring prebiotic supplementation over placebo (Fig. 2). Inclusion of two trials finding no change in satiety after prebiotic consumption( Reference Peters, Boers and Haddeman 37 , Reference Verhoef, Meyer and Westerterp 46 ) was not possible in the meta-analysis, as they did not report study data and did not provide results when contacted by the reviewers. An additional trial carried out in obese subjects was also excluded from the meta-analysis because it reported only qualitative improvements in satiety( Reference Genta, Cabrera and Habib 30 ). Of the five trials measuring energy intakes in normal-weight and overweight participants and those with type 2 diabetes, three found a significant reduction in total energy consumption during the prebiotic intervention when compared with placebo( Reference Cani, Joly and Horsmans 22 , Reference Dehghan, Gargari and Jafar-Abadi 26 , Reference Parnell and Reimer 36 ). However, the reduction in energy intake lost statistical significance after the meta-analysis (n 208) yielded a pooled SMD of − 0·51 (95 % CI − 1·20, 0·19; P= 0·16). The duration of one trial finding no difference in energy intake between control and intervention groups was 2 d( Reference Peters, Boers and Haddeman 37 ), and the trials finding reduced energy intake by the intervention groups lasted a minimum of 2 weeks. Available evidence supported that dietary prebiotic supplementation for at least 2 weeks' duration increases circulating peptide YY concentrations in normal-weight and overweight adults( Reference Cani, Lecourt and Dewulf 23 , Reference Parnell and Reimer 36 , Reference Verhoef, Meyer and Westerterp 46 ), but the effect was not statistically significant after the meta-analysis (n 100), with a combined SMD of − 0·96 (95 % CI − 1·98, 0·06; P= 0·07). Of four high-quality trials, two found increased GLP-1 concentrations after prebiotic supplementation in healthy and overweight subjects( Reference Cani, Lecourt and Dewulf 23 , Reference Piche, des Varannes and Sacher-Huvelin 38 ). The increase in GLP-1 concentrations was not significant after the meta-analysis (n 117), with a pooled SMD of − 0·32 (95 % CI − 0·87, 0·23; P= 0·25). Each of the trials reported significant reductions in ghrelin concentrations( Reference Parnell and Reimer 36 ) and increased GLP-2 concentrations( Reference Russo, Linsalata and Clemente 42 ) in subjects consuming dietary prebiotics. Contradictory results were reported by five trials examining the effect of prebiotic intervention on body weight. Significant reductions in body weight after prebiotic supplementation in comparison with placebo were reported by two trials( Reference Genta, Cabrera and Habib 30 , Reference Parnell and Reimer 36 ), while no change in body weight was observed in three trials( Reference Dehghan, Gargari and Jafar-Abadi 26 , Reference de Luis, de la Fuente and Izaola 28 , Reference Seidel, Boehm and Vogelsang 44 ). Trials of longer duration (12–17 weeks) were more likely to observe reductions in body weight than shorter trials lasting 4–8 weeks. The meta-analysis (n 191) indicated a non-significant reduction in body weight after prebiotic supplementation, with a pooled SMD of − 0·48 (95 % CI − 1·19, 0·23; P= 0·19).
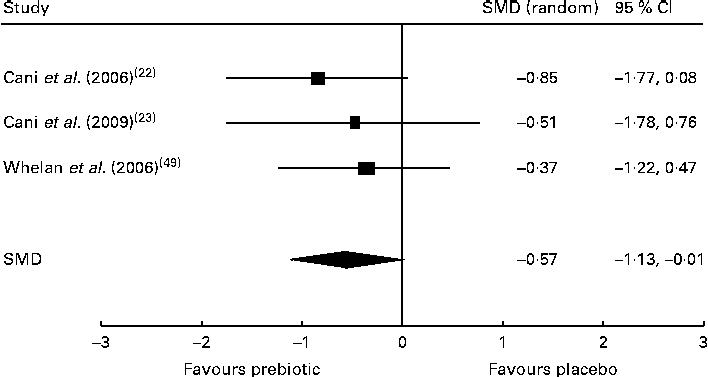
Fig. 2 Effects of dietary prebiotic supplementation on self-reported satiety. Forest plot of standardised mean differences (SMD, 95 % CI) for individual and pooled trials.
Outcomes associated with glucose homeostasis
Of the four studies measuring the effect of prebiotic supplementation on postprandial glucose concentrations, two reported significant reductions in glycaemia in normal-weight and obese participants( Reference Cani, Lecourt and Dewulf 23 , Reference Dewulf, Cani and Claus 29 ). Following the meta-analysis (n 131), the pooled SMD for postprandial glucose concentrations was − 0·76 (95 % CI − 1·41, − 0·07; P< 0·05), indicating a statistically significant effect supporting that prebiotic consumption results in the reduction of postprandial glucose concentrations (Fig. 3). Of three studies, two reported significant reductions in postprandial insulin concentrations following prebiotic intervention in overweight and hypercholesterolaemic subjects( Reference Giacco, Clemente and Luongo 31 , Reference Parnell and Reimer 36 ). Meta-analysis of these trials (n 121) indicated a statistically significant reduction in postprandial insulin concentrations, with a combined SMD of − 0·77 (95 % CI − 1·50, − 0·04; P< 0·05) (Fig. 4). Significant delays in gastric emptying times in healthy males consuming prebiotic supplements were found in two trials carried out by the same study group( Reference Russo, Riezzo and Chiloiro 40 , Reference Russo, Clemente and Linsalata 41 ). Studies investigating fasting glucose and fasting insulin concentrations and insulin resistance (HOMA-IR) reported conflicting results. Significant reductions in HbA1c levels in healthy participants after only 5 weeks of prebiotic supplementation( Reference Russo, Riezzo and Chiloiro 40 ) and in women with type 2 diabetes after 8 weeks( Reference Dehghan, Gargari and Jafar-Abadi 26 ) were found by two trials, while no change in HbA1c levels in obese women after prebiotic supplementation lasting 3 months was found by another trial( Reference Dewulf, Cani and Claus 29 ).
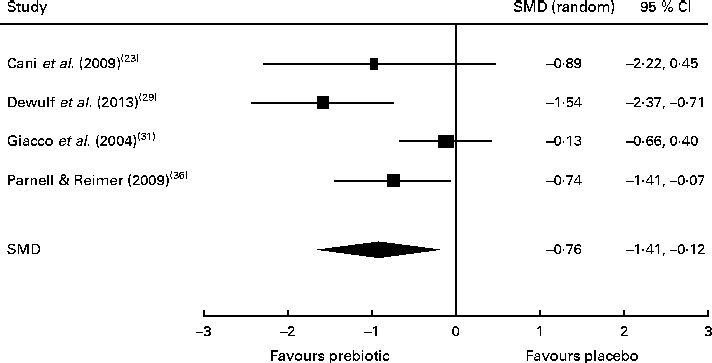
Fig. 3 Effects of dietary prebiotic supplementation on postprandial glucose concentrations. Forest plot of standardised mean differences (SMD, 95 % CI) for individual and pooled trials.
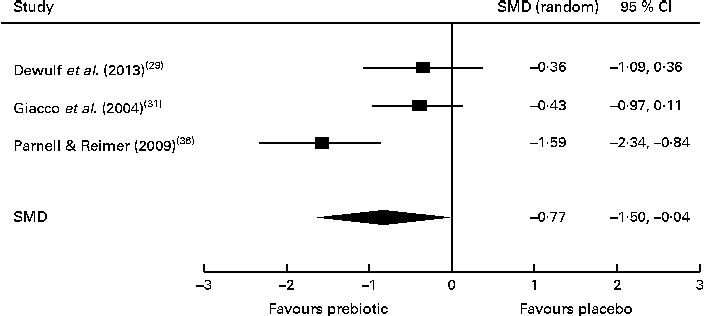
Fig. 4 Effects of dietary prebiotic supplementation on postprandial insulin concentrations. Forest plot of standardised mean differences (SMD, 95 % CI) for individual and pooled trials.
Outcomes associated with cardiovascular and hepatic health
There was insufficient evidence to support that prebiotic supplementation reduces total cholesterol or LDL concentrations in healthy, obese or dyslipidaemic individuals, with the majority of studies finding no change in the concentrations of these lipids after intervention. Of the eleven trials investigating the effect of prebiotic supplementation on circulating TAG concentrations, five reported significant reductions in healthy, overweight or hypercholesterolaemic individuals compared with controls( Reference Causey 24 , Reference Letexier, Diraison and Beylot 33 , Reference Russo, Chimienti and Riezzo 39 , Reference Tovar, Caamano Mdel and Garcia-Padilla 45 , Reference Vulevic, Juric and Tzortzis 48 ). However, the remaining six trials that failed to detect changes in TAG concentrations were also carried out in healthy, overweight or hypercholesterolaemic subjects( Reference Daubioul, Horsmans and Lambert 25 , Reference de Luis, de la Fuente and Izaola 28 – Reference Giacco, Clemente and Luongo 31 , Reference Luo, Van Yperselle and Rizkalla 35 ). These trials were subjected to meta-analysis (n 402), resulting in a non-significant pooled SMD for TAG concentrations of − 0·11 (95 % CI − 0·31, 0·08; P= 0·26) (Fig. 5). A significant reduction in serum aspartate aminotransferase concentrations was reported by one small trial carried out in people with NASH( Reference Daubioul, Horsmans and Lambert 25 ).
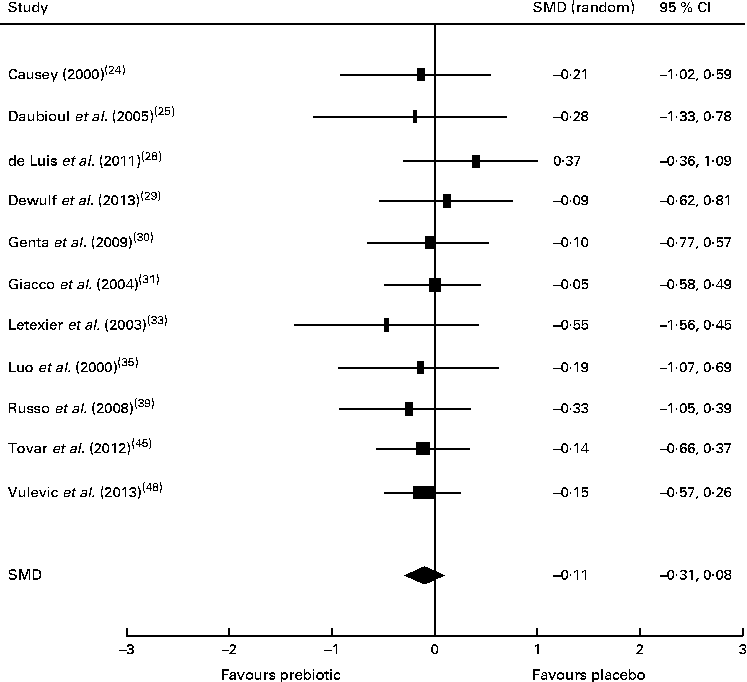
Fig. 5 Effects of dietary prebiotic supplementation on TAG concentrations. Forest plot of standardised mean differences (SMD, 95 % CI) for individual and pooled trials.
Outcomes associated with inflammation and immune function
Of the four trials investigating the impact of dietary prebiotic supplementation on circulating C-reactive protein (a biochemical marker of inflammation) concentrations, three found significant reductions in overweight and obese adults and women with type 2 diabetes in comparison with controls( Reference Dehghan, Gargari and Jafar-Abadi 26 , Reference de Luis, de la Fuente and Izaola 28 , Reference Vulevic, Juric and Tzortzis 48 ). Meta-analysis of these trials (n 181) indicated a non-significant reduction in C-reactive protein concentrations after prebiotic supplementation, however, with a pooled SMD of − 0·85 (95 % CI − 2·11, 0·42; P= 0·19). Studies measuring the production of pro-inflammatory cytokines (TNF-α and IL) and immune cell activity (T-cell activation and natural killer cell activation) yielded contradictory results. Significant increases in the measures of antioxidant status (total antioxidant capacity( Reference Pourghassem Gargari, Dehghan and Aliasgharzadeh 27 ), total radical-trapping antioxidant parameter and photosensitive chemiluminescence( Reference Seidel, Boehm and Vogelsang 44 )) were found by two studies, and a decrease in small-intestinal permeability( Reference Russo, Linsalata and Clemente 42 ) following prebiotic interventions was reported by one trial. Significant reductions in circulating lipopolysaccharide (LPS) concentrations after dietary prebiotic supplementation in healthy adults( Reference Lecerf, Depeint and Clerc 32 ) and women with type 2 diabetes( Reference Dehghan, Gargari and Jafar-Abadi 26 ) were identified by two studies.
Discussion
Simple, safe and effective interventions are urgently needed to prevent and treat obesity and its associated co-morbidities. The human gut microbiota and its metabolites influence host physiology, energy homeostasis, inflammatory processes and immune function both locally and within distal tissues. The use of dietary prebiotic supplements to promote the selective proliferation of beneficial intestinal microbes might represent an important nutritional strategy in the management of metabolic abnormalities and chronic disease.
Prebiotics and overweight/obesity
The SCFA acetate, proprionate and butyrate are produced as by-products of bacterial prebiotic fermentation in the colon. In addition to representing a source of energy for the host, these SCFA play a number of beneficial roles including the maintenance of human intestinal health and modulation of metabolic and immune processes. SCFA are the only known ligands for two G protein-coupled receptors, GPR41 and GPR43, which are expressed in a variety of gastrointestinal cells and stimulate the secretion of hormones involved in the regulation of energy intake and expenditure. Binding of SCFA to GPR41 increases the production of peptide YY and GLP-1, hormones that reduce appetite, delay gastric emptying and increase insulin sensitivity( Reference Flint, Scott and Louis 50 ). SCFA also promote the differentiation of intestinal L-cells, contributing to increased endogenous GLP-1 production( Reference Cani, Hoste and Guiot 51 ).
In animal studies, dietary supplementation of the SCFA butyrate has been found to prevent diet-induced obesity and improve insulin sensitivity with a concomitant increase in energy expenditure and fatty acid oxidation and an increase in mitochondrial respiration( Reference Gao, Yin and Zhang 52 ). In mice, the selective growth of certain Lactobacillus species in the colon has been found to reduce body fat storage through the up-regulation of Fiaf (fasting-induced adipose factor) gene expression and inhibition of lipoprotein lipase( Reference Backhed, Ding and Wang 53 , Reference Aronsson, Huang and Parini 54 ). Indeed, several animal studies have demonstrated the protective effects of prebiotics on the development of obesity and insulin resistance( Reference Parnell and Reimer 55 , Reference Everard, Lazarevic and Derrien 56 ); however, more robust human studies are required to confirm the protective effects of prebiotics on these pathways in human physiology.
The present review found consensus among three of the five high-quality trials supporting that the daily consumption of a prebiotic supplement for a minimum of 2 weeks increases satiety cues in healthy adults. However, these findings were based on self-reports from relatively small numbers of subjects (n 81) and prebiotic supplementation failed to result in significant weight reduction. Weight reduction was unlikely to be observed in these trials due to the short duration of their prebiotic interventions (2 d–2 weeks). The addition of pea fibre to the prebiotic supplement confounded one trial reporting an increase in satiety after 2 weeks of prebiotic consumption, making it difficult to draw conclusions about the action of either type of fibre individually( Reference Whelan, Efthymiou and Judd 49 ). Of the two trials that did not detect any change in satiety after prebiotic supplementation, one involved an intervention period of 2 d( Reference Peters, Boers and Haddeman 37 ), which may have been insufficient time to modify the growth and activity of intestinal bacteria to influence changes in host physiology. Increases in breath hydrogen production in the intervention group, indicative of enhanced intestinal bacterial fermentation, were detected by one of the trials investigating the effects of prebiotic supplementation on satiety sensations( Reference Cani, Lecourt and Dewulf 23 ). However, no trials analysed the stool samples of participants for changes in microbial growth; therefore, alterations in hunger and fullness reported by the subjects in these studies may have occurred independently of any changes in gut microbial fermentation. Prebiotics are soluble fibres capable of modifying the intestinal transit of food due to their water-binding and bulking capacity( Reference Nyman 57 ). Indeed, study participants consuming prebiotics within a single meal have reported increased levels of satiety, well before any changes in colonic bacterial growth could have taken place( Reference Perrigue, Monsivais and Drewnowski 58 , Reference Tarini and Wolever 59 ). Trials quantitatively evaluating the effect of prebiotic consumption on satiety and gut bacterial growth in overweight and obese individuals are now required.
Of the five high-quality trials, two provided consistent evidence favouring dietary prebiotic consumption for at least 2 weeks' duration for the reduction of total energy intake in normal-weight and overweight individuals and in women with type 2 diabetes. However, the pooled reduction in energy consumption was not statistically significant after the meta-analysis. A longer prebiotic supplementation period lasting 12 weeks was required before participants' reduced energy intake resulted in significant weight reduction( Reference Parnell and Reimer 36 ). Where body composition was evaluated, the weight lost was predominantly fat mass rather than lean tissue or fluid( Reference Parnell and Reimer 36 ). Trials of extended duration are now needed to determine whether dietary prebiotic consumption is a safe and effective therapeutic option for long-term weight and body fat reduction or whether physiological adaptations by the host eventually compensate for this energy imbalance to minimise weight loss.
The majority of trials investigating the effect of dietary prebiotic supplementation on the regulation of intestinal peptide (peptide YY) and incretin (GLP-1) secretion reported significant increases in the production of these molecules after 2 weeks, but the combined changes were not significant after the meta-analysis. The unique role played by prebiotics and specific bacteria in gut hormone kinetics requires further investigation, as non-prebiotic dietary fibres have also been reported to be associated with increased SCFA, peptide YY and GLP-1 production in human feeding studies( Reference Costabile, Klinder and Fava 60 , Reference Freeland, Wilson and Wolever 61 ).
Prebiotics and glucose intolerance
Reduced levels of bifidobacteria and lactobacilli and increased gastrointestinal permeability are found in mice consuming a diet high in saturated fat when compared with those consuming a standard diet. The provision of dietary prebiotic supplements subsequently restores the growth of these beneficial bacterial species and improves the integrity of the gut barrier( Reference Cani, Neyrinck and Fava 11 , Reference Serino, Luche and Gres 62 ). Animal studies have shown a causal link between the consumption of a high-fat diet and increased intestinal levels of LPS-containing bacteria, or concentrations of circulating LPS, and the development of obesity and insulin resistance( Reference Cani, Amar and Iglesias 63 ). LPS is the major component of the outer membrane of Gram-negative bacteria and is composed of a hydrophobic lipid (lipid A), a hydrophilic core oligosaccharide and a repeating hydrophilic polysaccharide side chain (O-antigen). In the setting of a high-fat diet, LPS is able to translocate from the intestine into the host circulation, resulting in ‘metabolic endotoxaemia’( Reference Cani, Osto and Geurts 64 ). LPS stimulates the overproduction of reactive oxygen species and pro-inflammatory cytokines by macrophages, resulting in subclinical systemic inflammation, weight gain and insulin resistance development( Reference Nakamura and Omaye 65 , Reference Liang, Hussey and Sanchez-Avila 66 ). Human subjects with type 2 diabetes have been found to possess serum endotoxin levels that are 2-fold higher than those observed in non-diabetic controls( Reference Creely, McTernan and Kusminski 67 ). Metabolic endotoxaemia is also positively correlated with total energy intake and fasting insulin concentrations in the non-diabetic population( Reference Amar 68 ). In mice with high-fat diet-induced metabolic endotoxaemia, nutritional supplementation with prebiotics restores intestinal levels of Gram-positive bacteria, improves glucose tolerance and reduces circulating concentrations of LPS and pro-inflammatory cytokines( Reference Everard, Lazarevic and Derrien 56 ).
Prebiotics and their fermentation products have been shown to reduce gastrointestinal permeability by a variety of mechanisms. The SCFA butyrate is involved in the maintenance of gut epithelial integrity by acting as the principal fuel for colonocytes and promoting the transcription of tight junction proteins between gastrointestinal cells( Reference Delzenne and Cani 69 ). Butyrate also reduces gastrointestinal permeability by enhancing the activation of the peroxisomal proliferator-activated receptor gamma (PPARγ) gene, a nuclear factor receptor involved in the attenuation of inflammation in colonic epithelial cells( Reference Eun, Han and Lee 70 , Reference Lewis, Lutgendorff and Phan 71 ). Prebiotic-induced changes in gut microbiota also increase the endogenous production of GLP-2, which enhances gut barrier function by promoting the proliferation of crypt cells( Reference Cani, Possemiers and Van de Wiele 12 , Reference Cani 72 ).
The present review found general agreement among trials supporting that the consumption of dietary prebiotic supplements reduces postprandial glucose and insulin concentrations in healthy and overweight individuals. Pooled reductions in postprandial glucose and insulin concentrations were statistically significant after the meta-analysis. High-quality randomised controlled trials conducted in subjects with either impaired glucose tolerance( Reference Garcia, Otto and Reich 73 ) or type 2 diabetes( Reference Lu, Walker and Muir 74 ) have also found reduced postprandial serum insulin concentrations after the consumption of arabinoxylan (a potential prebiotic fibre). Whether these results were mediated by alterations in intestinal bacterial growth or activity is unclear, as stool samples were not analysed in these trials. Significant delays in gastric emptying times after prebiotic supplementation in healthy males were found by two studies. However, these trials were conducted by the same research group, and it is unclear whether some subjects participated in both the studies. Therefore, further independent research is required before definitive conclusions can be drawn about the effects of prebiotic consumption on gastric emptying. The findings of studies investigating fasting glucose and fasting insulin concentrations and insulin resistance (HOMA-IR) after prebiotic supplementation were contradictory. Long-term prebiotic intervention studies in people with pre-diabetes or the metabolic syndrome are now required to determine whether prebiotics confer some protection against the future development of type 2 diabetes in high-risk individuals.
Prebiotics and dyslipidaemia
The abundance of particular bacterial species in the gut has been shown to be positively correlated with serum total cholesterol and LDL-cholesterol concentrations in subjects with CVD( Reference Guo, Liu and Zhang 75 ). It has also been hypothesised that bacteria found in atherosclerotic plaques may have originated from the gastrointestinal tract, as the DNA of specific micro-organisms can be found in both the colon and coronary atheroma of the same individual( Reference Koren, Spor and Felin 76 ). As the development of CVD involves multiple pro-inflammatory pathways, it is plausible that pathogenic microbes may potentiate inflammation within atherosclerotic plaques by delivering macrophages to the arterial wall and stimulating their production of reactive oxygen species and cytokines or their conversion to foam cells( Reference Wang, Klipfell and Bennett 77 ).
Proprionate, a SCFA product of prebiotic fermentation, may play a significant role in the modification of hepatic lipid metabolism. In the liver, proprionate is a possible substrate for gluconeogenesis and may contribute to the inhibition of cholesterol synthesis by altering the activity of 3-hydroxy-3-methylglutaryl-CoA reductase( Reference Roberfroid, Gibson and Hoyles 5 ). In addition, prebiotic supplementation might attenuate cholesterol and TAG production by stimulating the synthesis of cis-9, trans-11-conjugated linoleic acid from PUFA by beneficial bacterial species. This isoform of cis-9, trans-11-conjugated linoleic acid has been shown to reduce cholesterol and TAG concentrations in animal studies( Reference Baddini Feitoza, Fernandes Pereira and Ferreira da Costa 78 ), but the results of human trials are less conclusive. Gut microbes are also an essential requirement for the production of secondary bile acids in the colon. These bile acids are de-conjugated and are therefore unavailable for enterohepatic recirculation. As a result, the liver is forced to produce additional bile acids from circulating cholesterol( Reference Fava, Lovegrove and Gitau 79 ).
The findings of human intervention studies investigating the effect of dietary prebiotic supplementation on circulating total and LDL-cholesterol concentrations were contradictory. Of the two studies reporting significant reductions in total cholesterol concentrations, one found a reduction in only male subjects and was complicated by the use of an intervention containing both prebiotics and α-linolenic acid( Reference de Luis, de la Fuente and Izaola 28 ). α-Linolenic acid may have contributed to the cholesterol-lowering effect in this instance. There is limited evidence to support that prebiotic supplementation reduces total or LDL-cholesterol concentrations in hypercholesterolaemic individuals, as the only two trials conducted in participants with hypercholesterolaemia found no significant changes in total cholesterol, LDL-cholesterol or HDL-cholesterol concentrations( Reference Causey 24 , Reference Giacco, Clemente and Luongo 31 ). However, these trials involved short-term prebiotic intervention periods (3–8 weeks' duration) and studies of longer duration are therefore required.
The present review found conflicting evidence describing the effect of prebiotic supplementation on circulating TAG concentrations in healthy, overweight/obese and hypercholesterolaemic subjects. In addition to a prebiotic supplement, one study( Reference Tovar, Caamano Mdel and Garcia-Padilla 45 ) provided a low-energy diet co-intervention to all the trial participants, making it difficult to establish whether the TAG-lowering effect was associated with prebiotic-induced intestinal microbial changes alone or whether weight reduction or a reduced fat intake together with the action of the modified microflora produced a synergistic effect. Additional systematic reviews exploring this topic have also reported equivocal conclusions. The use of prebiotics for the reduction of TAG concentrations in humans regardless of health condition was favoured by one review of trials published between 1995 and 2005( Reference Brighenti 80 ), with the majority of trials being conducted in normolipidaemic individuals. The other meta-analysis of trials published between 1999 and 2010 supported the TAG-lowering effects of inulin in only hypercholesterolaemic subjects( Reference Guo, Liu and Zhang 75 ), but the reduction in TAG concentrations lost significance when results from both hyperlipidaemic and normolipidaemic subjects were combined. The present review also found a non-significant reduction in TAG concentrations after the meta-analysis of pooled trials. Future trials must simultaneously quantify lipid concentrations and gut bacterial growth and activity to determine whether prebiotic-induced modulation of the intestinal flora contributes to the reduction of serum TAG concentrations.
Prebiotics and non-alcoholic steatohepatitis
NASH is an asymptomatic disease characterised by fatty infiltration of the liver and inflammation, which can eventually lead to fibrosis, cirrhosis, portal hypertension, hepatocellular carcinoma and liver failure( Reference Henao-Mejia, Elinav and Jin 81 ). Obesity, dyslipidaemia, insulin resistance and diabetes have frequently been reported to be associated with the development of NASH. Increased plasma endotoxaemia, overproduction of inflammatory cytokines and excessive oxidative stress within hepatic cells are also thought to contribute to the pathogenesis of NASH. The use of dietary prebiotic supplements to restore an optimal microbial balance within the gastrointestinal tract of individuals with NASH may assist in the reduction of TAG accumulation in the liver, attenuate inflammation and promote hepatic secretion of lipoproteins such as VLDL( Reference Musso, Gambino and Cassader 15 ). The translocation of Gram-negative bacteria from the intestine into the circulation has been reported to be associated with an increased severity of cirrhosis( Reference Sheth and Garcia-Tsao 82 ). By maintaining gut barrier function and reducing bacterial translocation, prebiotics may be effective in the management of liver disease complications( Reference Gratz, Mykkanen and El-Nezami 83 ).
Studies exploring the effects of dietary prebiotic consumption on metabolic parameters in individuals with NASH are scarce. In the present review, one small trial involving seven adult males was included, which found a significant reduction in serum aspartate aminotransferase concentrations after prebiotic supplementation( Reference Daubioul, Horsmans and Lambert 25 ). This finding was supported by a larger randomised controlled trial (n 66), which administered a synbiotic (fructo-oligosaccharide+Bifidobacterium longum) to participants for 24 weeks( Reference Malaguarnera, Vacante and Antic 84 ). In addition to a significant reduction in serum aspartate aminotransferase concentrations, researchers found a reduction in the concentrations of circulating cytokines (TNF-α) and markers of inflammation (C-reactive protein), reduced concentrations of serum LDL-cholesterol and endotoxins, an improvement in insulin sensitivity (HOMA-IR) and a reduction in hepatic steatosis (determined by liver biopsy). More research is required in this potentially very promising area of study.
Prebiotics and immune cell dysfunction
Gut microbiota, innate immune function and metabolism are inextricably linked, with early pathological processes occurring at the molecular level (subclinical inflammation, immune cell activation, increased oxidative and endoplasmic reticulum stress, altered production of vascular adhesion molecules and advanced glycation end products) contributing to the eventual development of metabolic disturbances such as hyperlipidaemia, atherosclerosis, insulin resistance and weight gain. Colonic bacteria and their prebiotic fermentation products may play a key role in the modulation of immune function by both increasing host resistance to infection and down-regulating inappropriate immune responses in the case of allergic reactions or chronic inflammatory conditions( Reference Roberfroid, Gibson and Hoyles 5 ). By maintaining the integrity of the gastrointestinal barrier, prebiotics reduce the invasion of pathogenic intestinal bacteria and their products (including LPS) into the circulation, preventing downstream immune cell activation. Prebiotics are thought to encourage increased intestinal mucin production, protecting the intestinal wall from bacterial adherence and invasion( Reference Schley and Field 85 ). SCFA produced as a by-product of bacterial prebiotic fermentation interact with GPR41 and GPR43 receptors on neutrophils and inhibit NF-κB activation, reducing the production of pro-inflammatory cytokines( Reference Macfarlane and Macfarlane 86 ). Additional bacterial fermentation products such as polysaccharide A and peptidoglycan exert anti-inflammatory effects on the host immune system( Reference Maslowski and Mackay 87 ).
There is insufficient evidence at present to recommend dietary prebiotics for the modulation of immune function to improve cardiometabolic health. There are very few human trials available, and most have reported contradictory findings. Although individual studies have found significant increases in the measures of antioxidant capacity and reductions in small-intestinal permeability and circulating LPS concentrations after prebiotic interventions, further studies are required to verify these results. Inulin exhibits antioxidant properties independent of altering gut bacterial growth and is able to scavenge a number of reactive oxygen species, which may help to reduce lipid peroxidation in the stomach( Reference Stoyanova, Geuns and Hideg 88 ). Future studies must distinguish between health benefits derived solely from the consumption of soluble fibres and those associated with the growth and activity of beneficial gut microbes. Future intervention studies exploring the effect of dietary prebiotics on immune function need to be conducted in healthy individuals who are subsequently exposed to an immune challenge.
Conclusions
Although animal studies have provided convincing evidence to support the beneficial role of prebiotics in metabolic health, the results of human trials to date have been less conclusive. Research involving laboratory animals enables the provision of tightly controlled diets, whereas studies involving free-living humans are complicated by the variety of foods consumed by individuals from day to day. Some human studies have been complicated by the use of nutritional supplements containing prebiotics in combination with additional health-promoting components such as live bacteria, antioxidants and other dietary fibres, making it difficult to attribute changes in metabolism to prebiotics alone. To rule out cardiometabolic benefits associated with concomitant nutrients, prebiotic supplements in their pure form must be used in future trials.
In addition to bifidobacteria and lactobacilli, dietary prebiotics modulate the growth of numerous other gastrointestinal micro-organisms, the identity and function of which have not yet been fully characterised. Different species of bifidobacteria also have a variety of functions, which require further elucidation. Prebiotics are likely to undergo cross-fermentation by other microbial species of unknown benefit to the host. Bacterial analyses of human stool samples provide information only about the micro-organisms inhabiting the colon and are unlikely to accurately reflect the microbial composition of the proximal intestine. Responses to dietary prebiotics are variable in humans, with bifidogenic potential being affected by an individual's age, body weight, antibiotic use, dietary macronutrient intake, physical activity and baseline levels of colonic bifidobacteria( Reference Russell, Gratz and Duncan 89 , Reference Walton, van den Heuvel and Kosters 90 ). More research is required to determine host lifestyle behaviours capable of promoting intestinal normobiosis and to establish the optimal prebiotic dose required to maximise health benefits.
In conclusion, the present review found convincing evidence from short-term high-quality human trials supporting the use of dietary prebiotics as a potential therapeutic intervention for the regulation of appetite and the reduction of circulating postprandial glucose and insulin concentrations. Further studies are needed to correlate these findings with changes in the growth and function of specific gut bacteria. There is insufficient evidence at present to recommend dietary prebiotics for reducing energy intake and body weight, increasing gastric peptide YY and GLP-1 secretion, improving insulin sensitivity, lowering lipid levels and modulating immune function. Long-term prospective trials investigating primary metabolic end points are now required.
Acknowledgements
N. J. K. was the recipient of National Health and Medical Research Council (NHMRC) Postgraduate Public Health Scholarship APP1039709. N. J. K. thanks Mr Brendan Kellow for IT assistance.
N. J. K. was supported by NHMRC Postgraduate Public Health Scholarship APP1039709. The NHMRC had no role in the design and analysis of this work or in the writing of this article.
The authors' contributions are as follows: N. J. K. designed the review, conducted the literature search, extracted and analysed the data and drafted the manuscript; M. T. C. and C. M. R. provided comments on the manuscript. All the authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.









