Introduction
In tropical regions changes in land use have been the main cause of biodiversity loss, with the associated loss of habitats for wild flora and fauna (Arriaga & Gómez, Reference Arriaga, Gómez, Martínez and Fernández2004; González et al., Reference González, Briones-Salas, Alfaro, García-Mendoza, Ordóñez and Briones-Salas2004; INEGI, 2005). The principal causes of changes in vegetative cover and land use on the Isthmus of Tehuantepec in Oaxaca, Mexico, are human population growth (Mas et al., Reference Mas, Velázquez and Couturier2009; Nájera et al., Reference Nájera, Bojórquez, Cifuentes and Marceleño2010), which increases demand for space for housing and food production, and poor agricultural management, particularly with respect to livestock raising and prescribed burning of pastures (uncontrolled burning of grasses to promote new growth of grazing pasture for cattle) for seasonal crops (López-Granados et al., Reference López-Granados, Mendoza and Acosta2002; Peralta-Rivero et al., Reference Peralta-Rivero, Contreras-Servín, Galindo-Mendoza, Mas Caussel and Algara-Siller2014). Both of these matters have led to reduction, degradation and fragmentation of habitat (Bender et al., Reference Bender, Contreras and Fahrig1998; Rioja et al., Reference Rioja, Lorenzo, Naranjo, Scott and Carrillo-Reyes2011; Lorenzo et al., Reference Lorenzo, Carrillo-Reyes, Rioja-Paradela, de La Paz-Cuevas, Bolaños-Citalán and Álvarez-Castañeda2014), directly affecting the amount and quality of vegetation, as well as food availability for wild animals.
In the southern region of the Isthmus of Tehuantepec, habitat consists of scrub and mangrove, with submerged vegetation in coastal lagoons (Superior Lagoon and Inferior Lagoon; Ortiz et al., Reference Ortiz, Hernández, Figueroa, García-Mendoza, Ordóñez and Briones-Salas2004), as well as extensive areas of savannah with isolated shrubs and trees (Rzedowski, Reference Rzedowski2006; Sántiz et al., Reference Sántiz, González-Romero, Lorenzo, Gallina-Tessaro and Cervantes2012). Large areas of low tropical deciduous and low deciduous forest (Torres-Colín, Reference Torres-Colín, García-Mendoza, Ordóñez and Briones-Salas2004), as well as savannahs and human-induced grasslands (native or exotic species that develop in sites in which the original vegetation has been eliminated or in abandoned agricultural areas; Aristida, Bouteloua, Cathestecum, Cenchrus, Digitaria, Eragrostis, Panicum, Paspalum and Schizachyrium) are found in this region (Rzedowski, Reference Rzedowski2006; INEGI, 2012; Sántiz et al., Reference Sántiz, González-Romero, Lorenzo, Gallina-Tessaro and Cervantes2012).
Grasslands of the Isthmus of Tehuantepec have been transformed for agriculture and livestock raising, causing degradation and reduction of the original vegetation (Sántiz et al., Reference Sántiz, Lorenzo, Carrillo-Reyes, Navarrete and Islebe2016). One mammal species of the region that depends on savannahs and human-induced grasslands is the endemic Tehuantepec jackrabbit Lepus flavigularis (Anderson & Gaunt, Reference Anderson and Gaunt1962; Lorenzo et al., Reference Lorenzo, Retana-Guiascon, Cervantes, Vargas and Portales2000), which is categorized as Endangered on the IUCN Red List (Cervantes et al., Reference Cervantes, Lorenzo, Farías and Vargas2008). This lagomorph shares its habitat with other lagomorphs, such as the eastern cottontail Sylvilagus floridanus, as well as their predators: the coyote Canis latrans and the grey fox Urocyon cinereoargenteus (Lorenzo et al., Reference Lorenzo, Retana-Guiascon, Cervantes, Vargas and Portales2000; López et al., Reference López, Lorenzo, Barragán and Bolaños2009).
Interactions among taxa influence abundance and density (Erlinge et al., Reference Erlinge, Göransson, Högstedt, Jansson, Liberg and Loman1984; Daniel et al., Reference Daniel, Holechek, Valdez, Tembo, Saiwana, Fusco and Cardenas1993; Bailey, Reference Bailey2005) and can cause population fluctuations in response to environmental changes and the amount and quality of available food (Keith & Windberg, Reference Keith and Windberg1978; Keith, Reference Keith and Genoways1990). Therefore, identifying the impact of changes in vegetative cover and land use on distribution and population fluctuations of mammals, using basic ecological parameters such as population abundance and density, facilitates decision making for the conservation of endemic and threatened mammal species, such as L. flavigularis, as well as adequate management of agriculture and other land-based economic activities.
Densities of 6–13 individuals km−2 have been reported for L. flavigularis (Lorenzo et al., Reference Lorenzo, Retana-Guiascon, Cervantes, Vargas and Portales2000; Vargas, Reference Vargas2000; Sántiz, Reference Sántiz2002), and abrupt changes in density have been recorded over 6 years of monitoring. These changes have been partially attributed to management practices such as prescribed burning and grazing (Lorenzo et al., Reference Lorenzo, Rioja, Carrillo and Cervantes2008).
Given the importance for wildlife management and conservation of identifying long-term fluctuations in the population density of lagomorphs, the relationship of these fluctuations to those of carnivores, and the responses of lagomorph population densities to changes in vegetative cover and land use (such as grazing and prescribed burning), the objective of this study was to examine the relationships between fluctuations in population densities of mammals (L. flavigularis, S. floridanus, and their natural predators U. cinereoargenteus and C. latrans) and changes in land use and vegetative cover over a 16-year period.
Study area
Land use and vegetative cover were analysed in four municipalities in the southern region of the Isthmus of Tehuantepec in the Mexican state of Oaxaca: San Francisco del Mar, San Francisco Ixhuatán, San Dionisio del Mar, and Juchitán de Zaragoza. Mammals were monitored in Montecillo Santa Cruz in the municipality of San Francisco del Mar, and Huamuchil in the municipality of San Dionisio del Mar (Fig. 1). The study area comprised 33.1 km2 and the area surveyed for mammals was 1.6 km2. Climate is warm sub-humid, with a mean annual temperature of 25°C, and mean total annual precipitation of 932.2 mm (García, Reference García1988).
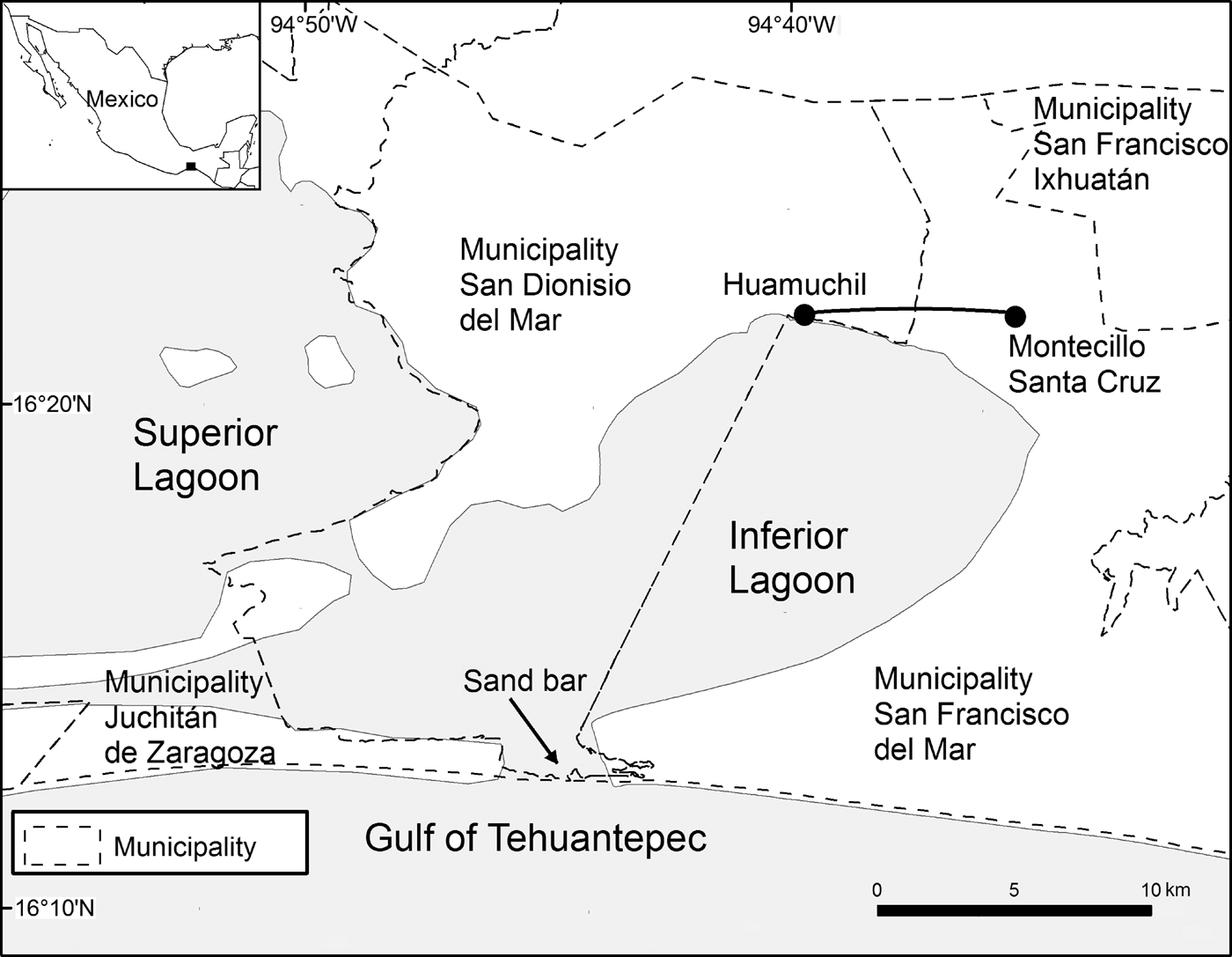
Fig. 1 Study area in Montecillo Santa Cruz, south-eastern Oaxaca, Mexico, in which vegetative cover and land use were analysed, with the location of an 8 km transect between Montecillo and Huamuchil along which lagomorphs and carnivores were monitored during 2001–2016.
Residents of Montecillo Santa Cruz are traditional fishers, although over the past decade they have begun to plant sorghum for sale and use as fodder for livestock, and maize and watermelon for family consumption (Vargas, Reference Vargas2001; Sántiz et al., Reference Sántiz, González-Romero, Lorenzo, Gallina-Tessaro and Cervantes2012). Cattle are raised extensively in all grassland areas, and prescribed burning is carried out to promote regeneration of grasses (Lorenzo et al., Reference Lorenzo, Retana-Guiascon, Cervantes, Vargas and Portales2000, Reference Lorenzo, Rioja, Carrillo and Cervantes2008; Vargas, Reference Vargas2000).
Within the study area a sand bar connects the Inferior Lagoon with the Gulf of Tehuantepec (Fig. 1). The sand bar closes and opens cyclically, changing in length as a result of natural events (e.g. changes in sea level, hurricanes). This process affects local people's socio-economic activities, as they principally engage in fishing. When the sand bar is closed, movement of fish and nutrients from the Gulf of Tehuantepec to the Inferior Lagoon is inhibited, and fish and shrimp populations decrease, and therefore local people turn to subsistence hunting, which has a negative effect on populations of lagomorphs, mainly L. flavigularis. Grey foxes and coyotes are also hunted, although not on a regular basis (J. Antonio, pers. comm.).
Methods
Surveys
The population density of L. flavigularis, S. floridanus and the carnivores U. cinereoargenteus and C. latrans was estimated annually during at least 1 month in the dry season (November–April) and 1 month in the rainy season (May–October) over 16 years (2001–2016). As there were few sightings of U. cinereoargenteus and C. latrans, these were grouped together as carnivores. We used the line transect method (Buckland et al., Reference Buckland, Rexstad, Marques and Oedekoven2015) to monitor mammal species along an 8 km transect of variable width between Montecillo Santa Cruz and Huamuchil. This transect was established according to accessibility (traversable roads) and visibility (flat open areas) (Fig. 1). Number of individuals was recorded while travelling along the transect in a pickup truck at 5–10 km per hour, at night, using halogen lanterns for illumination. Geographical location, altitude, habitat type, and perpendicular distance (the shortest distance from the individual to the transect, in m; Buckland et al., Reference Buckland, Rexstad, Marques and Oedekoven2015) were recorded for each individual observed. The perpendicular distance was recorded for jackrabbits and cottontail rabbits since 2001, and for carnivores since 2003. For this, an electronic distance measuring device (Bushnell Yardage Pro, Bushnell Corporation, Overland Park, USA) was used. All surveys were conducted during 20.00–02.00, when lagomorphs and carnivores are most active (Carrillo-Reyes et al., Reference Carrillo-Reyes, Lorenzo, Naranjo, Pando and Rioja2010; Rioja et al., Reference Rioja, Lorenzo, Naranjo, Scott and Carrillo-Reyes2011). Each night, the same 8 km transect was travelled in both directions, at least 2 hours apart to avoid duplicating data (in number of records and kilometers travelled); each mammal observed was recorded, and density was estimated using these data.
Data were analysed using Distance 6.0 (Buckland et al., Reference Buckland, Rexstad, Marques and Oedekoven2015) to estimate density for each mammal species, and we considered each direction travelled along the transect to be independent (i.e. we analysed the data for each direction independently). To evaluate the probability of detecting animals, the four models used by Distance were tested: half-normal, uniform, hazard rate, and negative exponential. Each model varies depending on homogeneity of sightings, probability of detecting individuals according to the distance from which they are observed, and number of sightings (Buckland et al., Reference Buckland, Rexstad, Marques and Oedekoven2015). In each case a cosine adjustment was assessed using likelihood ratio tests. The longest 5% of the perpendicular distances recorded throughout the analyses were eliminated to avoid bias introduced by atypical outlier distance sightings. The final model was chosen based on a combination of the lowest value for Akaike's information criterion (AIC) and the lowest variance (Rizo-Aguilar et al., Reference Rizo-Aguilar, Delfín-Alonso, González-Romero and Guerrero2016) for each year of monitoring. The population density obtained corresponds to the area monitored for mammals (1.6 km2; i.e. the length of the transect (8 km) multiplied by the maximum perpendicular distance (100 m each side of the transect) at which it is possible to identify observed animals). The results obtained for the density in the monitoring area (1.6 km2) were extrapolated to the total study area (33.1 km2).
Changes in vegetative cover and land use
Maps of vegetation and land use were created using analysis of a 2001 satellite image, from the Landsat Enhanced Thematic Mapper (ETM+), and a 2014 image from the Landsat Operational Land Imager (OLI). Changes in vegetative cover and land use were processed using the interdependent classification method (FAO, 1996), stratifying the two scenes with ArcGIS 10.2 (ESRI, Redlands, USA). Using this approach we delimited polygons for each type of vegetation and land use, to avoid bias in delimiting each thematic class. Estimating changes in surface area also involved the road layer, taken from a 2012 base map, using ArcGis 10.2, and the digital elevation model of Instituto Nacional de Estadística y Geografía (for 2013, with 30 m resolution).
To verify classification of the satellite image and ensure reliability of vegetative cover and land-use maps, 130 control points were recorded using a global positioning system in sites where the vegetation could potentially be interpreted correctly or incorrectly from satellite images (Velázquez et al., Reference Velázquez, Mas, Palacio, Díaz, Mayorga and Alcántara2002). For these points, types of vegetation and land use were described.
Once vegetation and land-use maps were drawn up for 2001 and 2014, along with the road layer and the digital elevation model, rates of change were estimated using the transition matrices (Bocco et al., Reference Bocco, Mendoza and Masera2001) by superimposing maps using the Land Change Modeler module of IDRISI Selva v. 17.02 (Clark Labs, Worcester, USA). An equation proposed by the FAO (1996) was used to obtain rates of percentage change in surface area for each type of vegetation and land use. This procedure was applied to all types of vegetation and land use: human-induced grassland, savannah, seasonal agriculture, human settlements, tropical dry forest, burned areas, areas without vegetation, secondary forest, bodies of water, and mangrove swamps. The results describe transitions from one type of land cover to another throughout the region (Velázquez et al., Reference Velázquez, Mas, Palacio, Díaz, Mayorga and Alcántara2002).
Results
In Montecillo Santa Cruz the mean estimated density of L. flavigularis during 2001–2016 was 9.14 ± SE 0.79 individuals per km2 (range 1.44–34.37; model hazard rate/cosine; AIC = 5375.7; CV = 8.65%) and of S. floridanus 12.28 ± SE 1.49 individuals per km2 (range 3.57–40.01; model hazard rate/cosine; AIC = 2164.9; CV = 12.12%). The mean estimated density of the carnivores U. cinereoargenteus and C. latrans during 2003–2016 was 1.91 ± SE 0.40 individuals per km2 (range 0.35–5.06; model hazard rate/cosine; AIC = 599.94; CV = 23.5%). The total sampling effort (for all 16 years) was 568 km travelled for L. flavigularis and S. floridanus, and 472 km travelled for the carnivores.
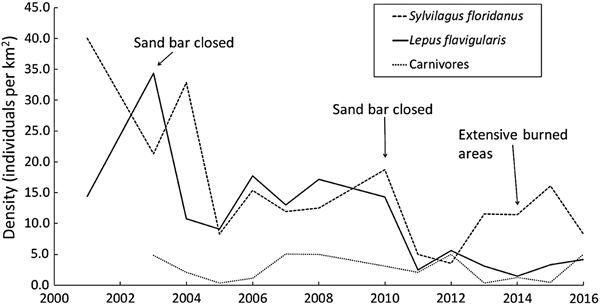
Fluctuations in mean annual population density for each mammal species/group are shown in Fig. 2. Although several fluctuations occurred during 2001–2016, as a general trend the density of L. flavigularis indicates a general decline; Sylvilagus floridanus has also declined but appears to be stable since 2005; and carnivores tended towards stability throughout the study period (Fig. 2).
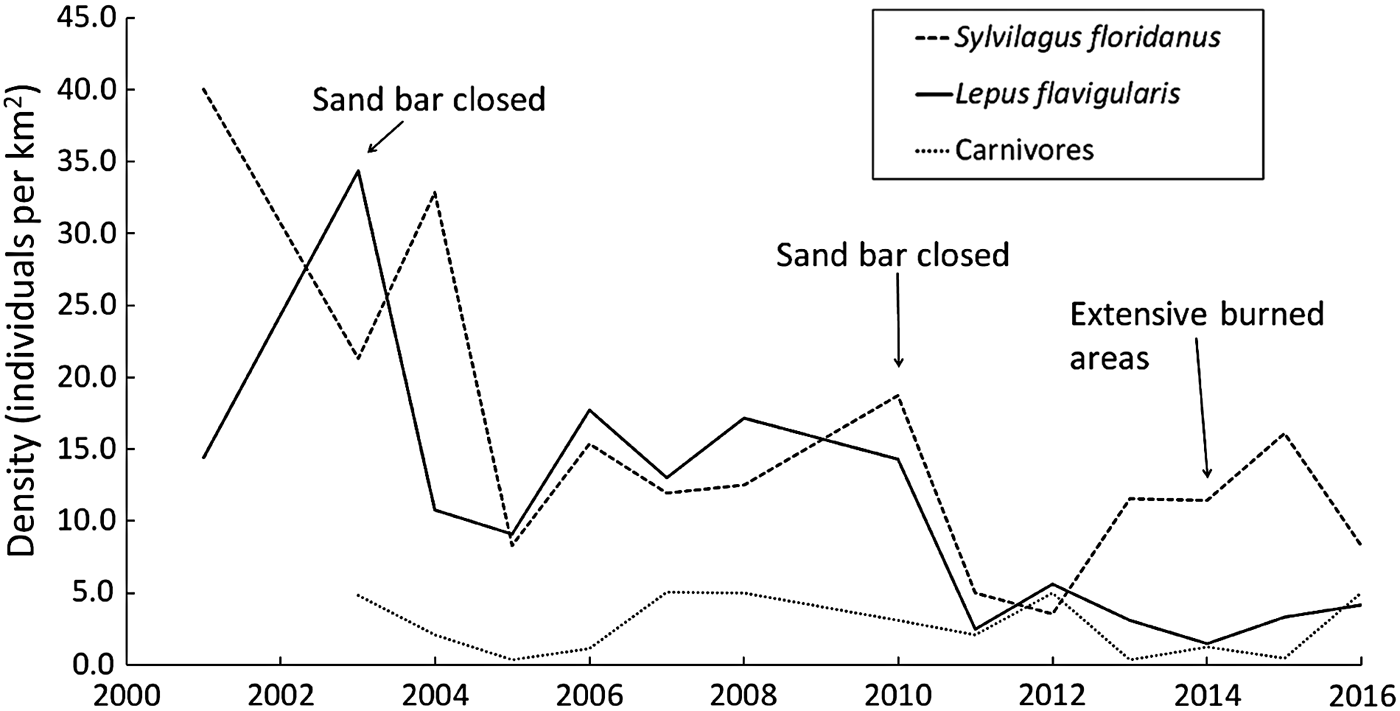
Fig. 2 Fluctuations in population densities of the Tehuantepec jackrabbit Lepus flavigularis, the eastern cottontail Sylvilagus floridanus, and carnivores (Canis latrans and Urocyon cinereoargenteus) in Montecillo Santa Cruz, Municipality San Francisco del Mar, and Huamuchil, Municipality San Dionisio del Mar, Oaxaca, Mexico (Fig. 1) during 2001–2016, with years indicated in which the sand bar was closed and in which there was extensive burning.
Savannah (8,174.5 ha in 2001; 6,823.4 ha in 2014) and human-induced grassland (6,806.5 ha in 2001; 6,804.8 ha in 2014) were the dominant cover types in the study area (Table 1), accounting for 11.6 and 10.5% of total surface area on average, respectively. Almost all vegetative cover types showed losses in surface area from 2001 to 2014, but the most drastic loss occurred in savannah, with a 1.4% decrease annually, and a total loss of 1,351.2 ha (16.5%) of the original surface area. This was principally a result of fires (burned area = 1,024.1 ha) and an increase in agricultural areas (302 ha). Tropical dry forest lost 513.4 ha (7.9%), with an annual loss of 0.7%, and an increase in human-induced grassland (238.8 ha) and burned areas (190.9 ha; Table 2).
Table 1 Percentage change (δ) in surface area (ha) for each type of vegetation and land use in Montecillo Santa Cruz, Oaxaca, Mexico (Fig. 1), in 2001 and 2014, with the change in area (ha), the percentage lost or gained, and the change per year (ha).
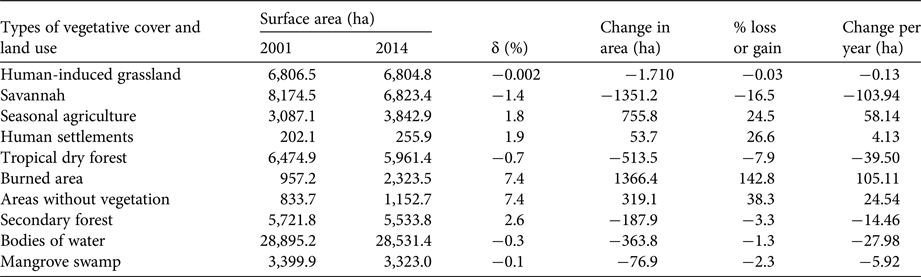
Table 2 Change in area (ha) from one type of vegetation and land use to another in Montecillo, Oaxaca, Mexico (Fig. 1), during 2001–2014.
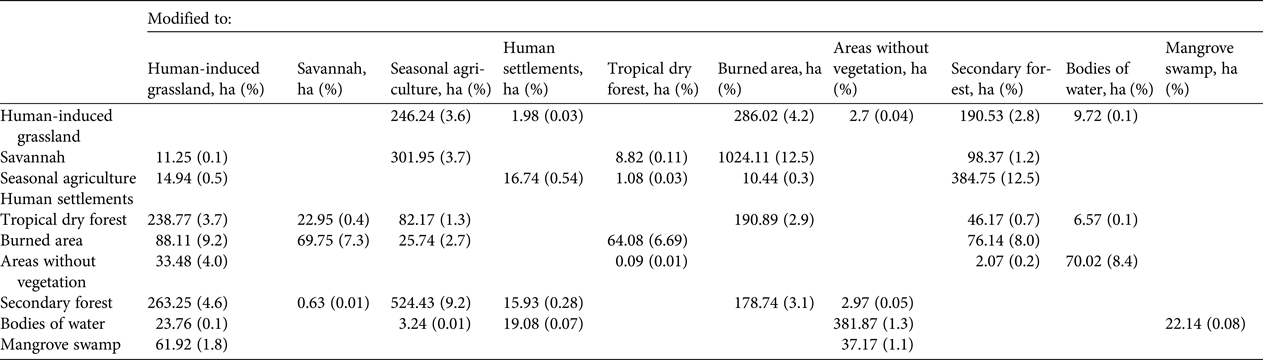
From 2001 to 2014 seasonal agriculture, human settlements, burned areas and areas without vegetation showed annual increases in surface area. Burned areas increased by 7.4% annually, with a total increase of 1,366.4 ha (142.8%). During the same time period, areas without vegetation increased by 7.4% annually, with a total increase of 319.1 ha (38.3%; Table 1). Burned areas were located mainly in savannahs (1,024.1 ha), human-induced grasslands (286 ha), and forests (190.9 ha; Table 2). All changes (losses and increases) in land use and surface area of vegetative cover from 2001 to 2014 are shown in Fig. 3.
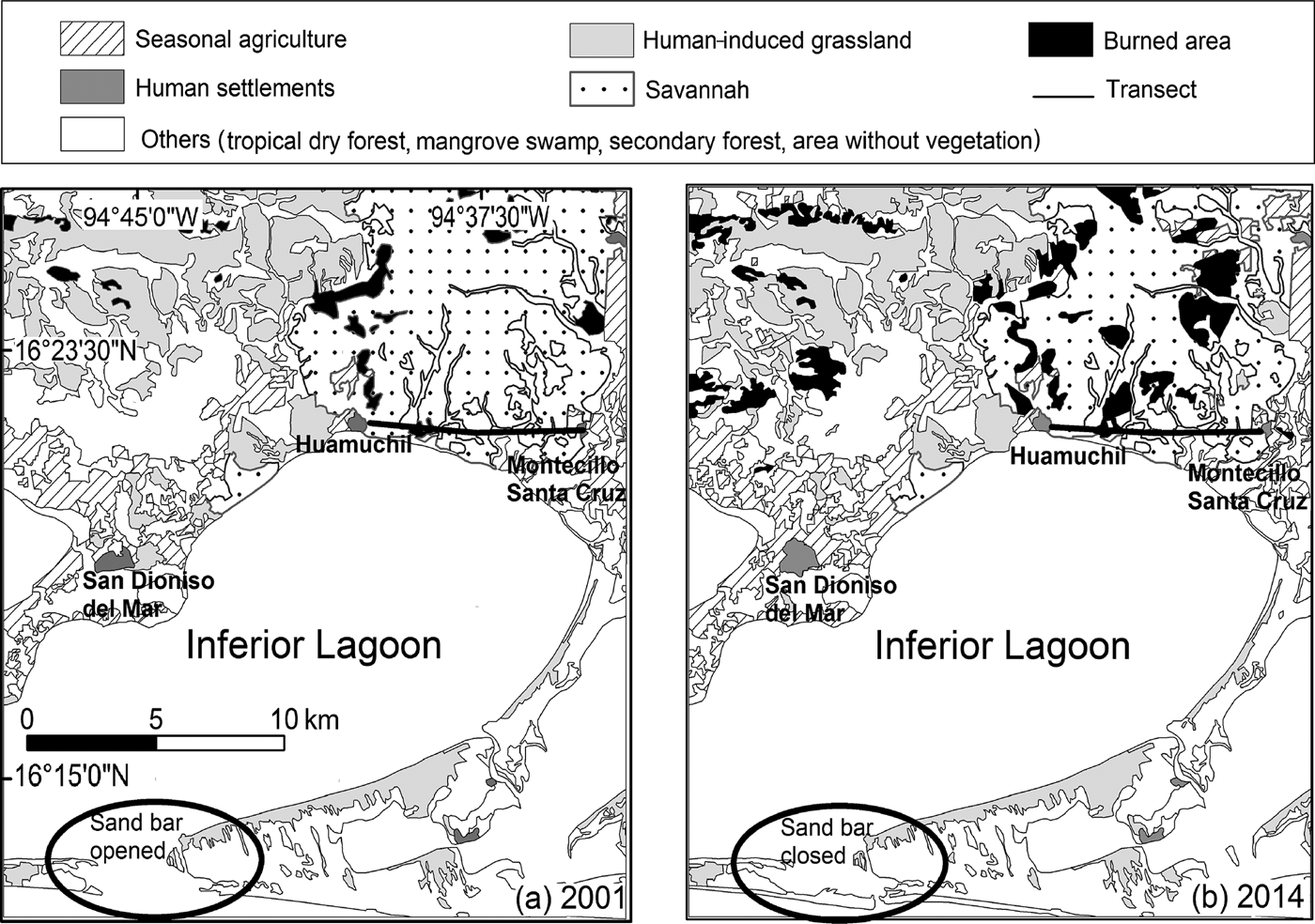
Fig. 3 Vegetative cover and land use in the municipalities of San Dionisio del Mar, San Francisco del Mar, San Francisco Ixhuatán, and Juchitán de Zaragoza, Oaxaca, Mexico, in (a) 2001 and (b) 2014.
Discussion
The estimated population density of L. flavigularis (9.14 individuals km−2) in the study region is similar to estimates from previous studies (1.3–11.5 individuals km−2; Vargas, Reference Vargas2000; Sántiz, Reference Sántiz2002; Lorenzo et al., Reference Lorenzo, Rioja, Carrillo and Cervantes2008). However, mean densities of L. flavigularis are low compared to other lagomorph species, such as Lepus californicus (20–154 individuals km−2; Hayden, Reference Hayden1966; Davis & Schmidly, Reference Davis and Schmidly1997; Portales-Betancourt et al., Reference Portales-Betancourt, Hernández, Laundré and Cervantes2012). The estimated population density for S. floridanus in this study (12.28 individuals km−2) is also low compared to other studies of the same species (27.5–2,000 individuals km−2; Chapman et al., Reference Chapman, Hockman, Edwards, Chapman and Feldhamer1982; Silvano et al., Reference Silvano, Acquarone and Cucco2000). Alteration and degradation of lagomorph habitat has probably led to a decrease in available food and space over time, resulting in lower population densities (Lorenzo et al., Reference Lorenzo, Retana-Guiascon, Cervantes, Vargas and Portales2000; Sántiz, Reference Sántiz2002).
The density of L. flavigularis decreased drastically over the study period, whereas the density of S. floridanus decreased significantly only during 2001–2005. Highs and lows in densities of the two species (Fig. 2) did not overlap except in 2005–2007 and 2011; this may be explained by the differential response of the two species to habitat use and food availability. Lepus flavigularis inhabits savannahs and human-induced grasslands (Lorenzo et al., Reference Lorenzo, Cervantes, Barragán and Vargas2006; Carrillo-Reyes et al., Reference Carrillo-Reyes, Lorenzo, Naranjo, Pando and Rioja2010; Sántiz et al., Reference Sántiz, González-Romero, Lorenzo, Gallina-Tessaro and Cervantes2012) and feeds mainly on grasses (Lorenzo et al., Reference Lorenzo, Carrillo-Reyes, Gómez-Sánchez, Velázquez and Espinoza2011), whereas S. floridanus inhabits human-induced grasslands, tropical forests, and scrub-brush, feeding on herbaceous and woody plants (Chapman et al., Reference Chapman, Hockman and Ojeda1980; Chapman & Ceballos, Reference Chapman, Ceballos, Chapman and Flux1990). It is likely that the two species avoid competing with each other.
The combined population density of the carnivores U. cinereoargenteus and C. latrans was stable over time. These species are adapted to a range of environments and can travel long distances in search of food, without excessive energy expenditure (Villa & Cervantes, Reference Villa and Cervantes2003). In our study area, at least, they do not appear to be greatly affected by changes in land cover.
In the study area, burned areas, areas without vegetation, and human settlements increased in surface area from 2001 to 2014. Undisturbed land has been converted to agriculture, with an increase in surface area of 755.8 ha during this period. This land was initially planted with corn; however, as a result of changes in geographical and environmental conditions (sandy soil, drought and intense rainfall), cultivation of sorghum has increased since 2005 (Vargas, Reference Vargas2001; Lorenzo et al., Reference Lorenzo, Cervantes, Barragán and Vargas2006; Rioja-Paradela et al., Reference Rioja-Paradela, Carrillo-Reyes and Lorenzo2012).
Savannahs and forests have increasingly been converted to pastures for livestock raising (Lorenzo et al., Reference Lorenzo, Cervantes, Barragán and Vargas2006; Rioja-Paradela et al., Reference Rioja-Paradela, Carrillo-Reyes and Lorenzo2012), affecting the coverage and diversity of vegetation (Altesor et al., Reference Altesor, Oesterheld, Leoni, Lezama and Rodríguez2005). Trampling by cattle modifies the soil's physical properties, causing it to lose its capacity for water retention, thereby facilitating erosion (Drewry & Paton, Reference Drewry and Paton2000). It has been observed that the permanence of the height and cover of species of bunchgrass such as Muhlenbergia macroura and Jarava ichu benefits the presence and abundance of other threatened lagomorph species, such as the volcano rabbit Romerolagus diazi, because it provides refuge from potential predators and nesting (Hoth et al., Reference Hoth, Velazquez, Romero, Leon, Aranda and Bell1987; Rizo-Aguilar et al., Reference Rizo-Aguilar, Guerrero, Hidalgo-Mihart and González-Romero2015).
To manage grazing areas, residents of Montecillo Santa Cruz carry out prescribed burning, which consists of deliberately causing fires to promote regeneration of grasslands during the dry season. However, these fires frequently become uncontrolled, affecting large areas of land (Lorenzo et al., Reference Lorenzo, Retana-Guiascon, Cervantes, Vargas and Portales2000, Reference Lorenzo, Rioja, Carrillo and Cervantes2008; Vargas, Reference Vargas2000).
Changes in land use to accommodate agriculture, including prescribed burning, have been the main cause of destruction of large areas of savannah (16.5% of the original surface area), which is the preferred habitat of L. flavigularis. This is a significant threat to this Endangered species, as lagomorphs are unable to quickly recolonize intensively burned areas (Meslow & Keith, Reference Meslow and Keith1968; Driessen, Reference Driessen1999; Salvatori et al., Reference Salvatori, Egunyu, Skidmore, De Leeuw and Van Gils2001). The time it takes for lagomorphs to recolonize these areas depends on how long it takes for habitat to recover its capacity to provide them with sufficient food and protection (Parker, Reference Parker1984), and burning results in temporary habitat fragmentation, which could affect population densities of several species (Fahrig & Paloheimo, Reference Fahrig and Paloheimo1988; Diffendorfer et al., Reference Diffendorfer, Gaines and Holt1995; Klok & De Roos, Reference Klok and De Roos1998). Our findings support this observation, as the population density of L. flavigularis decreased in 2014, when burned areas increased (Fig. 2). However, fires have had a positive effect on the abundance of R. diazi, as they maintain and promote new growth of the dominant zacaton bunchgrass, the main food source for this rabbit (Hunter & Cresswell, Reference Hunter and Cresswell2015). Additionally, the closing of the sand bar affects lagomorph population density. The sand bar closed in 2003 and 2010, and the following years the mean population density of L. flavigularis decreased (from 34.4 individuals km−2 in 2003 to 10.8 individuals km−2 in 2004, and from 17.2 individuals km−2 in 2008 to 2.5 individuals km−2 in 2011). This process appears to be affecting survival of the species, although the sand bar remained open in 2015 and 2016 and the population recovered slightly.
Conservation and management strategies
We offer several recommendations to conserve L. flavigularis and establish appropriate management strategies for grassland and savannah communities. (1) Grasses should be controlled annually by fire, taking care that fire does not spread beyond targeted areas. (2) Livestock-raising practices should be improved to avoid a negative impact on grasses; for example, by periodically rotating cattle among pastures to avoid overgrazing, thereby facilitating recovery of grasses to increase their vegetative cover and fodder productivity. (3) A programme of reproduction in semi-captivity in situ in protected enclosures should be developed. If successful, jackrabbits could be released and reintroduced into the habitat. Such a programme could be certified as a Wildlife Use and Management Unit by the Department of the Environment and Natural Resources, to obtain legal use permits for a threatened mammal species, and economic support for programme development. (4) As part of the Wildlife Use and Management Unit a Communal Ecological Reserve could be established, to be administrated by community authorities, with universities playing a technical advisory role. Local protected areas have been shown to promote appropriation of territory by communities, and the patrimony of natural resources, and their management could be a viable method of conserving habitat and the Tehuantepec jackrabbit, as well as improving the quality of life of local residents. This could ensure the persistence of the Tehuantepec jackrabbit, with economic support from the government.
Acknowledgements
We thank Miguel Aquino, Roberto Gutiérrez, Leyberto Gutiérrez, Juan Antonio, Julieta Vargas, Felipe Barragán, and the people of the municipality of San Francisco del Mar for their valuable help during fieldwork, Ann Greenberg for editorial support, and the families of Antonio Gutiérrez and Gutiérrez Vázquez for housing our crew. We thank El Colegio de la Frontera Sur, the Chicago Zoological Society, the Lincoln Park Zoo Neotropic Fund, and Mexico's Consejo Nacional de Ciencia y Tecnología, and Secretaría de Medio Ambiente y Recursos Naturales for funding this research. Recommendations and comments from Martin Fisher and an anonymous reviewer improved this article.
Author contributions
CL has provided funding for this study since 2001. She has monitored mammals in the field since 2001, and managed the mammal database. She supported the analysis of population density, and was the principal author. ECS has been monitoring mammals in the field since 2001. She managed the mammal database, reviewed satellite images and analysed changes in vegetation cover and land use, and assisted in writing the article. JBC has monitored mammals in the field since 2002. He managed the database, analysed density, and assisted in writing the article. DNG managed the database, analysed changes in vegetative cover and land use, and assisted in writing the article.
Biographical sketches
Consuelo Lorenzo is interested in the molecular systematics, taxonomy and conservation of lagomorphs and tropical rodents and has been monitoring populations and habitat of the Tehuantepec jackrabbit Lepus flavigularis for 20 years. Eugenia Sántiz studies the ecology and effects of global climate change on the distribution of threatened mammals, and the conservation and management of the Tehuantepec jackrabbit and its habitat. Jorge Bolaños-Citalán’s work is focused on the conservation and management of tropical mammals, especially bats and rodents. Darío Navarrete-Gutiérrez studies changes in distribution of mammals, as well as consequences of land-use changes.