Introduction
Although still containing some of the largest contiguous tracts of forested land in South-east Asia, the rainforests of Borneo are undergoing amongst the highest global levels of forest degradation and loss, principally as a result of selective timber extraction and subsequent conversion to oil palm Elaeis guineensis plantations (Gaveau et al., Reference Gaveau, Sloan, Molidena, Yaen, Sheil and Abram2014, Reference Gaveau, Sheil, Husnayaen, Salim, Arjasakusuma and Ancrenaz2016; Cushman et al., Reference Cushman, Macdonald, Landguth, Malhi and Macdonald2017). The intricate ecological responses to selective logging of Borneo's forests remain unclear for most species, yet several studies have indicated that many can persist after such management, with only a minority of species studied so far exhibiting markedly reduced post-logging densities (e.g. Meijaard et al., Reference Meijaard, Sheil, Nasi, Augeri, Rosenbaum and Iskandar2005; Costantini et al., Reference Costantini, Edwards and Simons2016). In comparison, the conversion of these forests to oil palm production has been shown to result in a substantial reduction in biodiversity and functional diversity (Fitzherbert et al., Reference Fitzherbert, Struebig, Morel, Danielsen, Brühl, Donald and Phalan2008; Yue et al., Reference Yue, Brodie, Zipkin and Bernard2015), a pattern mirrored region-wide (Wilcove et al., Reference Wilcove, Giam, Edwards, Fisher and Koh2013). Thus, although logged forest undoubtedly has lower intrinsic value to biodiversity conservation than pristine forest, it is becoming increasingly clear that further gains to conservation could be achieved if management of production forests were improved to minimize negative impacts on biodiversity (Meijaard & Sheil, Reference Meijaard and Sheil2008). However, such an optimization approach, based on an understanding of how biodiversity responds to forest management practices and other anthropogenic disturbances, is currently lacking for many species, and remedying this knowledge gap remains a priority.
The Sunda clouded leopard Neofelis diardi is a medium-sized felid, endemic to the islands of Borneo, where it is the terrestrial apex predator, and Sumatra. The species is categorized as Vulnerable on the IUCN Red List, based on a presumed small and declining population size (Hearn et al., Reference Hearn, Ross, Brodie, Cheyne, Haidir and Loken2016a). However, assessment of its conservation status and development of effective conservation actions are hindered by a lack of understanding regarding the species’ abundance, distribution and responses to anthropogenic disturbance (Hearn et al., Reference Hearn, Ross, Macdonald, Bolongon, Cheyne and Mohamed2016b). Records of Sunda clouded leopards inhabiting a diverse range of forest types, including both pristine and selectively logged forests (e.g. Brodie & Giordano, Reference Brodie and Giordano2012; Wilting et al., Reference Wilting, Mohamed, Ambu, Lagan, Mannan, Hofer and Sollmann2012; Cheyne et al., Reference Cheyne, Stark, Limin and Macdonald2013, Reference Cheyne, Sastramidjaja, Muhalir, Rayadin and Macdonald2016; Sollmann et al., Reference Sollmann, Linkie, Haidir and Macdonald2014; McCarthy et al., Reference McCarthy, Wibisono, McCarthy, Fuller and Andayani2015; Hearn et al., Reference Hearn, Ross, Brodie, Cheyne, Haidir and Loken2016a), indicate that they exhibit some capacity to tolerate anthropogenic disturbance. However, local-scale abundance has been found to be lower in logged forest sites compared to unlogged sites (Brodie et al., Reference Brodie, Giordano, Zipkin, Bernard, Mohd-Azlan and Ambu2015b). In addition, the movements of Sunda clouded leopards from a fragmented landscape were shown to be positively and strongly associated with forest, including highly disturbed forest types, but negatively associated with various non-forest vegetation types (Hearn, Reference Hearn2016), thus confirming earlier predictions that forest loss and conversion to oil palm plantations are one of the greatest threats to this felid (Rabinowitz et al., Reference Rabinowitz, Andau and Chai1987; Hearn et al., Reference Hearn, Ross, Brodie, Cheyne, Haidir and Loken2016a,Reference Hearn, Ross, Macdonald, Bolongon, Cheyne and Mohamedb). It is likely that the increasing prevalence of vast tracts of oil palm plantations throughout the species’ range is resulting in the fragmentation of habitat and the consequent isolation of individual populations, potentially making them increasingly vulnerable to demographic stochastic processes and inbreeding depression. Robust spatial ecology data are lacking for the Sunda clouded leopard but preliminary analyses suggest that individuals have relatively large home ranges (Hearn et al., Reference Hearn, Ross, Pamin, Bernard, Hunter and Macdonald2013). It is thus conceivable that as forests become increasingly fragmented and forest patches decline in size they become less able to support viable populations, resulting in reduced population densities and, ultimately, local extirpation.
Although research has provided new insights into how anthropogenic pressures influence Sunda clouded leopard abundance and habitat selection at a local scale, how these responses translate into changes in population density remains unknown. Sollmann et al. (Reference Sollmann, Linkie, Haidir and Macdonald2014) estimated densities of 0.8–1.6 individuals per 100 km2 in two primary and two mixed forest (primary and secondary) areas in Sumatra, but found no statistical support for differences in density between the populations. In the Malaysian state of Sabah, northern Borneo, Brodie & Giordano (Reference Brodie and Giordano2012) estimated the density of Sunda clouded leopards in an area of primary forest was 1.9 individuals per 100 km2, whereas Wilting et al. (Reference Wilting, Mohamed, Ambu, Lagan, Mannan, Hofer and Sollmann2012) reported densities from two selectively logged forests of c. 0.8 and 1.0 individuals per 100 km2. However, akin to Sollmann et al. (Reference Sollmann, Linkie, Haidir and Macdonald2014), the relatively large, overlapping variances of these estimates for Sabah suggest that the population densities were not significantly different. Such low-precision estimates reflect the difficulty of obtaining sufficiently large sample sizes. This is typical of studies of elusive forest felids (Foster & Harmsen, Reference Foster and Harmsen2012) and hinders our ability to draw robust conclusions regarding the Sunda clouded leopard's responses to disturbance, potentially masking any underlying problems.
As obligate carnivores, the abundance of large felids is directly affected by prey density under a wide range of ecological conditions (Carbone & Gittleman, Reference Carbone and Gittleman2002; Karanth et al., Reference Karanth, Nichols, Kumar, Link and Hines2004), and thus it is reasonable to assume that prey densities are a key limiting factor for Sunda clouded leopards. Quantitative data regarding the diet preferences of Sunda clouded leopards are lacking but incidental reports and observations from Borneo (e.g. Rabinowitz et al., Reference Rabinowitz, Andau and Chai1987; Yeager, Reference Yeager1991; Matsuda et al., Reference Matsuda, Tuuga and Higashi2008; Burnham et al., Reference Burnham, Hinks and Macdonald2012) suggest that they exploit a diverse array of mammals, and studies of temporal activity overlaps and patterns of co-occurrence with potential prey (Ross et al., Reference Ross, Hearn, Johnson and Macdonald2013) indicate that ungulates may be a key resource. Thus, the response of Sunda clouded leopards to anthropogenic disturbance may be mediated largely by the responses of their prey to such habitat modification. Responses of Bornean mammals to selective logging vary greatly, but their sensitivity to disturbance is positively correlated with their phylogenetic age and dietary specificity, and negatively correlated with their ecological niche width (Meijaard & Sheil, Reference Meijaard and Sheil2008; Meijaard et al., Reference Meijaard, Sheil, Marshall and Nasi2008). Brodie et al. (Reference Brodie, Giordano, Zipkin, Bernard, Mohd-Azlan and Ambu2015b) showed that, compared to estimates in unlogged forest, abundance of muntjac Muntiacus spp. and mousedeer Tragulus spp. declined, and of bearded pigs Sus barbatus and sambar deer Rusa unicolor increased in old logged forests. The abundance of all four ungulates was lower in recently logged forests. An increased abundance of some species in logged forest may benefit the Sunda clouded leopard and result in elevated abundances compared to primary forest. Conversely, the dense network of logging roads and skids present in production forests facilitates greater access and thus hunting opportunities for poachers (Laurance et al., Reference Laurance, Goosem and Laurance2009), of which ungulates are a favoured quarry (Corlett, Reference Corlett2007). In this balance, increased exploitative competition with humans in selectively logged forests without adequate protection against such threats could result in reduced densities of Sunda clouded leopards.
Here, we develop estimates of Sunda clouded leopard population density using spatially explicit capture–recapture analyses of camera-trap data from multiple forest areas in Sabah to investigate how density varies across the landscape and in response to anthropogenic disturbance. We test our a-priori hypotheses that the population density will be lower in forests with (1) higher hunting pressure and (2) higher levels of forest fragmentation. We also hypothesize that (3) among selectively logged forests, time since logging will be positively associated with Sunda clouded leopard density. We combine our results with those from previously published studies to develop an estimate of Sunda clouded leopard population size in Sabah.
Study areas
During May 2007–December 2013 we conducted intensive, systematic camera-trap surveys of eight protected forest areas in the Malaysian state of Sabah, northern Borneo (Fig. 1, Table 1). We selected survey areas that provided a broadly representative sample of the spectrum of forest types, elevations, anthropogenic disturbance and fragmentation present in the state. We surveyed three primary forests: one predominantly lowland hill (Danum Valley Conservation Area, henceforth Danum Valley) and two largely hill dipterocarp and submontane forests (Tawau Hills Park, henceforth Tawau, and Crocker Range Park, henceforth Crocker). We surveyed five forest areas that had been exposed to selective logging, including the Lower Kinabatangan Wildlife Sanctuary (Kinabatangan), Tabin Wildlife Reserve (Tabin), and Kabili–Sepilok, Malua and Ulu Segama Forest Reserves.
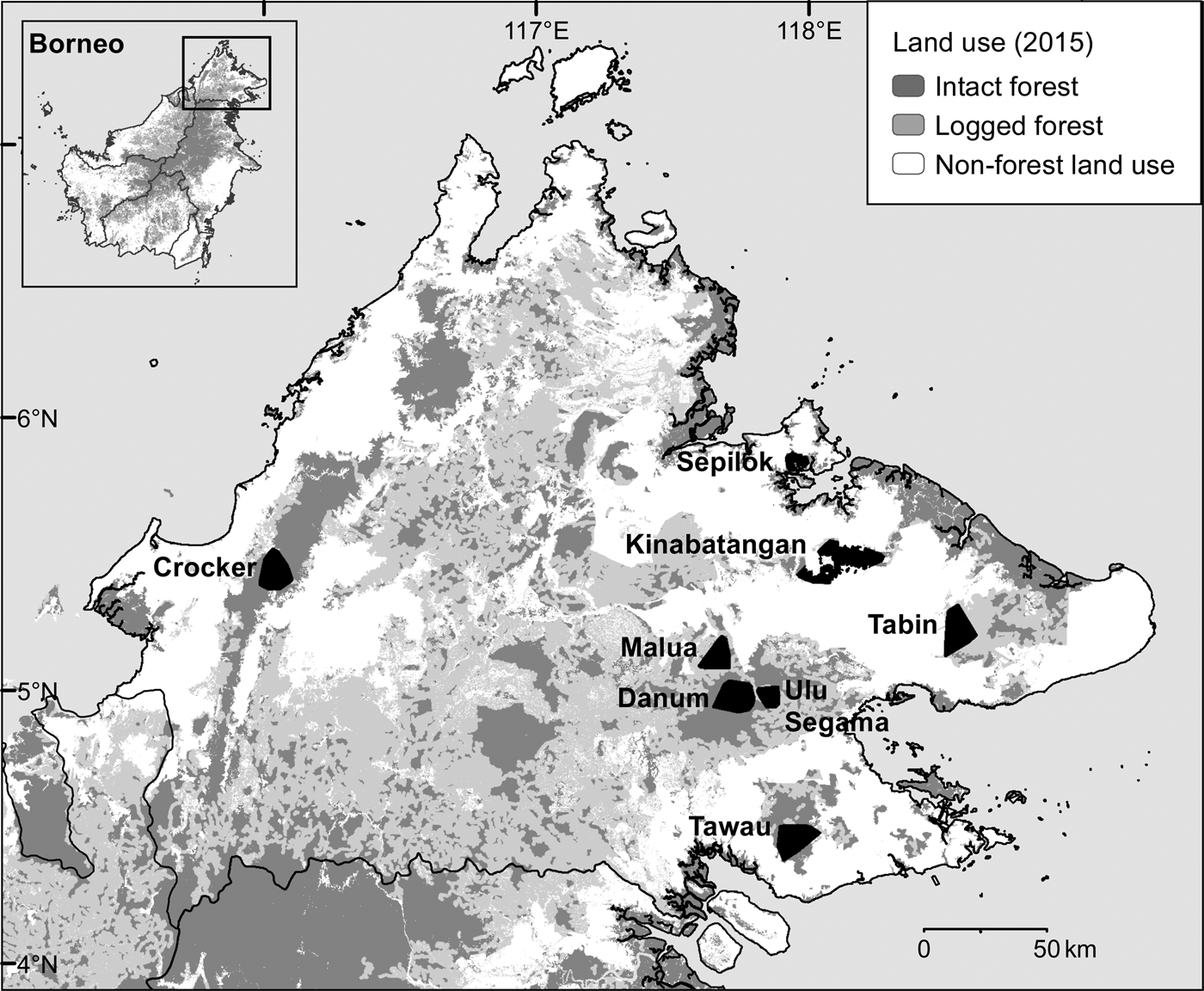
Fig. 1 The locations of the eight areas in Sabah, Malaysian Borneo, where camera-trap surveys of the Sunda clouded leopard Neofelis diardi were conducted, showing land use in 2015 (Gaveau et al., Reference Gaveau, Sheil, Husnayaen, Salim, Arjasakusuma and Ancrenaz2016). Intact forest includes both primary forest and previously logged forest, the impacts of which were no longer visible via analysis of satellite images in 2015; see Gaveau et al. (Reference Gaveau, Sheil, Husnayaen, Salim, Arjasakusuma and Ancrenaz2016) for further details.
Table 1 Details of the eight forest study areas in Sabah, Malaysian Borneo (Fig. 1), with location, size, level of isolation/fragmentation, dominant landcover type, and time since logging.

* In approximate order of increasing disturbance (level of fragmentation and exposure to selective logging practices).
Methods
Camera survey protocol
We undertook camera-trap surveys designed specifically to estimate the population density of Bornean felids (Hearn et al., Reference Hearn, Ross, Bernard, Bakar, Hunter and Macdonald2016c). Depending on logistical constraints, we deployed cameras according to one of two protocols, applying either a split-grid approach, where the entire grid is surveyed sequentially in two halves, or a simultaneous approach, where all camera stations are deployed in a single phase (Table 2). We deployed cameras primarily along established and newly cut human trails and ridgelines, and occasionally along old, unsealed logging roads, particularly in two of the selectively logged sites (Malua and Ulu Segama; Table 2). Camera stations were spaced c. 1.5–2.0 km apart, to balance the need for a sufficiently large sampling grid with the need to ensure that each individual's home range contained several stations (e.g. Foster & Harmsen, Reference Foster and Harmsen2012). Cameras were positioned c. 40–50 cm above the ground and arranged in pairs so that both flanks of an animal could be photographed simultaneously, to facilitate individual identification.
Table 2 Details of camera-trap sampling regimes, and Sunda clouded leopard Neofelis diardi photographic capture data derived from surveys of eight forest study areas in Sabah, Malaysian Borneo (Fig. 1).
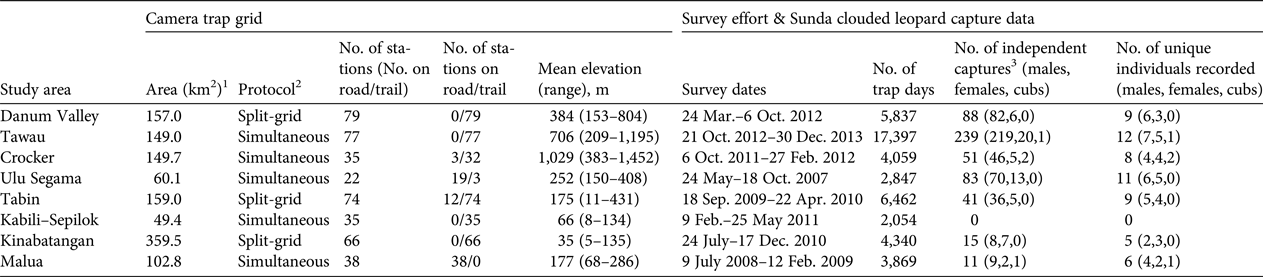
1 Camera trap grid area defined by a 100% minimum convex polygon around all camera stations.
2 Split-grid, the entire grid was surveyed sequentially in two halves; Simultaneous, all camera stations were deployed in a single phase.
3 Number of photographic captures of unique individuals, or images obtained more than 1 hour apart.
Assessment of poaching pressure
We followed the approach of Brodie et al. (Reference Brodie, Giordano, Zipkin, Bernard, Mohd-Azlan and Ambu2015b) and analysed our camera-trap data to provide an estimate of poaching pressure for each study area and to facilitate comparison with estimates of poaching pressure recorded in their previous studies. Our assessment was based on the photographic encounter rate of presumed poachers, calculated as the mean proportion of days that one or more poachers were recorded at each camera station. Hunting of birds or mammals of any species is prohibited by law in all our study areas, and people did not live in or use the forest for any legal purpose other than limited tourism, research and forest management at any of our sites. Excluding obvious records of unarmed park staff, field personnel and tourists, we assumed that any person photographed within the forest was a poacher. In most (86%) cases, people in the forest illegally were photographed carrying shotguns or spears, and/or accompanied by dogs. This approach does not facilitate assessment of historical poaching pressure, which arguably may be a more important parameter to measure, but it provides a useful, non-subjective assessment of current poaching levels.
Spatially explicit capture–recapture analyses
We developed estimates of Sunda clouded leopard population density using a spatially explicit capture–recapture approach (Efford, Reference Efford2004; Royle & Young, Reference Royle and Young2008), undertaken within a Bayesian framework (Royle et al., Reference Royle, Karanth, Gopalaswamy and Kumar2009). We used the package SPACECAP v. 1.1.0 (Gopalaswamy et al., Reference Gopalaswamy, Royle, Hines, Singh, Jathanna, Kumar and Karanth2012) in R v. 3.1.2 (R Development Core Team, 2014) to conduct all spatially explicit capture–recapture analyses. We used pelage markings and morphology to identify and sex individual animals and developed a unique capture history for each individual. Detections of cubs were recorded but only adults were included in the analysis. Although it has been shown that gender can affect detection parameters in felids, and inclusion of sex as a covariate can consequently improve parameter estimation precision (e.g. Sollmann et al., Reference Sollmann, Furtado, Gardner, Hofer, Jácomo, Tôrres and Silveira2011), we were unable to model sex-specific detection parameters because of the low number of female recaptures, and therefore data for both sexes were pooled and analysed together. We assigned each 24-hour period as a unique sampling occasion, as short sampling intervals may improve model precision (Goldberg et al., Reference Goldberg, Tempa, Norbu, Hebblewhite, Mills, Wangchuk and Lukacs2015). We limited our sampling duration to 90 days, apart from at one site (Tabin), where the lengthy transition period, and consequent reduction in camera-trapping effort, necessitated a period of 120 days to provide sufficient detection frequencies. Such sampling durations are in line with similar studies to approximate population closure (e.g. Royle et al., Reference Royle, Magoun, Gardner, Valkenburg and Lowell2011; Wilting et al., Reference Wilting, Mohamed, Ambu, Lagan, Mannan, Hofer and Sollmann2012).
We developed a state space, a polygon defined by the addition of a buffer to the outermost coordinates of each trapping grid, within which we established potential home range centres by delineating a grid of regularly spaced points, with a mesh size of 0.25 km2. Following Gopalaswamy et al. (Reference Gopalaswamy, Royle, Hines, Singh, Jathanna, Kumar and Karanth2012) we eliminated potential home-range centres from areas predicted to be unsuitable for Sunda clouded leopards, using ArcMap 10.2 (ESRI, Redlands, USA) in conjunction with habitat data derived from field knowledge and high-resolution aerial images from Google Earth (Google Inc., Mountain View, USA). We assumed that Sunda clouded leopards are restricted to forest cover and do not occur in oil palm plantations (Hearn et al., Reference Hearn, Ross, Macdonald, Bolongon, Cheyne and Mohamed2016b), and therefore we considered forested areas (both pristine and disturbed) as habitat, and all other non-forest land uses as unsuitable. During a sequence of preliminary runs we systematically increased buffer size until the probability of detection at the state space boundary was negligible. Accordingly, buffer size was 12–30 km.
We ran all SPACECAP density estimation analyses using a half normal detection and Bernoulli's encounter model, with 100,000 Markov-Chain Monte Carlo iterations and a thinning rate of 1. We varied burn-in for each survey until adequate parameter convergence was attained, which we assessed by means of Geweke tests; z scores between –1.64 and 1.64 were deemed acceptable. SPACECAP applies a data augmentation process in which a theoretical population of zero-encounter-history individuals is added to the dataset of known individuals (Gopalaswamy et al., Reference Gopalaswamy, Royle, Hines, Singh, Jathanna, Kumar and Karanth2012). We varied data augmentation values for each survey, assigning a final value following a series of preliminary runs, increasing data augmentation where necessary to ensure that ψ, the ratio of the estimated abundance within the state space to the maximum allowable number defined by the augmented value, did not exceed 0.8. We examined the Bayesian P-value provided by SPACECAP, which measures the discrepancy between observed data and expected values, to assess the goodness-of-fit of the model; models presenting P-values of c. 0 or 1 were considered to be inadequate (Gelman et al., Reference Gelman, Meng and Stern1996; Gopalaswamy et al., Reference Gopalaswamy, Royle, Hines, Singh, Jathanna, Kumar and Karanth2012). For each parameter estimated, we present the posterior mean, standard deviation and 95% Bayesian highest posterior density interval. The highest posterior density interval is the shortest interval enclosing 95% of the posterior distribution. Following Sollmann et al. (Reference Sollmann, Linkie, Haidir and Macdonald2014) we consider parameters from each site to be significantly different if the 95% highest posterior density interval of one does not include the mean of the other.
Estimation of population size in Sabah
We developed an estimate of Sunda clouded leopard population size for Sabah based on extrapolation of an estimate of this species’ density to an estimate of available habitat. Following a meta-analysis approach, we calculated a weighted mean population density estimate from estimates developed here (n = 6) and from previously published estimates from Sabah (Brodie & Giordano, Reference Brodie and Giordano2012, n = 1; Wilting et al., Reference Wilting, Mohamed, Ambu, Lagan, Mannan, Hofer and Sollmann2012; n = 2), by weighting each unique value by the inverse of its coefficient of variation, based on the 95% highest posterior density values. Using the same weighted approach, we calculated a mean upper and lower density estimate, based on each value's upper and lower quantiles. For an approximation of available habitat for the Sunda clouded leopard we assumed that these felids are restricted to forest habitats, and used an estimate of Sabah forest cover for 2015 developed by Gaveau et al. (Reference Gaveau, Sheil, Husnayaen, Salim, Arjasakusuma and Ancrenaz2016), based on analysis of LANDSAT imagery. The definition of forest used by Gaveau et al. (Reference Gaveau, Sheil, Husnayaen, Salim, Arjasakusuma and Ancrenaz2016) included closed-canopy, old-growth and selectively logged dipterocarp, heath, freshwater and peat swamp forests and mangrove forests, but excluded young forest regrowth, scrublands, tree plantations, agricultural land, and non-vegetated areas, and thus closely matches current predictions for clouded leopard habitat associations (Hearn et al., Reference Hearn, Ross, Macdonald, Bolongon, Cheyne and Mohamed2016b). It is important to note that this definition of available habitat includes forest types from which no robust density estimates are currently available (i.e. heath forests, peat swamp forests and mangrove), and therefore our population estimate should be treated with appropriate caution.
Results
Photographic capture success
We recorded 528 independent photographic captures of Sunda clouded leopards, with records from all survey areas apart from Kabili–Sepilok (Table 2). We found evidence of breeding activity at three sites, recording two cubs in Crocker, one in Malua and one in Tawau (Table 2). The number of independent photographic captures within the closed survey period varied considerably across the sites (10–101; mean = 41), and 5–10 individuals were recorded within this period (Table 3). We could assign individual identity to all but one of the photographic captures, a female from Malua. At most sites we recorded more males than females, and males typically had higher recapture rates than did females (Table 3).
Table 3 Sampling specifications and Sunda clouded leopard capture data from the closed survey periods from seven study areas in Sabah, Malaysian Borneo (Fig. 1).

1 Number of independent photographic captures that were used in the spatially explicit capture–recapture analysis.
2 Values in parentheses represent the number of different camera stations that each individual was recorded at during the closed survey period.
Assessment of poaching pressure
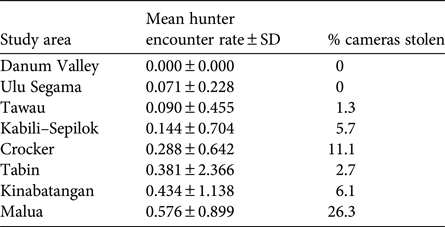
We found evidence of probable poaching activity in all forest areas apart from Danum (Table 4). The lowest poacher detection rates were found in Danum, Ulu Segama and Tawau, where camera theft was also low, and the highest were in Kinabatangan and Malua, where camera theft was high. Camera theft from Crocker was also relatively high. Tabin had a relatively high poacher detection rate but a relatively low incidence of camera theft.
Table 4 Indication of relative poaching pressure in each study area (Fig. 1), based on photographic detection rate of presumed poachers and percentage of camera traps stolen; see Methods for full description.
Density estimates
We developed estimates of Sunda clouded leopard density at all study sites at which the species was detected apart from Malua, where low numbers of photographic captures prevented spatially explicit capture–recapture model convergence, and was therefore removed from subsequent analyses. At all other sites Bayesian P-values indicated that the models were of an adequate fit (Table 5) and Geweke tests indicated that all model parameters converged. Sunda clouded leopard density across these six sites was 1.39–3.10 individuals per 100 km2 (Table 5). The two highest density estimates were from the enrichment-planted Ulu Segama (3.10 ± SD 1.11 individuals per 100 km2) and selectively logged Tabin (2.66 ± SD 1.11), and the lowest were from the primary upland Crocker (1.39 ± SD 0.41) and the highly degraded and fragmented Kinabatangan (1.54 ± SD 0.70). Sunda clouded leopard density was significantly higher in Ulu Segama than Crocker, Danum and Kinabatangan, and density in Tabin was significantly higher than in Crocker and Kinabatangan, but we found no statistical support for differences in density between any other sites. The movement parameters from Kinabatangan and Tabin were significantly larger than those from all other sites, and the estimate from Kinabatangan was significantly larger than that from Tabin, by almost a factor of two (Table 5).
Table 5 Posterior summaries of the Bayesian spatially explicit capture–recapture model parameters of camera-trap data on the Sunda clouded leopard from six study areas in Sabah, Malaysian Borneo (Fig. 1).

σ, movement parameter, related to home range radius; λ 0, baseline trap encounter rate, the number of independent photographic detections per day; ψ, the ratio of the estimated abundance within the state space to the maximum allowable number defined by the augmented value; N, number of individuals in the state space; D, density (individuals per 100 km2).
Estimation of population size in Sabah
The weighted mean population density developed from nine available density estimates was 1.90 individuals per 100 km2, and the weighted lower and upper 95% posterior intervals were 0.82 and 3.37 individuals per 100 km2, respectively. Based on data derived from Gaveau et al. (Reference Gaveau, Sheil, Husnayaen, Salim, Arjasakusuma and Ancrenaz2016), the amount of available habitat in Sabah in 2015 was 39,693 km2. Extrapolation of the weighted density estimate to this habitat assessment yielded an estimated population size of 754 (95% posterior interval 325–1,337) individuals for Sabah.
Discussion
Influence of anthropogenic disturbance on Sunda clouded leopard density
We present estimates of Sunda clouded leopard population density from six of eight forest areas we surveyed in Sabah, Borneo, including the first for this species from enrichment-planted, highly fragmented, and submontane forest types. Our density estimates from forest areas exposed to varying levels of anthropogenic disturbance are 1.39–3.10 individuals per 100 km2, and are thus comparable with those from previous studies in Sabah (0.84–1.9; Brodie & Giordano, Reference Brodie and Giordano2012; Wilting et al., Reference Wilting, Mohamed, Ambu, Lagan, Mannan, Hofer and Sollmann2012), the Indonesian province of Central Kalimantan (0.72–4.41; Cheyne et al., Reference Cheyne, Stark, Limin and Macdonald2013), and Sumatra (0.8–1.6; Sollmann et al., Reference Sollmann, Linkie, Haidir and Macdonald2014). Nevertheless, statistically significant differences in Sunda clouded leopard population density were evident between several of our study areas.
Although the absence of replication in our study approach limits our ability to draw robust conclusions about the possible influence of anthropogenic disturbance on Sunda clouded leopard densities, our results support our first a-priori hypothesis that population density is negatively impacted by poaching pressure. The two areas with the lowest estimates, the primary uplands of the Crocker Range Park and the low-lying logged forests of the Lower Kinabatangan, were subject to some of the highest levels of poaching pressure, whereas forest areas with a relatively low incidence of poaching (e.g. Danum Valley, Ulu Segama and Tawau) yielded some of the highest densities. In the case of Ulu Segama, the estimate of density was statistically higher than that of the two lowest density sites. The comparatively low density found in Crocker Range may also be a reflection of higher-elevation forest supporting lower productivity. Although we are unable to disentangle the possible influence of low detection probabilities as a result of other factors unrelated to abundance (Sollmann et al., Reference Sollmann, Mohamed, Samejima and Wilting2013), the low photographic capture success from Malua Forest Reserve, where poaching intensity was highest among our study areas, is indicative of a low population density relative to our other sites. The high density estimate from Tabin Wildlife Reserve, which was also significantly higher than that of our two lowest density sites, despite the site being subject to moderate levels of poaching, appears to contradict this trend. However, unlike other areas where poaching activity was more diffuse, most records of poaching activity in Tabin typically involved poachers spot-lighting from four-wheel-drive vehicles along the single access road within the Reserve, or occasionally along the western border with an oil palm plantation. It is therefore possible that the impact of poaching was not widespread throughout the study area.
Our data also tentatively support our second a-priori hypothesis, that Sunda clouded leopard population density is lower in forests with higher levels of forest fragmentation. Firstly, the Lower Kinabatangan, which is composed of several relatively small forest patches embedded within a largely oil palm plantation landscape, supported the second lowest density of all our study areas. Secondly, we found no evidence of Sunda clouded leopards within the Kabili–Sepilok Forest Reserve, a small (42.76 km2), potentially isolated dipterocarp forest fragment contiguous with a coastal chain of mangrove and nipah palm but otherwise surrounded by oil palm plantations. Forestry Department staff stationed in the area reported that the species had been recorded there in the past, so it is likely that gradual loss of surrounding forest and conversion to oil palm plantations has led to local extirpation. Kabili–Sepilok Forest Reserve is a probable harbinger of the effects of ongoing fragmentation, which will be detrimental to Sunda clouded leopard populations across much of the species’ remaining range.
The low number of photographic captures from Malua Forest Reserve, which was surveyed just 1 year after selective logging operations ceased, provides tentative support for our third a-priori hypothesis, that time since logging is positively related to Sunda clouded leopard density in selectively logged forests. Furthermore, our two highest density estimates were from two forests surveyed 16 and 20 years post logging activities, of which one, the enrichment-planted Ulu Segama Forest Reserve, had a statistically higher density than the primary Danum Valley Conservation Area. The survey of the Tangkulap–Pinangah Forest Reserve in Sabah by Wilting et al. (Reference Wilting, Mohamed, Ambu, Lagan, Mannan, Hofer and Sollmann2012), just 8 years after logging operations stopped, yielded a density of 0.84 individuals per 100 km2, which is lower than any of our estimates, and Brodie et al. (Reference Brodie, Giordano, Zipkin, Bernard, Mohd-Azlan and Ambu2015b) showed that, compared to unlogged forest areas, the abundance of four ungulate species was lower in recently logged areas, whereas bearded pigs and sambar deer were more abundant, and muntjac and mousedeer less abundant in old logged areas. Thus, although we cannot be sure by what mechanism the effect may operate, one hypothesis is that following recent logging there is a direct negative effect on prey abundance and/or availability, which declines over time. Another, not mutually exclusive, hypothesis is that logging operations, and the associated proliferation of roads, increase both the number of poachers and their penetration of the forest, reducing prey populations and perhaps also inflicting a bycatch on Sunda clouded leopards themselves, and that the relative impact of these roads diminishes over time as the roads become unnavigable. Brodie et al. (Reference Brodie, Giordano, Dickson, Hebblewhite, Bernard and Mohd-Azlan2015a) found that an increase in road density in Borneo was associated with reduced local occurrence of Sunda clouded leopards, and in Sumatra, Haidir et al. (Reference Haidir, Dinata, Linkie and Macdonald2013) found that this felid's habitat use was positively affected by distance to forest edge. In another Sumatran study, McCarthy et al. (Reference McCarthy, Wibisono, McCarthy, Fuller and Andayani2015) reported that this species occurred most commonly at moderate distances from roads, rivers and forest edges, all features that facilitate the movement of people.
Our results confirm earlier suggestions (e.g. Wilting et al., Reference Wilting, Fischer, Bakar and Linsenmair2006; Hearn et al., Reference Hearn, Ross, Brodie, Cheyne, Haidir and Loken2016a,Reference Hearn, Ross, Macdonald, Bolongon, Cheyne and Mohamedb) that selectively logged forest provides an important resource for Sunda clouded leopards, and suggest that appropriate management of these commercial forests could further enhance their conservation value. Our results suggest that the overriding priority is to reduce poaching pressure, both on these felids and their prey, by reducing access to the forest interior along logging roads. Reduction of vehicular access could be achieved through the installation of gates and the destruction of bridges following the cessation of logging activities. This is particularly important in more recently logged forests, which will have a more extensive network of gravel roads that are still passable. Such efforts will not prevent access on foot, and so measures such as anti-poaching patrols, although expensive, are also essential to reduce the threat from poaching in these forests.
Estimation of population size in Sabah
We provide the first estimate of Sunda clouded leopard population size for the Malaysian state of Sabah based on robust spatially explicit capture–recapture density estimates from nine forest areas within the state. Our estimated population size of c. 754 individuals (95% posterior interval 327–1,337) is a significant methodological improvement on the approximate estimate of 1,500–3,200 individuals provided by Wilting et al. (Reference Wilting, Fischer, Bakar and Linsenmair2006), based on extrapolation of a track-based assessment of density from Tabin Wildlife Reserve. Our basic model of population size does not include a minimum patch size or measure of proximity to other patches in its calculation, as such data are currently lacking. Nevertheless, the apparent absence of the species from the relatively small forest fragment of Kabili–Sepilok suggests that our estimate of available habitat may be slightly inflated, and with it our population estimate. In addition, although we made efforts to survey a range of forest types and levels of anthropogenic disturbance, there are a number of forest types that were not included. Of these, mangrove forest, given its potential role in connecting otherwise isolated populations, is particularly important. Surveys within these habitats, and efforts to determine minimum patch sizes for this felid, are therefore a priority.
As forest cover on Borneo declines, there is an increasing need to assess the population size of the Sunda clouded leopard across the entire island, and thus the conservation status of the Bornean subspecies, Neofelis diardi ssp. borneensis (Hearn et al., Reference Hearn, Sanderson, Ross, Wilting and Sunarto2008). The Sabah bias of our data, and the lack of robust spatially explicit density estimates from outside this region, currently hinders such assessment. Although the overall nature of the forests within Sabah broadly reflects those of the island as a whole, outside this state there are stark differences in forest management and patterns of deforestation (Cushman et al., Reference Cushman, Macdonald, Landguth, Malhi and Macdonald2017). Furthermore, the threat from hunting and/or poaching, which we have shown to be a potentially important factor influencing Sunda clouded leopard density, is likely to vary considerably throughout the island. There is increasing evidence that Sabah's forests have hitherto been subjected to lower influences of hunting and poaching than elsewhere and that population densities may be significantly lower outside this region. The mean encounter rates of hunters/poachers in five areas in Sarawak were more than an order of magnitude higher than that described here (Brodie et al., Reference Brodie, Giordano, Zipkin, Bernard, Mohd-Azlan and Ambu2015b). Furthermore, Cheyne et al. (Reference Cheyne, Sastramidjaja, Muhalir, Rayadin and Macdonald2016) surveyed eight forest areas in Kalimantan with a comparable effort and approach to that used in our study, and recorded an exceptionally low number of Sunda clouded leopards (≤ 3) from six of these forests, which could be indicative of low population densities. Efforts should thus be made both to establish the incidence of poaching across this felid's range, and to derive robust, spatially explicit estimates of its density outside Sabah to inform the conservation of this elusive wild cat.
Acknowledgements
We are indebted to our research assistants, especially Gilmoore Bolongon. We thank Glen Reynolds and the Royal Society's South East Asia Rainforest Research Partnership for logistical support, and Rahel Sollmann and Paul Johnson for statistical advice. We thank Sabah Parks, Sabah Forestry Department, Sabah Wildlife Department, Yayasan Sabah, the State Secretary, the Sabah Chief Minister's Department, and the Sabah Biodiversity Centre for permission to conduct research. This research was funded primarily by the Darwin Initiative, the Recanati-Kaplan Foundation, the Robertson Foundation, and the Sime Darby Foundation, with additional funding from the Clouded Leopard Project, the Felidae Conservation Fund, Houston Zoo, HG Wills International Trust for Nature Conservation, Panthera, Point Defiance Zoo and Aquarium, and Wild About Cats.
Author contributions
AJH, JR and DWM conceived and designed the research. AJH and JR conducted the fieldwork and analysed the data. AJH wrote the first draft of the article, and all authors commented on and improved it.
Biographical sketches
Andrew Hearn’s interests lie in the distribution, status, spatial ecology and conservation of the guild of sympatric felids on Borneo. Joanna Ross’s research focuses on the ecology and conservation of members of the Bornean felid guild and other threatened Bornean mammals. Henry Bernard has research interests in Bornean small mammal communities and proboscis monkeys. Soffian Bakar’s core interests lie in the conservation and sustainable management of wildlife in Sabah. Benoit Goossens is Director of the Danau Girang Field Centre in Sabah, where he is running long-term programmes on an array of tropical forest species to understand their biological responses to rainforest fragmentation and oil palm monoculture. Luke Hunter oversees the direction and strategy of Panthera's global wild cat conservation programmes. David Macdonald has a background in behavioural ecology, with an emphasis on carnivores.








