Cognitive decline is an important public health issue.Reference Jacqmin-Gadda, Alperovitch, Montlahuc, Commenges, Leffondre and Dufouil1 Progressive deterioration of cognitive function can affect an individual's overall health and well-being, including daily self-care activities (for example dressing, bathing and housework), as well as the ability to effectively manage existing medical conditions and to participate actively in society. Increasing evidence suggests that cardiometabolic factors, including central obesity,Reference Whitmer, Gustafson, Barrett-Connor, Haan, Gunderson and Yaffe2 dyslipidemia,Reference van Vliet3 hypertension,Reference Birns and Kalra4 insulin resistance or diabetesReference Bordier, Doucet, Boudet and Bauduceau5 and inflammationReference Tegeler, O'Sullivan, Bucholtz, Goldeck, Pawelec and Steinhagen-Thiessen6 might accelerate cognitive decline in midlife and at older ages. Similarly, the metabolic syndrome, a cluster of metabolic abnormalities that increase cardiovascular risk, has been associated with cognitive decline in prospective cohort studies.Reference Dik, Jonker, Comijs, Deeg, Kok and Yaffe7
Emerging evidence suggests that the association between cardiometabolic dysregulation and cognitive decline may include indirect pathways via depressive symptoms.Reference Tuligenga, Dugravot, Tabak, Elbaz, Brunner and Kivimaki8 Specifically, a meta-analysis by Pan et al Reference Pan, Keum, Okereke, Sun, Kivimaki and Rubin9 found evidence for a bidirectional association between metabolic syndrome and depression. There is also considerable evidence linking depression with cognitive impairment. Meta-analyses suggest that depression at older ages may be a risk factor for Alzheimer's diseaseReference Ownby, Crocco, Acevedo, John and Loewenstein10 and have found moderate impairment in executive function, memory and attention in individuals with depression relative to controls.Reference Rock, Roiser, Riedel and Blackwell11 However, recurrent or increasing levels of depressive symptoms may also be a prodrome of dementia.Reference Mirza, Wolters, Swanson, Koudstaal, Hofman and Tiemeier12 We hypothesise that depressive symptoms are one pathway that links cardiometabolic factors to cognitive decline. Given that previous studies have examined associations of cardiometabolic factors with depression and cognitive functioning separately, the temporal relationships between these factors are unknown. Using data from two longitudinal community-based samples of adults in the Netherlands (Rotterdam Study) and the UK (Whitehall II study), this study evaluated if depressive symptoms mediate the association between cardiometabolic factors and cognitive decline while controlling for other risk factors.
Method
Design/setting and participants
Rotterdam study
The Rotterdam Study is a prospective population-based cohort study of adults in Rotterdam, the Netherlands. More details are provided elsewhere.Reference Hofman, Murad, van Duijn, Franco, Goedegebure and Ikram13 Briefly, in 1990 all inhabitants aged 55 years and older living in a catchment area in Rotterdam were invited to participate. A total of 7983 (78%) individuals agreed to participate. The initial cohort was expanded by 3011 individuals aged 55 years and older in 2000. This study used the third data wave of the first cohort (1997–99) and the first wave of the expanded cohort (2000–01) as baseline. All participants underwent home interviews and an extensive set of examinations in a research centre at baseline and after approximately 5 and 12 years (2002–04 and 2009–11; original cohort) and after approximately 4 and 11 years (2004–05 and 2011–12; extension cohort). The Rotterdam Study has been approved by a medical ethics committee, in accordance with the Population Screening Act: Rotterdam Study, executed by the Netherlands Ministry of Health, Welfare and Sports. All participants in the present analysis provided written informed consent.
Depression was assessed using a validated Dutch 20-item version of the Center for Epidemiologic Studies Depression (CES-D) scale,Reference Beekman, Deeg, VanLimbeek, Braam, DeVries and VanTilburg14 which measures self-reported frequency of depressive symptoms experienced during the past week. The CES-D is scored on a four-point Likert scale, ranging from ‘rarely or none of the time’ to ‘most or all of the time’. Cognitive function was assessed twice (second and third wave) at separate centre visits.Reference Hoogendam, Hofman, van der Geest, van der Lugt and Ikram15 The test battery included the Stroop test,Reference Houx, Jolles and Vreeling16 letter-digit substitution task (LDST),Reference Hoogendam, Hofman, van der Geest, van der Lugt and Ikram15 verbal fluency test (VF),Reference Welsh, Butters, Mohs, Beekly, Edland and Fillenbaum17 15-word verbal learning test (15-WLT)Reference Bleecker, Bollawilson, Agnew and Meyers18 and the Purdue pegboard test.Reference Tiffin and Asher19 Higher scores on each test indicate a better performance, except for the Stroop test in which a higher score indicates a worse performance. Scores for the Stroop test were therefore inverted. All tests were scored so that higher scores indicated better cognitive function.
Cardiometabolic dysregulation was defined using the facets of the metabolic syndrome:Reference Alberti, Eckel, Grundy, Zimmet, Cleeman and Donato20 elevated blood pressure (BP>130/85 mmHg or use of antihypertensive medication), impaired glycaemic control (fasting blood glucose concentration greater than 5.6 mmol/L (38 mmol/mol) or diagnosed type 2 diabetes), low high-density lipoprotein cholesterol (<1.03 mmol/L in men and <1.30 mmol/L in women), elevated triglycerides (>1.7 mmol/L) and central obesity (waist circumference ≥102 cm (men), ≥88 cm (women)). Systemic inflammation was added as an additional risk factor (C-reactive protein >3 mg/L). These measures were collected during research centre visits.
Level of education was categorised into low (primary education), medium (intermediate general education) and high (higher vocational education or university) levels. Smoking status was assessed by interview and categorised as smoker (current smoker) and non-smoker (never or former smoker). Physical activity levels were assessed with an adapted version of the Zutphen Physical Activity Questionnaire, which assigns a metabolic equivalent of task (MET) to all activities.Reference Koolhaas, Dhana, Golubic, Schoufour, Hofman and van Rooij21 Participants were classified into three groups of physical activity as low physical activity (<10 Met-hours per week); medium physical activity (between 10 and 50 Met-hours per week) and high physical activity (>50 Met-hours per week).
The Whitehall II study
The Whitehall II study is a prospective cohort study of London-based office staff working in 20 civil service departments. More details are provided elsewhere.Reference Marmot and Brunner22 Briefly, a total of 10 308 individuals (73% response rate) aged 35–55 years were recruited between 1985 and 1988. Ten follow-up assessments were conducted; participants were assessed approximately every 2 to 3 years. A clinical assessment was conducted every second phase. Informed consent was obtained from all participants, and the University College London Medical School Committee on the Ethics of Human Research approved the protocol.
Wave five (1997–1999) was used as the baseline for our analyses where the age range was between 45 and 69 years. Cardiometabolic factors were categorised as in the Rotterdam Study. Missing waist circumference was substituted with body mass index (BMI >30 kg/m2 for obesity). Depression was assessed using the 20-item CES-D scale,Reference Beekman, Deeg, VanLimbeek, Braam, DeVries and VanTilburg14 which was administered at wave seven for the first time. Therefore, we have used the four-item depression subscale of the 30-item General Health Questionnaire (GHQ)Reference Nicholson, Fuhrer and Marmot23 to control for depressive symptoms at baseline.
Educational attainment was grouped into three levels (no formal education or lower secondary education, intermediate education and higher degree). Ethnicity was drawn from wave one and categorised as White/Black minority ethnic. Smoking status was assessed by interview and coded as never, former and current. Intensity of physical activity was assessed via questionnaires. Participants were asked about the frequency and duration of their participation in ‘mildly energetic’, ‘moderately energetic’ and ‘vigorous’ physical activity. The measures were combined and categorised high, medium and low activity.
Cognitive function was assessed by four standard tasks at waves five, seven and nine. Short-term verbal memory was assessed with a 20-word free recall test. The Alice Heim 4-I (AH4-I)Reference Heim24 was used to assess inductive reasoning. Phonemic and semantic verbal fluency was assessed via ‘S’ words for phonemic fluency and via ‘animal’ words for the semantic fluency.Reference Borkowski, Benton and Spreen25 More details can be found elsewhere.Reference Akbaraly, Kivimaki, Shipley, Tabak, Jokela and Virtanen26 The most recent waves (i.e. seven and nine) were used in the present study.
Statistical analyses
Structural equation modelling was used to examine a potential mediating role of depression in the association between cardiometabolic dysregulation and cognitive decline. Mediation analyses requires a temporal relationship between exposure (cardiometabolic dysregulation, wave one), mediator (depressive symptoms, wave two) and outcome (cognitive decline, wave three). Therefore, longitudinal data from three waves was used for each cohort.Reference Kraemer, Stice, Kazdin, Offord and Kupfer27 Structural equation models are an extension of regression analysis that can handle measurement error, latent variables and relationships among latent and observed variables. Latent variables summarise the information from observed variables and account for measurement error and the individual contribution of each measure.Reference Bollen28 Elevated blood pressure, impaired glycaemic control, low high-density lipoprotein cholesterol, elevated triglycerides, central obesity and systemic inflammation were used as indicators for the baseline latent cardiometabolic dysregulation measure. The cognitive tests in each study were used as indicators for a general cognitive function (g-factor).
Change in cognitive function between two assessments was modelled using a latent change score approach.Reference McArdle29 In these models, a latent difference score is created by a distinct latent construct representing the difference between the two cognitive g-factors. Briefly, the model constrains the association between the first latent score and the second latent score to 1 such that any variance in the second latent score that was not explained by the first latent score would be accounted for by the latent change score for a given participant.
The conceptual framework is shown in Fig. 1: first, two latent constructs representing the cognitive g-factors at time 2 (T 2) and time 3 (T 2) were created. Second, a latent change score (cognitive decline) was constructed so that the cognitive g-factor at T 3 is considered to be the sum of the cognitive g-factor at T 2 plus the latent change score. Regression coefficients between the T 2 and T 3 cognitive functioning g-factors were constrained to 1, and regression coefficients between the T 3 cognitive functioning g-factor and the latent change score was also constrained to 1 so that the latent change score represented the nature of the change in cognitive functioning g-factors from T 2 to T 3. The cognitive g-factor at T 2 was standardised to a mean of 1 and a s.d. of 1 so that higher scores indicate better cognitive functioning. Third, the latent change score was correlated with the cognitive functioning g-factors from T 2 to account for individual changes over time that are associated with the initial cognitive functioning score at T 2. A negative change score indicates a cognitive decline.
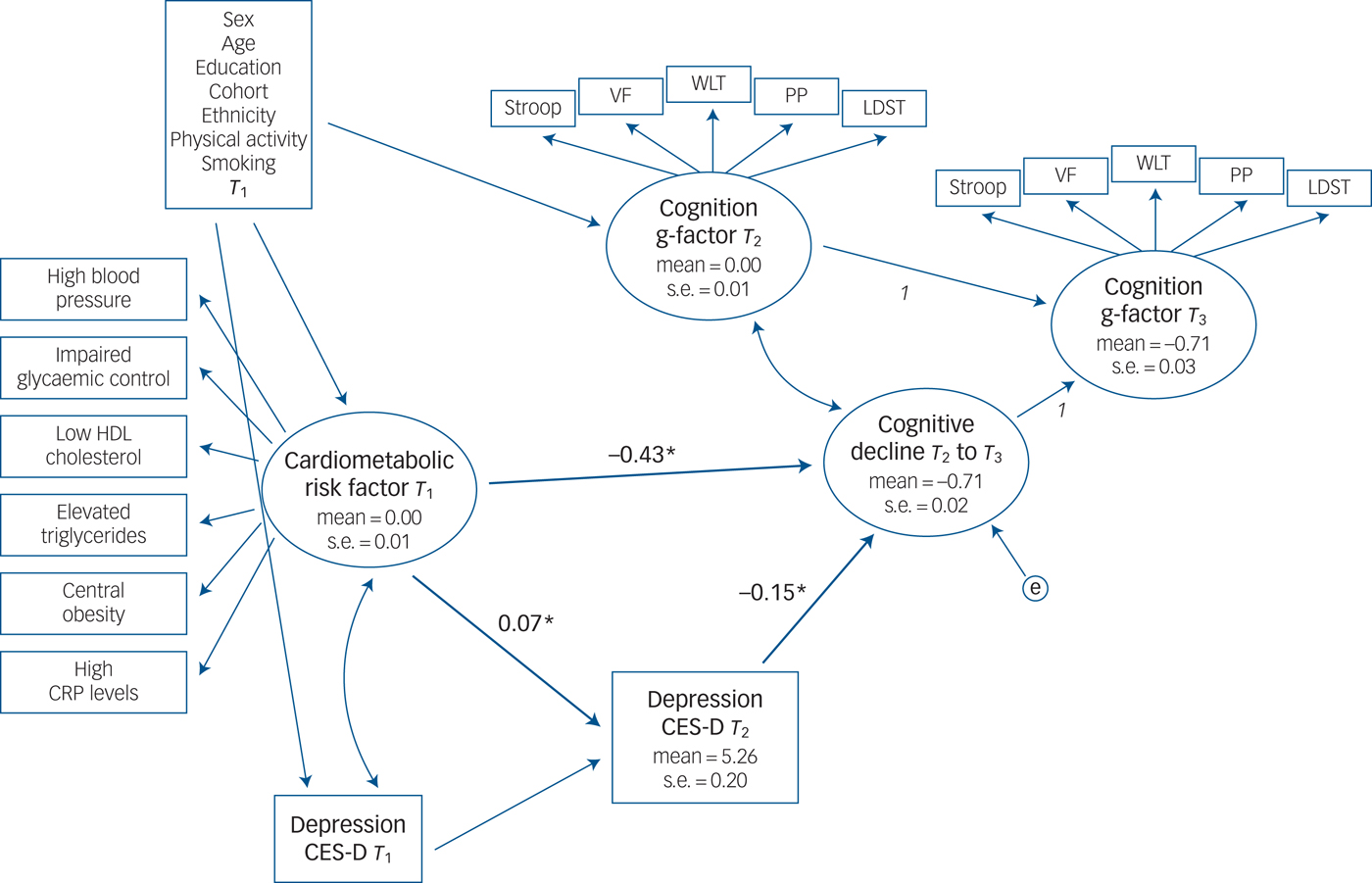
Fig. 1 Depressive symptoms as a potential mediator between cardiometabolic risk factors at baseline and cognitive decline at follow-ups in the Rotterdam Study cohort.
Depressive symptoms at baseline were included in all models to account for previous depression symptoms. In a final step, associations between cardiometabolic risk factor at time 1 (T 1) (latent variable), depression at T 2 (manifest variable) and cognitive change (latent variable) are modelled using a regression framework. We controlled for gender, age, education, ethnicity, smoking and physical activity in our analyses. Depressive symptoms at baseline were included in all models to account for previous depression symptoms. In the Rotterdam Study, the cohort source was also controlled for.
Depression was modelled as a mediator between cardiometabolic dysregulation and cognitive decline. A direct association between the metabolic risk factor at T 1 and cognitive decline (T 2– T 3) was estimated, as well as an indirect association through depression at T 2 (for example metabolic risk factor at T 1 predicts depression at T 2, which in turn predicts cognitive decline from T 2 to T 3). Indirect association coefficients were calculated by multiplying the standardised coefficients for the paths from the predictor to the mediator (for example metabolic risk factor to depression) and from the mediator (depression) to the outcome (cognitive decline).Reference Preacher and Hayes30
The general goodness of fit of each model was evaluated using the comparative fit index (CFI) and the root mean square error of approximation (RMSEA). A CFI of 0.90 or more and RMSEA of 0.08 or lower indicates adequate fit.Reference Marsh31 Multiple imputed values for the missing data from the variables used in the analysis were generated (PROC MI in SAS). In sensitivity analyses, analyses were rerun without imputed values for missing covariates. All analyses were conducted with SAS (version 9.4) and MPlus (version 7.4). Because observed cardiometabolic indicators were dichotomous, the weighted least squares estimator with a diagonal weight matrix, robust standard errors and a mean- and variance-adjusted χ2 test statistic (WLSMV) and theta parameterisationReference Muthén LKaM32 were used for parameter estimation in MPlus.
Results
Findings from the Rotterdam study
A total of 2940 individuals participated in all three assessments of the Rotterdam Study and had data on cardiometabolic factors, depression and cognitive functioning (see flow chart for sample selection in supplementary Fig. 1; available at http://dx.doi.org/10.1192/bjp.2017.26). Mean ages at baseline, first and second follow-ups were 65.0 years (s.d. = 5.9), 69.3 years (s.d. = 6.1) and 75.8 years (s.d. = 6.1), respectively. There were more women than men in the sample (57%).
Table 1 presents baseline characteristics of participants. There were sociodemographic and health differences at baseline between those who participated in all three assessments and those who participated only in one or two assessments; individuals who participated at all assessments were younger (65 v. 73 years), more likely to have a high educational level (16.0 v. 10.6%), more likely to have a high physical activity level (74.6 v 59.1%), less likely to have elevated depressive symptoms (5.8 v. 15.3%) and were more likely to have no metabolic risk factors (15.4 v. 9.6%). See supplementary Fig. 1 for a flow chart of the sample selection.
Table 1 Baseline characteristics of the Rotterdam Study cohort and the Whitehall II Study cohort
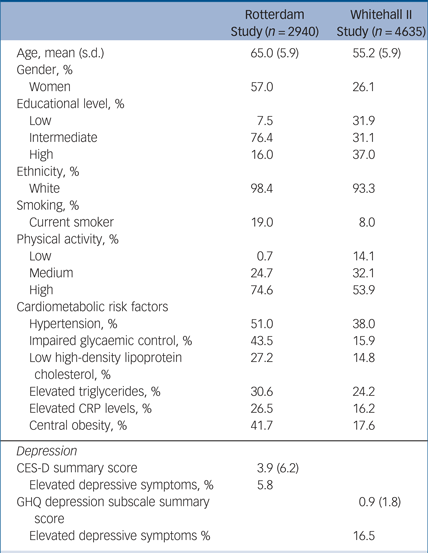
CRP, C-reactive protein; CES-D, Center for Epidemiologic Studies – Depression; GHQ, General Health Questionnaire.
Mean depression scores increased between the first (mean 3.9, s.d. = 6.2) and second assessment (mean 5.3, s.d. = 6.9; P < 0.001). Individuals with no metabolic risk factor had lower mean depression scores at the second assessment (mean 4.5, s.d. = 5.7) than those with one or two metabolic risk factors (mean 5.2, s.d. = 6.8) and those with three or more metabolic risk factors (mean 5.7, s.d. = 7.5) (test for a linear trend across the cardiometabolic risk factors groups: F(1,2937) = 13.4, P < 0.001).
Cognitive decline was observed between the second and third assessment on all cognitive functioning subtests: the effect sizes of change (difference between first and second cognitive functioning subtest score, divided by standard deviation of the first score) were 0.41, 0.18, 0.39, 0.12 and 0.50 for the letter-digit substitution task, verbal fluency test, Stroop test, 15-word verbal learning test and Purdue pegboard test, respectively.
Table 2 describes the associations between the number of cardiometabolic risk factors at baseline and change in cognitive functioning (g-factors controlled for age and gender). Individuals with no cardiometabolic risk factors had the highest cognitive functioning scores at T 2 and T 3 whereas individuals with three or more cardiometabolic risk factors had the lowest cognitive functioning scores at T 2 and T 3. The largest cognitive decline was observed for those with three or more cardiometabolic risk factors.
Table 2 Association between cardiometabolic risk factors and depression and cognitive functioning in the Rotterdam Study and the Whitehall II study cohorta

a. A g-factor for cognitive functioning was computed from the five cognitive function tests using confirmatory factor analyses as shown in Fig. 1. Scores were standardised to have a standard deviation of 1 (time 2 (T 2) and time 3 (T 3)) and a mean of 0 (T 2) so that negative scores indicate poorer cognitive function. A test for a linear trend of g-factor decline across the cardiometabolic risk factors groups indicated a statistically significant decrease of g-factor scores across groups (Rotterdam Study: F(1,2937) = 49.6, P < 0.001; Whitehall II study: F(1,4632) = 31.1, P < 0.001)).
Results of the mediation analyses are presented in Fig. 1. The model fit was acceptable (CFI = 0.933, RMSEA = 0.041). Results show that a poorer cardiometabolic status at baseline was associated with greater cognitive decline, after controlling for age, gender, education, ethnicity, cohort, smoking and physical activity. There was a direct association from the latent variable cardiometabolic risk factor to cognitive decline (standardised regression coefficient of −0.43, P < 0.001) and an indirect association through depression; poorer cardiometabolic status at baseline was associated with a higher level of depressive symptoms at T 2 (standardised regression coefficient of 0.07, P < 0.001) which in turn was associated with cognitive decline (standardised regression coefficient of −0.15, P < 0.001).
The indirect association coefficients were small but significant: −0.011 (95% CI −0.002 to −0.020), suggesting that depression partially mediated the association between cardiometabolic status and cognitive decline.
Findings from the Whitehall II study
A total of 4469 individuals participated in waves five, seven and nine of the Whitehall II study and had data on cardiometabolic factors, depression and cognitive functioning. The mean age at baseline (wave five, mean 55.2, s.d. = 5.9) was almost 10 years lower compared with the Rotterdam Study and there were almost three times more men than women in this cohort. Mean ages at wave seven and nine were 61 (s.d. = 6.0) years and 66 (s.d. = 6.0) years, respectively.
Table 1 shows sociodemographic and clinical characteristics of participants at wave five (the baseline). Those who participated in all three assessments were more often male (73.9% v. 63.3%), more likely to be younger (55 v. 57 years), more likely to have a high educational level (38.6% v. 30.7%), more likely to have a high physical activity level (56.5% v. 47.8%), less likely to have elevated depressive symptoms (16.1 v. 20.5%), and were more likely to have no metabolic risk factors (31.7 v. 23.5%) than those who participated only in wave five. Individuals with no metabolic risk factor had lower mean depression scores at the second assessment (mean 7.7, s.d. = 7.5) then those with three or more metabolic risk factors (mean 8.4, s.d. = 7.8). See supplementary Fig. 1 for a flow chart of the sample selection.
Cognitive decline was observed between the second and third assessment on all cognitive functioning subtests: the effect sizes were 0.07, 0.29, 0.16, and 0.14 for the Alice Heim 4-I task, short-term verbal memory task, phonemic and semantic verbal fluency tasks, respectively. Associations between the number of cardiometabolic risk factors at baseline and the change in cognitive functioning (g-factors controlled for age and gender) are presented in Table 2. Associations were similar as in the Rotterdam Study, although changes in cognitive functioning g-factors were smaller. Individuals with no cardiometabolic risk factors had the highest cognitive functioning scores at T 2 and T 3 compared with those with one or more cardiometabolic risk factors.
The mediation analyses (Fig. 2) also suggested a potential mediating effect of depression in the association between metabolic risk factors and cognitive decline. Model fit was acceptable (CFI = 0.917, RMSEA = 0.049). There was a direct association from the latent variable cardiometabolic risk factor to cognitive decline (standardised regression coefficient of −0.38 P < 0.001) and an indirect association through depression: poorer cardiometabolic status at baseline was associated with a higher level of depressive symptoms at T 2 (standardised regression coefficient of 0.06, P = 0.003) which, in turn, was associated with cognitive decline (standardised regression coefficient of −0.41, P < 0.001). The indirect association coefficient was −0.023 (95% CI −0.005 to −0.041), which if anything, was higher than in the Rotterdam Study.
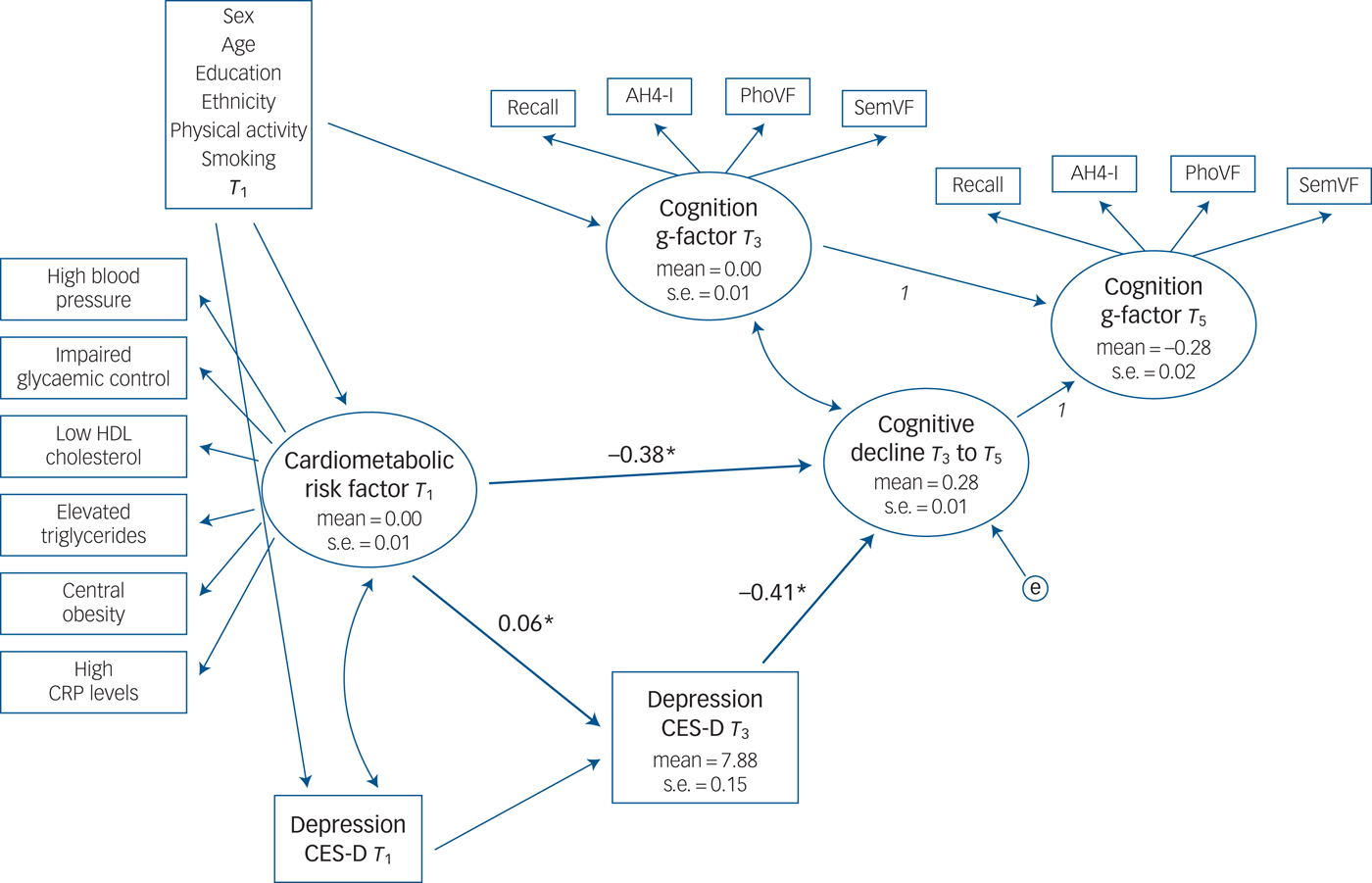
Fig. 2 Depressive symptoms as a potential mediator between cardiometabolic risk factors at baseline and cognitive decline at follow-ups in the Whitehall II study cohort.
Discussion
Main findings
This study evaluated temporal associations between cardiometabolic risk factors, depressive symptoms and change in cognitive function in two community studies of individuals aged 45 years and above in the Netherlands and the UK. Evidence from both studies suggest a direct association between cardiometabolic risk factors and cognitive decline and an indirect association through depressive symptoms: cardiometabolic risk factors at T 1 predicted depressive symptoms at T 2 (4–5 years later), which, in turn, predicted cognitive decline from T 2 to T 3 (4–6 years later).
These results suggest that depression might be one potential pathway through which cardiometabolic dysfunction is associated with cognitive decline. To our knowledge, this is the first study that simultaneously estimated longitudinal associations between cardiometabolic functioning, depressive symptoms and change in cognitive functioning. The congruence of the results from the Rotterdam cohort with those from the Whitehall II cohort, which is comprised individuals 10-years younger, suggests that the mediating role of depression apply to elderly populations as well as middle-aged adults who are not yet experiencing accelerated cognitive decline.
Strengths and limitations
The main strength of this study is the repeated measurement of cognitive function using validated cognitive batteries,Reference Singh-Manoux, Fayosse, Sabia, Canonico, Bobak and Elbaz33 as well as the availability of depression assessments and cardiometabolic variables in two large population-based samples. Strengths of the analysis include a structural-equation modelling approach that permitted the modelling of the dynamic relationship between cardiometabolic factors, depressive symptoms and change in cognitive function. This approach accounted for measurement error by including latent variables for cardiometabolic functioning and cognitive functioning. A latent-change score approach for the evaluation of cognitive decline is a more flexible approach than a simple difference-score approach because latent-change score methods separate out the portion of change that is correlated with the initial measurement and thereby removing a source of unreliability of a simple change score.
There are also several study limitations. Depressive symptoms were assessed using a self-report scale, and not diagnostic interviews that assessed depressive symptoms experienced in the past week and does not account for treatment of depression and history of depression. The overall level of depression was low, given that we examined non-clinical cohorts. In addition, lifestyle-related behaviours were also assessed by self-report, and thus may be subject to some bias. Cognitive functioning was only assessed at the second and third assessment. Therefore, we were not able to control for baseline cognitive functioning.
Attrition is an expected issue in large elderly population cohorts. Indeed, there were substantial sociodemographic and clinical differences between those who participated in all three assessments and those who did not, indicating that individuals with a better cardiometabolic functioning and a lower level of depressive symptoms were included in our cohort. This might have resulted in an underestimation of the underlying associations.
The time interval (4–5 years) between our single measurement of cardiometabolic functioning and the measurement of cognitive functioning could also be considered as a limitation. For example, Akbaraly et al have demonstrated that only persistent metabolic syndrome was associated with lower cognitive performance in late midlife.Reference Akbaraly, Kivimaki, Shipley, Tabak, Jokela and Virtanen26 Cardiometabolic functioning might have changed after the assessment because of clinical treatment or change of lifestyle-related behaviours. Finally, the applicability to other ethnic groups may be limited since the majority of the participants in the present study were White.
Comparison with findings from other studies
Our findings are consistent with previous prospective studies that have focused on comorbid depression as risk factor for cognitive decline or dementia in people with diabetes, a clinical form of cardiometabolic dysregulation. A recent systematic review on the association between depressive symptoms and cognitive functioning in people with diabetes found evidence that the presence of depressive symptoms in people with diabetes was associated with poorer cognitive outcomes.Reference Danna, Graham, Burns, Deschenes and Schmitz34 Sullivan et al Reference Sullivan, Katon, Lovato, Miller, Murray and Horowitz35 described an interaction between depression and diabetes on cognitive outcomes in the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) trial; patients with type 2 diabetes and comorbid depression were at higher risk for cognitive decline than those with type 2 diabetes without depression. Similar results have been reported from two studies using administrative databases.Reference Katon, Lin, Williams, Ciechanowski, Heckbert and Ludman36, Reference Katon, Lyles, Parker, Karter, Huang and Whitmer37
Our study adds to this evidence by considering temporality and suggesting that depression might explain some of the association between metabolic factors and cognitive decline (partial mediation). Management of cardiometabolic risk factors requires patient engagement. Depression is associated with poorer self-care behaviours (for example following a healthy diet, not smoking, engaging in exercise and medication adherence), which could worsen the management of the metabolic risk factors course and increase the risk of cognitive decline through a long-term exposure to metabolic risk factors.Reference Akbaraly, Kivimaki, Shipley, Tabak, Jokela and Virtanen26
Cardiometabolic dysregulations and depression also share common pathophysiological mechanisms, including dysregulation of the hypothalamic–pituitary–adrenocortical (HPA) axis.Reference Musselman, Evans and Nemeroff38 The co-occurrence of both conditions might adversely have an impact on both conditions, which can in turn result in several metabolic dysregulations and might amplify the risk of cognitive decline.Reference Epel39 Vogelzangs et al Reference Vogelzangs, Beekman, Dortland, Schoevers, Giltay and de Jonge40 have shown that metabolic dysregulations predicted a more chronic course of depressive disorders. Recurrent or chronic depressive symptoms are associated with prolonged exposure to psychosocial and other life stresses, which can cause wear and tear to different circulatory, inflammatory, immune and psychological regulatory systemsReference McEwen41, Reference Lesage42 and increase allostatic load.Reference McEwen41 Allostatic load has been associated with cognitive decline in population studies.Reference Juster, McEwen and Lupien43 Liu et al Reference Liu, Carvalho and McIntyre44 concluded in a recent review that several other overlapping physiological mechanisms in addition to HPA-axis disturbances may explain the association of metabolic dysfunction with worse cognition in individuals with depression, including abnormalities in brain-derived neurotrophic factor signalling, adipose-derived hormones, insulin signalling, inflammatory cytokines, and oxidative and nitrosative stress.
Implications
In conclusion, our findings show that people with metabolic dysregulation are at increased risk of cognitive decline and that depressive symptoms may partially mediate this decline. This has important implications for investigating the pathways that could link metabolic dysregulation and increased risk of cognitive decline. For adequate prevention of cognitive decline both cardiometabolic and mental health should play a key role.
Funding
The Rotterdam Study is funded by the Erasmus Medical Center and Erasmus University, Rotterdam, the Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. Continuing data collection on the Whitehall II study is funded by the Medical Research Council (K013351), National Institute on Aging (AG13196), National Heart Lung and Blood Institute (HL36310) and the British Heart Foundation. No funding was received to complete the analysis and interpretation of data for this paper. The funding agencies had no role in the design or conduct of the study, in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2017.26.







eLetters
No eLetters have been published for this article.