The worldwide prevalence of the metabolic syndrome and type 2 diabetes is increasing. Currently, 27 % of adults in the United States and 20–25 % of adults worldwide suffer from the metabolic syndrome, and evidence suggests that the rates are on the rise(1, Reference Pradhan2). Similarly, current estimates predict a further 50 % increase in the worldwide prevalence of type 2 diabetes by the year 2030(Reference Shaw, Sicree and Zimmet3). It is estimated that individuals with the metabolic syndrome have on average a fivefold increase in the risk of developing diabetes(1). The presence of type 2 diabetes increases the risk of CVD two- to fivefold, especially for women(Reference Barrett-Connor and Wingard4, Reference Pan, Cedres and Liu5). Despite these dangerous trends in the Western world, there is even more concern now that the incidence of diabetes is increasing in the Indian subcontinent, China, Central and South America, and Africa, with a particularly rapid growth in the Middle East(Reference Adeghate, Schattner and Dunn6). As it stands, the toll of diabetes on global health and economy is enormous, and will continue to rise.
Both diabetes and the metabolic syndrome are associated with a myriad of other health complications that include hypertension and stroke, cancers of breast, prostate and colon, limb amputation, blindness, and renal and gallstone disease(Reference Kannel and McGee7–Reference Shaffer15). Therefore, the resulting burden of these conditions on the health care systems not only has been onerous on the Western nations, but may also prove disastrous for nations with limited resources. Currently, there is no available cure for diabetes. Therefore, primary prevention through modifications in diet and lifestyle is of paramount importance.
Fortunately, diabetes prevention trials in high-risk groups have shown that modest dietary changes, weight loss and exercise lead to a 45–60 % reduction in the incidence of diabetes over a 3- to 6-year period(Reference Pan, Li and Hu16–Reference Knowler, Barrett-Connor and Fowler18). Moreover, an assessment of the Nurses' Health Study (NHS) cohort from 1980 to 1996 demonstrated that good diet, healthy BMI ( < 25 kg/m2), moderate to vigorous exercise ( ≥ 30 min/d), no smoking and modest alcohol intake ≥ 5 g/d (1/2 drink/d)(Reference Hu, Manson and Stampfer19) can prevent 91 % of type 2 diabetic cases. Unfortunately, compliance has been a major problem, with as few as 3·4 % of the cohort following these seemingly simple choices(Reference Hu, Manson and Stampfer19).
The metabolic syndrome is composed of the most dangerous risk factors for CVD, including diabetes, elevated fasting plasma glucose, abdominal adiposity, low HDL-cholesterol (HDL-C) and high blood pressure (BP)(Reference Alberti, Zimmet and Shaw20). The International Diabetes Federation clinical practice guidelines for the diagnosis of the metabolic syndrome include ethnic-specific risk values for abdominal obesity(1). For instance, European men and women with a waist circumference >94 and 80 cm, respectively, are considered to be at high risk. Furthermore, large waist circumference must be linked with two of the following four conditions in order to meet the diagnostic criteria: TAG >1500 mg/l; HDL-C below 400 and 500 mg/l for men and women, respectively; systolic or diastolic BP >135 and 85 mmHg, respectively; and fasting blood glucose >1000 mg/l or previously diagnosed type 2 diabetes(1).
With the rapidly rising prevalence of the metabolic syndrome and type 2 diabetes, there is an urgent need to identify dietary and lifestyle strategies that promote their prevention. Based on the current scientific evidence, nuts may play an important role in improving the risk factors for these diseases. The commonly used term ‘nut’ encompasses a wide range of seeds that based on botanical definitions may not actually be nuts. While hazelnuts meet the botanical definition of nuts, almonds, pistachios and walnuts, which are all seeds of drupe fruits, do not. Despite this inconsistency, this variable group of seeds has been clustered together under the collective term ‘tree nuts’. Despite small variations in their micro- and macronutrient profiles, tree nuts as a whole are healthy foods because of their good fatty acid profile (low in saturated fats and high in mono- and polyunsaturated fats (MUFA and PUFA, respectively)) and low available carbohydrate content, as well as being good sources of vegetable protein, fibre, phytosterols, polyphenols, vitamins and minerals (Table 1)(Reference Phillips, Ruggio and Ashraf-Khorassani21–23). Nuts may therefore be a useful component of a dietary strategy aimed at improving the risk factors of the metabolic syndrome, diabetes and CVD.
Table 1 Nutritional profile of commonly consumed, whole, raw nuts (per oz/28·4 g)*

* US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. http://www.nal.usda.gov/fnic/foodcomp/search (accessed on 12 January 2010).
† Values are for dried Brazil and pine nuts.
‡ Adapted from Segura et al. (Reference Segura, Javierre and Lizarraga22).
§ Adapted from Phillips et al. (Reference Phillips, Ruggio and Ashraf-Khorassani21); this value includes steryl glycosides (the reported values for the other nuts do not).
∥ Unless otherwise stated, mg.
Nuts and heart disease
Large cohort studies have indicated that the consumption of nuts is protective against CVD and CHD. A pooled analysis of the results of cohorts from the Adventist Health Study, Iowa Women's Health Study (IWHS), NHS and Physicians' Health Study demonstrated that in comparison to little or no nut consumption, the highest intake group for nut consumption had approximately a 35 % reduction in the risk of CHD incidence (relative risk (RR) 0·65; 95 % CI 0·47, 0·85)(Reference Kris-Etherton, Hu and E24). Worth noting is that a dose–response relationship was reported for all RR of CHD and nut consumption(Reference Kris-Etherton, Hu and E24). Furthermore, dietary intervention studies have supported the findings in these cohort studies by showing that nut consumption can reduce the risk of heart disease by improving serum lipid profile(Reference Kris-Etherton, Zhao and Binkoski25, Reference Griel and Kris-Etherton26), endothelial function(Reference Ros27) and BP(Reference Estruch, Martinez-Gonzalez and Corella28), in addition to lowering oxidative stress(Reference Jenkins, Kendall and Marchie29, Reference Jenkins, Kendall and Josse30) and inflammation(Reference Ros27, Reference Jiang, Jacobs and Mayer-Davis31). Two systematic reviews showed that the intake of 40–100 g of nuts, five or more times per week, can reduce LDL-cholesterol (LDL-C) 3–19 % in comparison to the Western or lower fat diets(Reference Griel and Kris-Etherton26, Reference Mukuddem-Petersen, Oosthuizen and Jerling32). Specific nuts have also been linked to a reduced risk of heart disease. A 2009 meta-analysis showed that the intake of 25–168 g/d of almonds leads to significant reductions in total cholesterol (TC) and LDL-C (P = 0·03 and 0·05, respectively)(Reference Phung, Makanji and White33). Similarly, in another meta-analysis, walnut consumption that provided 10–24 % of total daily energy was linked to a significantly greater decrease in TC and LDL-C in comparison to the control diets(Reference Banel and Hu34). Moreover, despite the absence of a pooled analysis, a number of recent trials have shown that 50–100 g/d of pistachio nuts can significantly improve HDL-C levels(Reference Sheridan, Cooper and Erario35–Reference Aksoy, Aksoy and Bagci37). The strength of the current evidence on the possible beneficial role of nuts for individuals at risk of developing heart disease has resulted in the United States Food and Drug Agency allowing a qualified health claim for nuts and serum cholesterol reduction(Reference Ternus, McMahon and Lapsley38). However, these findings have brought about a reassessment of the possible role of nuts not only in heart disease but also in the metabolic syndrome and type 2 diabetes.
Nuts, metabolic syndrome and diabetes
While current evidence indicates that the frequent intake of nuts is protective against CHD, the effect of nuts on specific risk factors of the metabolic syndrome and the overall risk of developing type 2 diabetes is not as conclusive. Two cohort studies have addressed this issue directly and evaluated the effect of nut consumption on the risk of developing type 2 diabetes(Reference Jiang, Manson and Stampfer39, Reference Parker, Harnack and Folsom40). Results from the NHS cohort show a 27 % reduction in the RR of developing diabetes in individuals who consumed nuts five or more times per week compared with those who rarely or never ate nuts, after adjustment for age, BMI, family history of diabetes, physical activity, smoking, alcohol and total energy intake. The effect seemed to be greatest in those with a normal body weight (45 % in RR). High intake of nuts was not associated with being overweight, and among the nurses diagnosed with diabetes, nut consumption five or more times per week tended to reduce the RR of CHD (multivariate RR 0·53, 95 % CI 0·24, 1·41; P for trend = 0·07)(Reference Hu, Stampfer and Manson41). Despite these findings, the analysis of the previously mentioned IWHS did not show a link between nut/peanut intake and the risk of type 2 diabetes(Reference Parker, Harnack and Folsom40). According to the authors, the inconsistency between the results from the NHS and IWHS cohorts may have been due to the fact that the IWHS included only one dietary measure, had older participants and used measures that were not as effective as those used by the NHS in the diagnosis of diabetes(Reference Parker, Harnack and Folsom40). Furthermore, the non-significant effect of nuts in the IWHS may in part be due to the overall low mean intake of nuts in this cohort. The reported mean nut intake was 0·75 (sd 1·75) servings per week(Reference Blomhoff, Carlsen and Andersen42).
Despite the inconsistencies in the cohort findings, there are reasons to believe that nuts may reduce the risk of developing diabetes. Adequate glycaemic control is important for prevention and management of type 2 diabetes(12). Foods that elicit low post-meal glucose and insulin responses, such as low-glycaemic index foods(Reference Jenkins, Wolever and Taylor43), have been effective in increasing insulin sensitivity, preventing hyperinsulinaemia and, overall, improving glycaemic control in patients with type 2 diabetes(Reference Jenkins, Kendall and McKeown-Eyssen44). This finding is supported by recent recommendations by the International Diabetes Federation, which highlight the significance of post-meal glycaemic control in diabetes risk reduction(45). Because nuts have low available carbohydrate content, they may contribute little to postprandial glycaemia when fed alone, and blunt postprandial glycaemia when consumed with carbohydrate-rich foods. This effect has been confirmed in acute studies involving almonds(Reference Jenkins, Kendall and Josse30, Reference Josse, Kendall and Augustin46). These studies have shown that almonds, taken alone or in combination with other carbohydrate-rich foods, can significantly lower postprandial glucose and insulin responses(Reference Jenkins, Kendall and Josse30, Reference Josse, Kendall and Augustin46). Another potential way by which nuts can improve glycaemic control is through their high MUFA content. It has been suggested that high-MUFA diets can also improve glycaemic control(Reference Garg, Bonanome and Grundy47), possibly by displacing the carbohydrate in the diet and thereby effectively reducing the glycaemic load. Despite these plausible mechanisms and evidence from acute studies, it is not clear whether improvements in postprandial glycaemia are indicative of long-term changes in insulin resistance. Larger and longer trials are required to determine whether these beneficial acute effects can be sustained in the long run. However, a longer study has shown that nuts in combination with other foods can improve glycaemic control in patients with type 2 diabetes(Reference Jonsson, Granfeldt and Ahren48). Overall, despite favourable evidence from acute studies, the results from long-term intervention studies that have examined the effects of nuts on glycaemic markers in patients with diabetes and the metabolic syndrome have not been as supportive. However, at the same time, in addition to approaches that help manage diabetes, it is important to determine whether the intake of nuts can produce similar cardioprotective effects, since this population is at a risk of developing CHD. A 2009 analysis of women with type 2 diabetes from the NHS cohort showed that those consuming five or more servings of nuts and peanuts per week had significantly lower serum LDL-C levels and overall a 44 % lower risk of developing CVD in comparison to those who almost never consumed nuts(Reference Li, Brennan and Wedick49). However, similar to the effect of nuts on glycaemic markers, the evidence from long-term clinical trials on the impact of nuts on serum lipid risk factors in patients with the metabolic syndrome and diabetes has been inconsistent.
We came across eight trials that examined the effect of nuts on both CHD risk factors and glycaemic markers in patients with diabetes or the metabolic syndrome (Table 2). In terms of CHD risk factors, three of the eight studies showed improvements in at least one marker of CHD risk in the nut group in comparison to the control(Reference Estruch, Martinez-Gonzalez and Corella28, Reference Lovejoy, Most and Lefevre50, Reference Tapsell, Gillen and Patch51). One study, however, showed improvements in BP in the control in comparison to the nut group(Reference Ma, Njike and Millet52). There were, however, a number of limitations associated with these studies. For instance, the study by Lovejoy et al. (Reference Lovejoy, Most and Lefevre50) compared the effect of various sources of MUFA on lipid parameters. It is possible that the beneficial effect of nuts on serum lipids may depend on the level of MUFA and not on their specific source. In the study by Scott et al. (Reference Scott, Balasubramanyam and Kimball53), almonds were given to the higher MUFA group only during the last 24 weeks of the study. The authors cited the small sample size, high dropout (twelve of thirty-five patients dropped out within the first 6 weeks) and weight loss as possible confounders(Reference Scott, Balasubramanyam and Kimball53). It is also important to consider that the control diet was a ‘heart healthy’ diet, which would expectedly result in significant reductions in blood lipids. Thus, the addition of almonds to this heart healthy diet provided no additional benefits with regard to blood lipids in patients with type 2 diabetes. In the two studies by Tapsell et al. (Reference Tapsell, Gillen and Patch51, Reference Tapsell, Batterham and Teuss54) and the one study by Ma et al. (Reference Ma, Njike and Millet52), the baseline LDL-C levels of the patients were relatively low ( < 2·9 mmol/l), and in the second study, the baseline HDL-C levels were somewhat high (approximately 1·5 mmol/l). Similarly, in the study by Mukuddem-Petersen et al. (Reference Mukuddem-Petersen, Stonehouse Oosthuizen and Jerling55), the participants had relatively low TC and LDL-C levels. This would make it difficult to attain statistically significant reductions in TC and LDL-C or further increases in HDL-C. The limited evidence from the present clinical studies on the link between nuts and an improved blood lipid profile for patients with type 2 diabetes points to the need for more long-term clinical interventions of nuts in these patients. However, it is possible that nuts may improve markers other than serum lipids in patients with type 2 diabetes, such as flow-mediated dilatation(Reference Casas-Agustench, Lopez-Uriarte and Bullo56), and that the changes in these risk factors may in part account for the strong inverse association between nut intake and the risk of CVD observed in the cohort studies.
Table 2 Effect of nuts on CHD risk factors and markers of glycaemic control in long-term clinical interventions
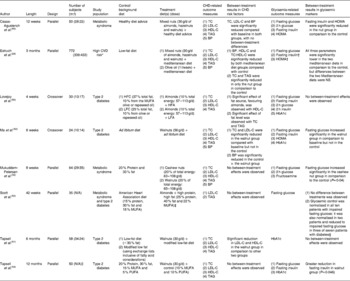
m, Male; f, female; TC, total cholesterol; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; BP, blood pressure; HOMA, homeostatic model assessment; N/A, not available, HFC, high-fat control; LFC, low-fat control, HFA, high-fat, high-almond diet; LFA, low-fat, high-almond diet.
* Included 421 patients with type 2 diabetes (55 % of subjects).
† Calculated for subjects without diabetes.
‡ Almonds were added to the diet during the last 24 weeks of the intervention; no dose was provided.
§ No other details with respect to this finding were provided.
∥ Only thirty-five subjects were analysed.
In terms of the potential effect of nuts on glycaemic markers, again, four of the studies showed significant improvements in one marker of glycaemic control(Reference Estruch, Martinez-Gonzalez and Corella28, Reference Scott, Balasubramanyam and Kimball53, Reference Tapsell, Batterham and Teuss54, Reference Casas-Agustench, Lopez-Uriarte and Bullo56). However, none of the studies have shown significant improvements in HbA1c (established marker of long-term glycaemic control). The studies by Tapsell et al. (Reference Tapsell, Batterham and Teuss54) and Casas-Agustench et al. (Reference Casas-Agustench, Lopez-Uriarte and Bullo56) did find a significant between-treatment effect with nuts in fasting insulin. The latter study also found improvements in homeostatic model assessment of insulin resistance(Reference Casas-Agustench, Lopez-Uriarte and Bullo56), while the study by Estruch et al. (Reference Estruch, Martinez-Gonzalez and Corella28) showed a significant reduction in fasting glucose in comparison to the control but not to the olive oil group. Some of the limitations include the fact that the study by Lovejoy et al. (Reference Lovejoy, Most and Lefevre50) was not long enough (4 weeks) to observe changes in HbAIc, which was one of the primary markers of glycaemic control. In the first study by Tapsell et al. (Reference Tapsell, Gillen and Patch51) and the one by Ma et al. (Reference Ma, Njike and Millet52), the lack of effect on HbA1c levels may be due to the relatively low baseline levels ( < 6·8 %). Moreover, some of the inconsistencies in the present findings may be attributed to the variations in the health status of the study participants, subject number, length of trial, outcome measures and the dose of nuts used. Overall, these inconsistencies make it difficult at this stage to reach definite conclusions on the role of nuts in glycaemic control in the long run.
However, there may be other mechanisms by which nuts can reduce the risk of type 2 diabetes and its complications. Inflammation has been linked to the risk of diabetes and heart disease(Reference Pradhan and Ridker57). Evidence suggests that nuts by themselves or as part of a cholesterol-lowering diet may significantly lower markers of inflammation including C-reactive protein(Reference Ros27, Reference Jenkins, Kendall and Marchie58). Furthermore, a recent meta-analysis of the prospective studies showed that a 100 mg/d increase in dietary Mg intake reduces the risk of developing diabetes by 14 % (RR 0·86; 95 % CI 0·77, 0·95)(Reference Larsson and Wolk59). Nuts are good sources of Mg (Table 1).
The findings presented here suggest that nuts may have a modest beneficial effect on serum lipids and markers of glycaemia in subjects with type 2 diabetes. Therefore, the moderate use of nuts as part of a healthy diet, and in very high-risk populations alongside medication, may help better manage diabetes. More importantly, however, since patients with the metabolic syndrome and diabetes are at a higher risk of developing heart disease, the addition of nuts to a healthy diet can help lower the risk of CHD, which is an effect that several hypoglycaemic medications have not been able to achieve(Reference Nissen and Wolski60–Reference Patel, MacMahon and Chalmers62). Overall, the present findings and the plausibility of the discussed mechanisms of action warrant further research, in the form of larger and longer randomised controlled trials that measure appropriate markers such as HbA1c, into the role of nuts in prevention and management of type 2 diabetes.
Key challenges and future direction
Even though major diabetes agencies, such as the American Diabetes Association, recognise nuts as sources of healthy fats, they have been somewhat hesitant in recommending nuts as part of the diabetic diet. This is perhaps due to several important research areas that need to be addressed when assessing the impact of nuts on health outcomes, and for the subsequent recommendation of their use as part of a healthy diet for diabetic patients.
As mentioned before, the term ‘nut’ encompasses a wide range of products that for most part have similar macro- and micronutrient profiles. However, there are some exceptions. For example, the PUFA content of 1 oz (about 30 g) of macadamia nuts is significantly lower than that of walnuts (0·4 v. 13·4 g) (Table 1). A key issue here is that the effects of all nuts on health outcomes have not been studied extensively. A search of MEDLINE database shows that almonds, walnuts and hazelnuts are the three most studied tree nuts, while pine nuts have been rarely examined. Therefore, making conclusions and recommendation for nuts as a whole may be too general based on the current evidence. At the same time, however, evidence from meta-analyses that demonstrate that both almonds and walnuts can improve TC and LDL-C, despite having significantly different unsaturated fatty acid profiles, cannot be overlooked(Reference Phung, Makanji and White33, Reference Banel and Hu34). It would be of great benefit for future studies to examine the effect of lesser studied nuts on health outcomes. Furthermore, the efficacy of mixed nut models should also be tested. This may also be beneficial from a compliance perspective, because it adds variation to the diet, and may allow participants to better adhere to the dietary recommendations.
This leads to the second key issue, which is the dose–response effect of nuts. The cohort studies assessing the impact of nut intake on CHD risk show a dose–response effect, with a reduction in risk with increasing nut intake frequency(Reference Kris-Etherton, Hu and E24). However, the long-term interventions involving nuts have not examined the dose–response relationship. This is important, because acute studies have shown a dose–response effect on glycaemic response(Reference Jenkins, Kendall and Josse30, Reference Josse, Kendall and Augustin46). Moreover, establishing a dose–response relationship is crucial before making nutritional recommendations for the general public.
Another significant issue is the preparation method of nuts. Different preparation methods may significantly alter the bioavailability of the bioactive compounds in nuts. There is currently no study that directly compares the effect of preparation methods of nuts on health outcomes. More studies are, therefore, required to directly compare the effects of roasting, blanching, and other processes on bioavailability of the active components of nuts and health outcomes.
Because of their high fat content and energy density, nuts have been viewed negatively in weight maintenance and contraindicated in energy-restricted diets. However, there is evidence suggesting that not only does nut consumption not lead to weight gain, but that it may in fact promote weight control. A recent analysis of the NHS cohort in an 8-year follow-up showed that women who consumed two or more servings per week of nuts had a lower risk of obesity in comparison to women who did not consume nuts (hazard ratio: 0·77; 95 % CI 0·57, 1·02; P = 0·003)(Reference Bes-Rastrollo, Wedick and Martinez-Gonzalez63). Also, a 24-week randomised study testing the effect of almonds v. complex carbohydrates on weight loss showed that a diet rich in almonds (84 g/d) is associated with greater reductions in weight (18 v. 11 %). The high-almond diet also resulted in greater reductions in waist circumference and systolic BP, both of which are risk factors for the metabolic syndrome(Reference Wien, Sabaté and Iklé64). Mechanistically, it has been suggested that the cell walls of almonds decrease the bioaccessibility of lipids by hindering their availability for digestion(Reference Ellis, Kendall and Ren65). This mechanism, however, needs to be verified in other nuts as well. Overall, it would be of great benefit if in the future all clinical studies on nuts published weight data in order to increase the evidence for a potential effect. Despite the potential role of nuts in weight loss, recent evidence from clinical weight loss interventions suggests that the level of energy restriction, and not the macronutrient profile of the diet, is responsible for weight loss(Reference Sacks, Bray and Carey66–Reference Dansinger, Gleason and Griffith68). Even if this is the case, a recent clinical trial demonstrated that an energy-restricted, high-fat (43 % of total energy), vegan diet in which mixed nuts (almonds, cashews, hazelnuts, macadamia, pecans and pistachios) provided 43·6 % of total fat was more effective in reducing serum LDL-C levels in comparison to an energy-restricted National Cholesterol Education Program Step II diet (20 v. 12 %), despite similar weight loss between both groups over the length of the study(Reference Jenkins, Wong and Kendall67). Therefore, moderate intake of nuts in energy-restricted diets may potentially help induce weight loss, but more importantly, it can help lower the risk of obesity-related risk factors such as heart disease. More clinical studies, however, are required to assess the impact of nuts on weight loss and, if applicable, to help determine the potential mechanisms by which nuts can help induce weight loss.
Overall, addressing these four major areas will help better clarify the role of nuts in health and disease. As such, it will allow governing agencies to make better recommendations to the general public.
Conclusion
Although randomised controlled trials have failed to demonstrate improvements in glycaemic control with nut consumption, nuts appear to be beneficial in terms of lowering TC, LDL-C and TAG, and raising HDL-C. Evidence also suggests that nuts may have a role in weight loss. Acute studies indicate that almonds have little effect on rising postprandial blood glucose levels independently, and diminish the rise in blood glucose levels when consumed with common carbohydrate foods. However, longer and larger studies examining appropriate long-term glycaemic markers (i.e. HbA1c) are required to determine whether these beneficial acute effects can be sustained in the long run. Furthermore, more clinical studies are required to test the cardioprotective effect of nuts in high-risk populations (i.e. patients with type 2 diabetes). Furthermore, these trials should test the dose–response effect of nuts on the aforementioned outcomes. Finally, due to their high fat content and energy density, more studies are required to determine the impact of nuts on obesity. While limited evidence suggests that nuts protect against obesity, more studies are required to validate these findings. The current evidence, however, does suggest that the moderate intake of nuts as part of a healthy diet can improve risk factors of heart disease, including serum lipids, inflammations, BP and obesity. As such, the moderate inclusion of nuts can be of benefit for healthy individuals and those with the metabolic syndrome or type 2 diabetes.
Acknowledgements
The present research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. D. J. A. J. has served on the Scientific Advisory Board of Loblaw Brands Limited, Sanitarium, Herbalife International, Nutritional Fundamentals for Health, Pacific Health Laboratories, Metagenics/MetaProteomics, Bayer Consumer Care, Almond Board of California, California Strawberry Commission, Orafti, Unilever and Solae. C. W. C. K. has served on the Scientific Advisory Board of Paramount Farms. D. J. A. J. and C. W. C. K. have received grants from Barilla, Solae, Unilever, Hain Celestial, Loblaw Brands, Sanitarium, Almond Board of California, International Tree Nut Council, California Strawberry Commission, the Western Pistachio Commission, Orafti, and the Canola and Flax Councils of Canada. David Jenkins has been on the speakers' panel for the Almond Board of California. C. W. C. K. has been on the speakers' panel for the Almond Board of California, Paramount Farms and the International Tree Nut Council. The authors' contributions are as follows: C. W. C. K., A. R. J., A. E. and D. J. A. J. were involved in the acquisition of data, analysis and interpretation of data, drafting of the manuscript and critical revision of the manuscript for important intellectual content.




