INTRODUCTION
Infection of wild animals by bovine tuberculosis (bTB), caused by Mycobacterium bovis, is raising concern worldwide. The pathogen has been demonstrated to infect a large number of free-ranging mammals across different ecosystems, often characterized by relatively moist conditions, such as North American forests [Reference Schmitt1], insular ecosystems in the Pacific [Reference Pfeiffer2] and Great Britain [Reference Delahay3], but also including drier ecosystems such as the Iberian peninsula [Reference Aranaz4] and semi-arid savannahs in Africa [Reference Bengis5]. The importance of bTB infection in wild animals relates to four aspects: (i) conservation issues, as the disease may negatively impact on wild mammal populations, including endangered species, (ii) impact on livestock productions, as some wild species can maintain TB and act as a reservoir of the pathogen for livestock, (iii) impact on public health, with wildlife acting as a reservoir of infection either directly or through livestock and (iv) economic impacts on private game ranchers. So far, more than 60 wild mammal species worldwide have been shown to be infected with M. bovis [Reference De Lisle, Mackintosh and Bengis6, Reference Thoen7], although only a few have been demonstrated to play the role of maintenance hosts. The best known examples of the important constraint that wild free-ranging hosts may represent for eradication of bTB are the European badger (Meles meles) in the UK and the Republic of Ireland [Reference Nolan and Wilesmith8, Reference Woodroffe9] and the possum (Trichosurus vulpecula) in New Zealand [Reference Coleman and Cooke10, Reference Ramsey and Efford11].
The first reported diagnosis of M. bovis infection in African free-ranging wild mammals was during the 1920s [Reference Paine and Martinaglia12, Reference Woodford13], followed by the confirmation of the African buffalo (Syncerus caffer), as a maintenance host during the late 1990s [Reference De Vos14]. But it is unclear when African wildlife first became exposed to the pathogen. Until recently it was believed that the origin of bTB in Africa was associated with the importation of infected cattle mainly from Europe and other continents, essentially during the past three centuries. New studies have revealed the existence of at least three clonal complexes of M. bovis which each appears to occur predominantly or exclusively in a geographically localized region of the world. The presence of M. bovis strains belonging to the European 1 clonal complex in South Africa, Tanzania and Zambia may therefore indeed be explained by historical livestock trade links between the UK and these African countries [Reference Smith15]. Two additional clonal complexes, African 1 [Reference Müller16] and African 2 [Reference Berg17], have been detected in several countries in West-central and East Africa, respectively, but very rarely outside Africa. The origin of these two M. bovis complexes is unknown and there is a possibility that their progenitors evolved in cattle in Africa any time between the appearance of classic M. bovis over 2000 years ago [Reference Brosch18, Reference Taylor19] and colonial times [Reference Hutcheon20]. Given current knowledge we cannot exclude the possibility that the exotic status of bTB in the indigenous African cattle population may have to be re-considered, at least in some subpopulations. It is therefore not possible to place the first M. bovis exposure of immunologically naive free-ranging African wildlife populations within a defined time period, but it was likely sporadic in nature with little or no chance of co-evolution [Reference Bengis, Kock and Fischer21]. Given the exceptional diversity of African wild mammal species, especially ruminants, presumed to be immunologically naive to the infection, the disease might spillover to wild African species, especially those that are taxonomically related to the domestic bovid reservoir host.
Livestock and wildlife have co-existed for at least 6500 years in Africa [Reference Bradley22] especially in arid and semi-arid lands, often sharing the same spaces and resources [Reference Kock, Osofsky, Cleaveland and Karesh23]. However, wildlife–livestock–human interfaces in Africa have been significantly altered during recent decades, with increasing encroachment of human activities into wildlife habitats due to global (e.g. human population demography, increased movements of people and products) and regional (e.g. development of Transfrontier Conservation Areas in Southern Africa [Reference Bengis, Osofsky, Cleaveland and Karesh24, Reference de Garine-Wichatitsky, Andersson, de Garine-Wichatitsky, Cumming, Dzingirai and Giller25]) dynamics. These changes in the nature, frequency and intensity of wildlife–livestock–human interactions may provide opportunities for pathogen spillover and spillback [Reference Nugent26]. Zoonotic risks due to M. bovis in Africa have been a concern for more than 15 years [Reference Cosivi27]. Because African countries often lack the resources to adequately control bTB, and because there are numerous populations living at wildlife–livestock–human interfaces, bTB represents a significant risk for veterinary and public health, and for conservation in Africa [Reference Michel, Müller and van Helden28, Reference Michel29], if indeed the disease does spillback from wildlife to livestock under African conditions.
In this paper, we review existing and recent information on emerging bTB in wildlife in Africa, with emphasis on the epidemiology and control of the disease at the wildlife–livestock–human interface. Based on official records and published information, we first compare the current situations and management of bTB in the different African subregions, illustrated by several well documented case studies. We then assess the drivers of bTB infection in wildlife and identify the main factors likely to increase the risk of transmission to livestock (‘livestock’ in this review refers to all domesticated hoofstock species used for human consumption, fibre, draught, etc.) and to human populations. We conclude by identifying the main knowledge gaps and technical limitations, and suggest several areas that should be targeted by scientific research in order to improve bTB control in the African context.
REGIONAL CONTEXTS FOR bTB IN WILDLIFE IN AFRICA
Overview
According to the World Animal Health Information databases collating reports of member states regarding outbreaks of transboundary animal diseases to the Office International des Epizooties (OIE) [30, 31], during the period 1996–2011 the majority of African countries (38/54) reported bTB in livestock (infection or clinical disease), with an additional four countries reporting suspected infection, while only four countries that implemented general or targeted surveillance reported the absence of the disease (Fig. 1a). During the same period, bTB (clinical cases) in wildlife were confirmed in only 6/54 countries, all located in southern and eastern Africa (Fig. 1b), with an additional five countries reporting suspected cases, while 11 countries that implemented general or targeted surveillance reported absence of the disease, and 33 African countries indicated unavailability of data regarding bTB in wildlife.
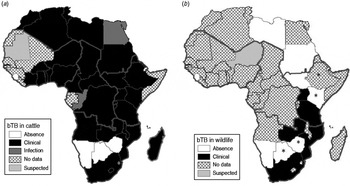
Fig. 1. Distribution map of bovine tuberculosis in Africa during 1996–2011 (large grey lines indicate the African subregions as referred to in the text: West, Central, East and Southern Africa). (a) Cattle status at country level; (b) wildlife status at country level. Asterisk (*) indicates countries (i.e. Botswana, Ethiopia, Kenya, Zimbabwe) where suspected and confirmed cases have been detected but not yet reported to OIE [Reference de Garine-Wichatitsky37, Reference Jori38, Reference Tschopp50, Reference Tanner59, Reference Tarara60]. No additional information (e.g. species) was available for suspected cases reported in wildlife for Niger, Equatorial Guinea and Guinea–Bissau and confirmed cases in wildlife in Mozambique. Data compiled from World Animal Health Information databases/OIE [30, 31, Reference Bengis42] and [Reference de Garine-Wichatitsky37, Reference Jori38, Reference Tschopp50, Reference Tanner59, Reference Tarara60].
Bovine TB is a legally notifiable or controlled disease in livestock for several African countries, but the information regarding bTB reports in wildlife should be treated cautiously owing mainly to the absence of simple and reliable diagnostic tests, and because the veterinary services of most countries lack the financial and human resources to carry out disease surveillance in wildlife. Nevertheless, published information seems to indicate that bTB is probably widespread in livestock at the continental level [Reference Ayele32, Reference Boukary33], whereas bTB infection of wildlife has only been confirmed in few countries of southern and eastern Africa [Reference Woodford13, Reference Bengis, Kock and Fischer21, Reference Gallagher34].
Southern Africa
In southern Africa, bTB in free-ranging wildlife has been confirmed in South Africa [Reference Paine and Martinaglia12, Reference Michel35], Zambia [Reference Munyeme36] and Zimbabwe [Reference de Garine-Wichatitsky37], while the presence of the infection has been suspected in African buffalo in Botswana [Reference Jori38] although not confirmed using gold standard techniques. Furthermore, in Botswana, Alexander et al. [Reference Alexander39] reported the emergence of M. mungi, a new member of the M. tuberculosis complex in banded mongooses (Mungos mungo). In South Africa, bTB was first diagnosed in greater kudu (Tragelaphus strepsiceros), and small antelopes in 1929 in the Eastern Cape of South Africa [Reference Paine and Martinaglia12]. In 1972, M. bovis infection was reported in Kafue lechwe antelopes (Kobus leche kafuensis), in the Lochinvar Game Reserve in Zambia [Reference Gallagher34], followed by the isolation of the pathogen from African buffalo in Gonarezhou National Park (GNP) in Zimbabwe in 2008 [Reference de Garine-Wichatitsky37].
The introduction of bTB into these wildlife populations has been largely ascribed to spillover from domestic cattle [Reference Munyeme36, Reference Michel40], with the exception of GNP where buffalo-to-buffalo transmission from the Kruger National Park (KNP) has been suggested [Reference de Garine-Wichatitsky37]. The establishment of bTB in a free-ranging ecosystem requires that the infection is maintained by at least one wildlife reservoir which serves as source of infection to a range of spillover hosts. Maintenance host status has been shown for buffalo [Reference De Vos14, Reference Michel and Bengis41] and lechwe, and suggested for greater kudu [Reference Michel35, Reference Michel40] and possibly common warthog (Phacochoerus africanus [Reference Bengis42]). Lions also became infected in KNP, although there is no evidence that they infect other species and could therefore be considered dead-end hosts (D. Keet, personal communication, 2012).
Transmission to wild spillover species may occur in different ways, including aerosol, ingestion and percutaneous. However, the exact mode of transmission, especially between ungulates remains poorly understood. Apart from direct transmission, which requires close contact between species, indirect transmission via environmental contamination is a possibility, although it has not been conclusively proven in Africa (see [Reference Woodford43, Reference Michel44]). In Zambia, it has been postulated that yearly seasonal floods play a role in the environmental propagation and dissemination of microorganisms (a point which needs further study and elaboration), while overcrowding of animals during lekking (mating season) with extra-large assemblages at watering points enhances the direct animal-to-animal transmission due to the contagious nature of the disease [Reference Munyeme36]. Environmental transmission of bTB could occur at locations where domestic and wild animals congregate to rest, drink or feed and is supported by the pathogen's ability to survive in the environment of KNP for between 5 days and 6 weeks, depending on temperature and moisture [Reference Tanner and Michel45]. In addition, the gregarious nature of most wild bovids with higher herd densities observed in drier seasons is thought to facilitate intraspecies transmission of M. bovis among wild ungulates.
In southern Africa, the interface between wildlife and domestic livestock is often defined by a game deterrent fence, restricting movement and minimizing contacts (for review see [Reference Ferguson and Hanks46]), thus reducing the risk of M. bovis transmission. There are, however, some potentially infected species such as greater kudu and common warthog, which are capable of crossing these man-made barriers, and whose role in M. bovis transmission at the interface remains to be quantified. In addition, the boundaries of several conservation areas in the region are either unfenced or very permeable to animal movements in both directions because they have been damaged and/or are not adequately maintained. Contacts between wildlife reservoirs and livestock populations living at the periphery of transfrontier conservation areas in southern Africa, do occur and occasionally result in the transmission of wildlife-borne diseases such as corridor disease or foot-and-mouth disease (e.g. [Reference de Garine-Wichatitsky, Andersson, de Garine-Wichatitsky, Cumming, Dzingirai and Giller25, Reference Caron47]). Thus, there is a risk of bTB spillover from wildlife to livestock, and vice versa, although the environmental pathways and frequency need to be determined. Hence, measures to mitigate against such transmission events may be required in order to protect local populations and their livestock living at the periphery of transfrontier conservation in the southern African region.
East Africa
Eastern Africa (see Fig. 1b) comprises a diversity of countries and ecological zones, but is primarily a moist to dry savannah ecosystem. Agriculture and settlement have encroached on the dry grasslands, where most livestock occur, putting considerable pressure on the resilience of these biological communities and land degradation is increasing as a result [Reference Norton-Griffiths48]. In many of the low rainfall zones the livestock and wildlife share the same space, usually at different times, which reduces the chance of direct physical contact. Here, overall densities of wildlife and livestock are relatively low, except in Ethiopia where cattle densities are locally very high, and infectious diseases tend towards endemicity. In some cultures large cattle aggregations occur seasonally (e.g. southern Sudan), and wildlife can locally reach high densities where mass migrations still occur (e.g. southern Sudan and in the Greater Serengeti-Mara ecosystem of Kenya and Tanzania). These conditions create frequent, even if indirect, contacts possibly leading to disease spread and pathogen spillover between species. There are few wildlife populations which are fenced or artificially supplemented, leading to high densities, but also mostly preventing contact between wildlife and livestock. Eastern Africa contains >50% of the total African livestock population. Wildlife–livestock interactions occur at low frequency and predominantly at unfenced areas. Most contacts are indirect through the environment, at water points and key forage resource areas, and are seasonally related.
In the East African region, the evidence generally suggests low prevalence of bTB in both wild [Reference Cleaveland49, Reference Tschopp50] and domestic animals, with notable exceptions in intensive husbandry systems (dairy) in some countries such as Ethiopia, Uganda and Tanzania [Reference Cleaveland51, Reference Wondewosen52] and in pastoral systems in Uganda [Reference Oloya53, Reference Oloya54]. The spillover of bTB into humans in the region is poorly documented [Reference Michel, Müller and van Helden28], and Ugandan studies report an above global average of ∼3% of human TB infection [Reference Oloya54–Reference Makita56]. In Ethiopia, despite the very high prevalence of extrapulmonary TB (EPBT) in humans, which is suggestive of bTB infection, M. bovis was only isolated in 4/964 EPTB patients [Reference Wondewosen52, Reference Firdessa57]. Three out of 173 pulmonary TB patients were M. bovis positive, and none of the suspected lymphadenitis TB cases were positive in another study among pastoralists in close physical contact with livestock in south-east Ethiopia [Reference Gumi58]. The disease is not a high priority for veterinary services, except in the dairy sector, but data is lacking.
Historically, bTB has been a concern in Queen Elizabeth National Park in Uganda [Reference Woodford13], affecting a range of wildlife species, with buffalo as a maintenance host. Recently bTB has caused detectable mortality in kob (Kobus kob thomasi): 12 deaths suspected to be due to bTB were reported in one locality, and a further eight noticeably sick kobs were observed and sampled over a period of 1 month from the same population (P. Atemnedi, personal communication, 2011). Overall, the decline in ungulate populations susceptible to bTB in the park is of concern, but the contribution of disease to this trend or the drivers for this are not known. Lion populations have also declined (R. Bengis, personal communication, 2012) but underlying causes (possibly disease or poaching) have not been conclusively determined. Bovine TB infection of buffalo and baboons has also been confirmed in Kenya [Reference Tanner59, Reference Tarara60] and several wildlife species have been found positive to serological rapid tests in Ethiopia [Reference Tschopp50].
Central Africa
Boukary et al. [Reference Boukary33] recently reviewed bTB studies in domestic stocks in sub-Saharan Africa. Only seven studies were related to bTB in livestock in Central African countries, and the reported prevalence in individual cattle ranged from 0·2% to 19·9% depending on the surveillance method adopted. The results also depended on the age of the animals tested and the type of production system considered, and on the breed of cattle tested [Reference Goutard61–Reference Ngandolo63]. No published information is available, to our knowledge, on bTB in wildlife populations, and according to the OIE database [30] only one ‘suspected’ bTB case in wildlife was reported (in Equatorial Guinea) for the period 2007–2012.
Wild ungulates (including African buffalo) still persist mainly in savannah ecosystems of southern Chad, North Cameroon and the Republic of Central Africa, and these largely decimated populations may be exposed to bTB spillover from livestock. In forest ecosystems, no study has been published on wildlife–livestock interactions, especially between forest buffalo and cattle. Wildlife–livestock interactions may be expected to be less frequent than in other parts of the continent because densities of both livestock and wildlife are lower and access to water usually does not represent a limiting factor in these forest ecosystems.
West Africa
Very little data has been published on bTB in wildlife for West African countries (Fig. 1b). According to the OIE database [30] only one ‘suspected’ bTB case in wildlife was declared (in Niger) for the period 2007–2012. For livestock populations, the studies that have estimated bTB prevalence in West Africa revealed significant variations according to the areas and the production system considered [Reference Boukary33, Reference Gidel64–Reference Fofana66]. Low bTB prevalence was estimated in the Torodi region of Niger (0·8% comparative intra-dermal skin test positive [Reference Boukary67]) and at Bamako's abattoir in Mali (1·8% with symptomatic gross lesions [Reference Müller68]). The highest bTB prevalence in cattle was found in peri-urban areas, in Ghana (13·8% comparative intra-dermal skin test positive [Reference Bonsu, Laing and Akanmori69]) and in Mali (Bamako, 19% prevalence comparative intra-dermal skin test positive [Reference Sidibé70]). The economic loss incurred by cattle dealers due to bTB infection in cattle was estimated at US$1 million in Togo in 1985, based on the meat condemned at abattoirs because of the presence of bTB lesions [Reference Thorel71].
In West Africa, bTB thus appears to infect livestock populations with a prevalence similar to other African regions depending on the type of livestock production system (i.e. higher in peri-urban vs. extensive systems [Reference Boukary67]). In addition, as for Central Africa, no reliable data is available regarding wildlife. This may reflect the absence of the disease in wildlife or it may be due to the absence of appropriate surveys to detect it. The abundance and distribution of most wildlife populations have markedly decreased in West Africa during recent decades [Reference Hibert72] due to over-harvesting of wildlife and increasing human populations in the periphery of protected areas [Reference Wittemyer73]. For example, African buffalo populations only persist in a handful of protected areas, where buffalo–cattle interactions appear to be very infrequent [Reference Hibert72]. The small size of remaining wildlife populations and their reduced distributions suggest that they are unlikely to act as a maintenance host or significant sources of bTB for cattle. In West Africa, bTB seems to be widespread and poorly controlled in cattle populations, and there is a risk that bTB could spillover to naive and locally endangered wildlife populations.
EPIDEMIOLOGY OF bTB AT AFRICAN WILDLIFE–LIVESTOCK–HUMAN INTERFACES
Conceptual model of bTB transmission at the wildlife–livestock–human interface
A disease reservoir may consist in a maintenance population or community (i.e. with more than one species involved) within which a given pathogen can be maintained and which acts as a potential source of infection for a target species [Reference Haydon74]. In the case of bTB in Africa, maintenance populations have been identified as cattle, lechwe and buffalo. The existence of a maintenance community remains unknown. The role played by other wild ungulate species, such as the greater kudu and the common warthog, is still debated although in theory, they could connect reservoir (e.g. buffalo) and target (e.g. naive cattle) populations, and so contribute to the maintenance community of bTB. Similarly, the maintenance community created by sympatric cattle and buffalo populations in frequent contacts may allow bTB spillover or spillback, and could act as a source of bTB for other target populations.
Within this maintenance community framework, bTB epidemiology may be represented as a dynamic multi-host system with three main ‘compartments’, namely wildlife, livestock and humans (Fig. 2). Bovine TB infections may be maintained (independently or not) within livestock populations and within wildlife populations, whereas human infections result from pathogen spillover from animals [Reference Biet, Guilloteau, Boschiroli and Thorel75], and very rarely from human-to-human transmission [Reference Michel, Müller and van Helden76]. In the following sections we review the main risk factors of bTB infections within the livestock and wildlife compartments, and identify the main drivers of interspecific transmission of bTB (‘spillover’ and ‘spillback’ [Reference Nugent26]; Fig. 2) at wildlife–livestock–human interfaces in Africa.
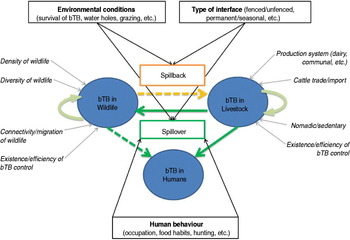
Fig. 2 [colour online]. Interspecific transmission of bovine tuberculosis (bTB) at wildlife–livestock–human interfaces in Africa. Bovine TB can be maintained in livestock (bTB in livestock) and in wildlife (bTB in wildlife), promoted by factors indicated in italics with a grey arrow (e.g. diversity of wildlife, production system). Risks of bTB spillover from livestock to wildlife or humans, and spillback from wildlife to livestock, are indicated in boxes with black arrows (e.g. environmental conditions, human behaviour, etc.).
Livestock as a source of bTB: spillover to wildlife and humans
The main bTB risk factors in African cattle populations (see Fig. 2; adapted from [Reference Boukary33, Reference Oloya53, Reference Tschopp77–Reference Gumi81]) include the type of production system (intensive, dairy farms, use of upgraded B. taurus breeds), animal movements (herds importing animals, transhumance) and absence or inefficiency of bTB surveillance and control.
Several studies have analysed bTB risks of transmission between livestock and humans [Reference Gumi58, Reference Boukary67, Reference Biet, Guilloteau, Boschiroli and Thorel75, Reference Tschopp80, Reference Mfinanga82], identifying husbandry practices (housing, mixing cattle herds with other small ruminants), food preferences (consumption of raw or soured milk) and overall health and hygienic conditions (HIV) as the main risk factors for humans (Fig. 2). The role of livestock as a source of infection for humans has been relatively well documented and established in Africa [Reference Cleaveland51, Reference Gumi58, Reference Mfinanga82]. It should be noted that these studies rarely determined with certainty (i.e. using molecular techniques) the animal source and the routes of transmission [Reference Oloya54, Reference Idigbe, Anyiwo and Onwujekwe83–Reference Rasolofo85]. In addition, it has been shown that several livestock species other than cattle may be infected by M. bovis in African conditions (e.g. camels in Kenya and Ethiopia [Reference Mamo86, Reference Paling87]) although apparently with lower prevalence than cattle (e.g. camels and goats in pastoralist herds in Ethiopia [Reference Gumi58]), but the epidemiological role that they might play in bTB multispecies systems remains unexplored.
Although bTB is currently established in several wildlife populations from several sub-Saharan African countries, the introduction of M. bovis into a community of free-ranging wild animals has been best documented in KNP, South Africa. The disease was first diagnosed in the early 1990s in the African buffalo population in the southern part of the park [Reference Bengis5]. The primary source of the pathogen is believed to have been an infected cattle population in the Komatipoort/Malelane region [Reference Renwick, White and Bengis88], located south of the Crocodile River, which forms the southern border of the park. Direct (or indirect) spillover from cattle to buffalo must have occurred in or prior to 1960 according to phylogenetic analyses of isolated strains. The genetic characterization of bTB strains isolated from wildlife in KNP indicated a common source in the African buffalo, followed by clonal expansion of the strain [Reference Michel40]. We have no indication of the route of transmission for this initial spillover of bTB from cattle to buffalo, but it is believed that they may have entered into close contact, sharing water and grazing resources at this unfenced interface. It is also speculated that only a limited number of transmission events between cattle and buffalo have occurred, as the disease took a long time to spread to other buffalo herds within the park.
Although detailed documentation of transmission risks only exists for South Africa and Uganda, we suggest that the main risk factors potentially leading to bTB spillover (Fig. 2) may be related to: (i) the type of wildlife–-livestock interface (absence of physical separation such as fence, allowing for repeated and prolonged direct or indirect contacts); (ii) the environmental conditions, leading to competition/sharing of common resources (water and grazing) and also compatible with the persistence of M. bovis in the environment.
Wildlife as a source of bTB: spillback to livestock or spillover to humans
So far, two free-ranging mammal species have been demonstrated to play a role as maintenance hosts in natural conditions, the African buffalo in KNP, South Africa [Reference De Vos14] and the lechwe in the Kafue basin, Zambia [Reference Gallagher34, Reference Munyeme36]. Two other species, greater kudu and common warthog, are also considered as potential maintenance hosts [Reference Bengis42], but no published data exist to confirm this. Since its introduction during the 1960s, bTB has increased its host range by infecting at least 14 species of wild mammals within the boundaries of the Greater KNP Complex [Reference Michel35] including a sporadic case in a bushbuck (Tragelaphus scriptus) [Reference Bengis42]. The epidemiology of bTB in African wildlife has been described as a multi-species host–pathogen system [Reference Caron47, Reference Renwick, White and Bengis88]. Although many of the mechanisms involved remain uncertain, the main risk factors leading to introduction and maintenance of the infection in a wildlife population (Fig. 2) are probably linked with species diversity (maintenance or spillover hosts), social behaviour of wildlife hosts, wildlife densities (threshold population/community densities), movements of animal populations (possible introduction through migratory individuals, confinement), and absence or inefficiency of wildlife bTB surveillance and control.
Few studies in Africa have specifically investigated the transmission of bTB from wildlife to livestock (spillback; Fig. 2). Although other diseases have been demonstrated to occasionally spread from KNP wildlife to neighbouring cattle populations (e.g. [Reference de Garine-Wichatitsky, Andersson, de Garine-Wichatitsky, Cumming, Dzingirai and Giller25]), so far no suspected cases of bTB spillback from wildlife to cattle living in contact with the KNP fence have been confirmed using gold-standard techniques. In 2008, the main bTB strain circulating in KNP buffalo populations was demonstrated to have spread to the GNP buffalo population in Zimbabwe [Reference de Garine-Wichatitsky37]. However, several surveys carried out in the surrounding communal lands indicated that bTB has not infected the cattle population [Reference de Garine-Wichatitsky89]. Factors associated with bTB spillover from livestock to wildlife (see above [Reference Munyeme90]) should also influence bTB spillback from wildlife to livestock (Fig. 2). The main risk factors are thus linked with: (i) the type of interface (fence, herding practices) and the distribution of resources (water and grazing), which directly influence contact patterns between livestock and wildlife; (ii) the environmental conditions, which directly influence the persistence of bTB in the environment. It is therefore of paramount importance to further understand the spatio-temporal overlap between buffalo and cattle (and other reservoir hosts) [Reference de Garine-Wichatitsky89].
Human infections with M. bovis from infected wildlife, acquired through ingestion or aerosol exposure, have been described only sporadically as an occupational and recreational hazard, mainly for wildlife veterinarians, hunters, taxidermists and people preparing and consuming venison [Reference Liss91]. To our knowledge, no cases have so far been described in Africa of M. bovis in people that are likely to have originated from wildlife, although specific investigation has been lacking at the wildlife–human interface, except in KNP [Reference Weyer92]. We assume that the zoonotic risks associated with wildlife bTB (Fig. 2) are similar to those involved in bTB spillover from livestock, mainly related to human behaviour and activities: occupation (national park staff, veterinarians), dietary habits (venison consumption) or leisure (hunting, ecotourism).
Ecology of bTB transmission in Africa: source or sink in multi-host systems?
Transmission of M. bovis at wildlife–livestock–human interfaces in sub-Saharan Africa is driven by biological, ecological and anthropological processes. Spillover, and spillback, events between wildlife and the two other compartments are probably relatively rare in Africa, with the limited interfaces prevailing in most African countries, but many aspects of this complex system are not well understood.
It is often difficult to accurately study the dynamics of pathogen spread in bTB outbreaks in livestock due to the implementation of control measures such as test-and-slaughter. In wildlife populations, it is mostly unknown when the pathogen was introduced and the situation may be further complicated by mixed and ongoing introductions from multiple sources. However, the bTB epidemic in KNP offers a unique opportunity to study the epidemiology of a single strain of this multi-host pathogen in a multi-species environment in the absence of human control interventions. Following its introduction into the naive buffalo population of KNP, M. bovis spread mainly between buffalo herds and spilled over into at least 13 other species [Reference Bengis42], the true extent of which is unknown as bTB monitoring and surveillance in wildlife are most effective in the more visible species showing signs of disease, and where active surveillance has taken place (mainly in buffalo). Molecular analysis of M. bovis isolates from affected herbivores, predators and omnivores was able to provide evidence for direct M. bovis transmission (e.g. between predator and prey) but also for indirect transmission (e.g. between buffalo and kudu [Reference Michel40]). Bovine TB epidemiology in wildlife is thus a complex multi-host pathogen system [Reference Renwick, White and Bengis88], partly driven by resource selection and spatial ecology of wild herbivores sharing common resources and by predator–prey interactions.
As bTB can be transmitted between (wild and domestic) hosts through indirect contact with the contaminated environment (e.g. grass, trees, water, faeces [Reference Courtenay93]), there is also a possibility that contact patterns between wildlife and livestock may result in directional bTB transmission, with spillback being prevented or likely to occur with a lower probability than spillover transmission. Cattle and buffalo may share the same space and resources, but not at the same time/season, and this temporal shift in use (e.g. night/day, season) may result in different risks of disease transmission through indirect contact if the survival of the bacteria in the environment differs according to time/season. In southern Africa, for instance, cattle are commonly confined in kraals at night and driven during the day by herders who decide to a large extent in which area cattle herds will graze and at which water hole they will drink, while buffalo roam freely. In addition, differential grazing behaviour between livestock and wildlife (e.g. avoidance of sites contaminated by faeces or different preferences for feeding patches) may also result in directional bTB transmission. This could explain why the dominant parental KNP strain has spread to buffalo populations within KNP and even to GNP in Zimbabwe, while current evidence suggests that it has not spilled-back to cattle populations adjacent to KNP or GNP. Similarly, indirect buffalo–cattle contacts and active test-slaughter control in the buffalo population of Hluhluwe–Umfolozi National Park (South Africa) could explain why bTB strains have spilled over from cattle to buffalo, as indicated by molecular analysis [Reference Munyeme36], while spillback from buffalo to cattle or human populations has not been reported to date.
Currently, even in southern Africa, bTB spillback from wildlife to livestock (and humans) has not been confirmed. But indirect contacts between cattle and buffalo do occur at the periphery of several large conservation areas in southern Africa, where no efficient fences separate conservation areas from adjacent communal lands. This could lead to a situation where domestic and wildlife reservoirs co-exist, creating a complex maintenance system that would be more difficult to control [Reference Haydon74]. In addition, wild spillover hosts, which are considered as unable to maintain bTB infection alone, could play a crucial epidemiological role as ‘bridge’ species, between the wild and domestic compartments. For instance impala (Aepyceros melampus), although rarely infected by bTB [Reference Bengis42], and greater kudu or common warthog (considered as potential maintenance hosts [Reference Bengis42]), are relatively abundant at wildlife–livestock interfaces and relatively tolerant of human activities. As these species use common water or food resources, they could potentially transmit bTB infection in both directions, although this has not been demonstrated so far.
MANAGEMENT AND CONTROL OF bTB AT WILDLIFE–LIVESTOCK–HUMAN INTERFACES
Why control bTB in wildlife?
Bovine TB is an alien disease in African wildlife [Reference Bengis, Kock and Fischer21], and there are numerous reasons that could justify implementing control measures in wildlife populations: impacts on conservation objectives/endangered species, tourism, wildlife trade, risk of spillback to livestock and spillover to humans. As it is a slow progressive and zoonotic disease, with limited tools for diagnosis and control, it makes sense to act proactively to prevent it from entering wildlife populations. Once M. bovis has been introduced in a wildlife population, it is very difficult to control the disease [31, Reference Munyeme90]. Consequently, every effort should be made to protect wildlife populations that are not infected, by reducing the risk of spillover from livestock.
What are the control options for bTB in African wildlife
The KNP example does help in understanding the complex environment in which we are trying to control the disease in wildlife populations. Due to the expense and difficulty of diagnosing TB in wildlife (non-validated tests and expense of immobilizing) it is impossible to apply test-and-slaughter approaches in large open ecosystems. In smaller parks like Hluhluwe-Umfolozi where herds are in distinct confined areas, test-and-slaughter has been implemented, resulting in a reduction of bTB incidence in the buffalo population but not in the eradication of the disease [Reference Cooper94]. Although fences may have severe negative ecological and socio-cultural impacts, they keep buffalo and cattle separated most of the time, which efficiently reduces the risks of disease spillover [Reference de Garine-Wichatitsky, Andersson, de Garine-Wichatitsky, Cumming, Dzingirai and Giller25]. However, when fences are damaged, buffalo and/or cattle may cross the boundaries of the protected area, increasing these risks. In the far northern region of KNP adjoining Mozambique and southern GNP there are no fences and spatial overlap between cattle and buffalo is a regular event. However, there is field evidence that buffalo and cattle do not mix readily. This also applies to buffalo that leave the fenced areas to the west of KNP, as most records of escaped buffalo are in areas where there are no cattle, pointing to possible active avoidance of close contact (State Veterinary Skukuza, personal communication).
Lessons learned from past and current measures/strategies
Once bTB was diagnosed in KNP in 1990, management conducted regular surveys in buffalo herds throughout the park. The disease was confined to the south of the park (south of the Sabie River) in 1992, but surveys carried out in 1994, 1996 and 1998 clearly demonstrated that the disease was spreading northwards through inter-herd transmission. By 2005, it had reached buffalo in the far northern part of the park (D. Keet, internal State Veterinary Report, 2005). In 2008, it was diagnosed in GNP in southern Zimbabwe. Various control methods were discussed, including culling all buffalo south of the Sabie River and putting up a fence to the north of known bTB herds. Even complete eradication of buffalo and restocking was considered but the ecological impact was considered more serious than the risk taken by not being able to fully eradicate the disease. In the last decade numerous studies have been conducted to determine the longitudinal impact of bTB in buffalo and no population effect in buffalo [Reference Cross95]. A similar study is now being conducted in lions in KNP [Reference Ferreira, Hofmeyr and Funston96].
Limited attention has been paid to the risk and incidence of spillback of bTB from buffalo to livestock, but bTB tests conducted in cattle to date to the west of KNP and southern Zimbabwe [Reference de Garine-Wichatitsky89] have failed to confirm bTB in the adjacent areas using gold-standard techniques. Lessons learnt from the KNP example showed that better surveillance in the 1950s to 1990s could have resulted in earlier disease detection with a possible test-and-slaughter control strategy. Once bTB has established itself, more localized risk management is the most cost-effective way to prevent spillover and spillback between wildlife and livestock. Fencing would be the primary line of defence to curb bTB spread. In the absence of fences, innovative ways of keeping buffalo and cattle apart should be considered (e.g. herding and kraaling cattle, or manipulation of water availability at the interface and supervised visits to water holes). The effort and impact of bTB control and risk management must also be seen in the context of preventing other livestock-related diseases from transmitting across the wildlife–livestock interface.
When bTB management and control measures are envisaged, it is important to consider deterministic factors at play in the epidemiology of the disease. Further, the choice of workable control measures and strategies despite being limited for wildlife, should take into account all key factors unique to each different ecosystem. A number of factors have been observed to be associated with bTB in cattle herds [Reference Omer97]. Oloya and co-workers observed that bTB was associated with different types of drinking water sources [Reference Oloya53]. The same study also indicated that risks of bTB in cattle are linked to specific geographical regions of production. This implies that bTB control may thus vary according to prevailing ecological conditions. Further, bTB has also been shown to be associated with communal grazing, animal breed and husbandry practices across most of the African continent [Reference Omer97]. Similarly, studies have also shown that herd size has an influence on the prevalence of bTB [Reference Kazwala79, Reference Ameni, Amenu and Tibbo98–Reference Firdessa100]. Taken together, these factors are vital in formulating workable control strategies for cattle bTB across African pastoral communities. However, control measures such as the test-and-slaughter schemes used to control bTB in cattle populations are impractical in free-living wildlife populations. Nevertheless, at the livestock–wildlife–human interface more detailed studies are needed to understand both key and proxy factors related to the maintenance, spread and transmission of the disease among susceptible hosts. Owing to the impracticality of other control measures, the key factor at the livestock–wildlife–human interface area is to reduce or to completely eliminate interspecies contact. Selective cropping of old, debilitated animals can also be used to remove what may be chronic shedders of the disease. BCG vaccination in buffalo has been shown to provide poor protection [Reference Courtenay93] and further work on approaches to vaccination is required, including target species, age of vaccinees and new vaccine formulations. BCG vaccination of cattle populations living in contact with wildlife could also be envisaged in order to prevent or reduce the risks of bTB spillback and spillover, but there is also a need to evaluate the efficiency of such control strategies. However, given the lack of scientific data and resources of most developing countries, the use of vaccines is impracticable at the moment. A more feasible option is the creation of double fences or buffer zones (e.g. with limited and managed grazing of livestock, with wildlife hunting activities), with a zone where there is sufficient distance to avoid environmental contamination with M. bovis.
Perspectives for a one health approach
Studies on bTB in Africa have so far focused rather narrowly, either on livestock, wildlife or human health research in separate compartments. Systems-based research involving both the animal and the public health sector have rarely been undertaken [Reference Weyer92]. Despite decades of research on bTB, there is still very little information on the epidemiology of bTB in Africa. Important knowledge gaps include the strains involved in humans and animals in the same areas, spillover and spillback dynamics, quantification of risk factors of disease transmission between wildlife–livestock–humans, impact of bTB on animal health and animal productivity and livelihoods, impact on conservation and dynamics of wildlife population. The absence of this information probably reflects the apparently low incidence of bTB in humans, and of bTB transmission between wildlife and livestock, although this picture may change given increasing overlap at the interface between species in many parts of Africa. Due to the nature of the disease (multi-host pathogen with impact on humans, animals and ecosystem health), collaboration between the public and animal health sectors, including wildlife specialists, is important from a sanitary, and probably also from an economic, point of view. Integrated research is paramount to gain a better understanding of the epidemiology of bTB at the human–livestock–wildlife interface, to be able to quantify the impact of the disease and to propose adequate and sustainable control approaches.
CONCLUSIONS, KNOWLEDGE GAPS AND THE WAY FORWARD
The information collated in this review confirms that bTB is widespread in livestock on the African continent [Reference Ayele32], whereas bTB infection of wildlife, which was historically limited to a few countries in southern and eastern Africa (South Africa, Tanzania, Uganda, Zambia) may be spreading in southern Africa (Mozambique [30], Zimbabwe [Reference de Garine-Wichatitsky37]). Although the impact of bTB may be significant in Africa, affecting the economy, the ecosystem and human health [Reference Michel, Müller and van Helden28], wildlife TB is currently not considered a priority for most African countries. M. bovis has been isolated from a wide range of wild free-ranging mammals, including maintenance hosts in natural conditions, which will considerably complicate future bTB control programmes [Reference De Lisle101], and possibly compromise the chances of eradication in sub-Saharan African countries. But to date, to our knowledge, no cases have been documented in sub-Saharan Africa demonstrating M. bovis spillback from infected wildlife to cattle or spillover to human populations.
Currently, bTB in African wildlife south of the Sahara is essentially a conservation issue, with social, welfare and economic implications for the affected areas/countries. Although the ecological impacts of bTB on infected free-ranging populations is still not clearly demonstrated (even for buffalo [Reference Caron, Cross and du Toit102] or lions [Reference Ferreira, Hofmeyr and Funston96]), there is concern in southern Africa that transboundary animal diseases spread by wildlife, and bTB in particular, may negatively impact on economic activities and livelihoods of local people, especially in the context of transfrontier conservation areas initiatives [Reference Michel35]. In other parts of Africa, it is likely that the risk of bTB transmission between wildlife and livestock (and possibly humans) will increase in the near future, with ever-increasing human encroachment into wildlife habitats due to the demand for grazing and cropping land, and possibly through dissemination of bTB-positive upgraded livestock into rural areas (e.g. dairy cattle breeds in Ethiopia). This will have serious consequences for biodiversity conservation, as bTB may potentially further threaten several small and isolated wildlife populations (including endangered species at a local or global scale), and also potentially for veterinary and public health as the wildlife reservoir will complicate future bTB control or eradication programmes.
Several major knowledge and technical gaps must be addressed before effective long-term control of bTB at the wildlife–livestock–human interface in Africa can be put in place. The first gap in knowledge relates to the absence of understanding of the role of individual wildlife species/populations in the epidemiology of bTB in complex multi-host systems [Reference Renwick, White and Bengis88]. This also applies to several livestock species other than cattle, like goats and pigs, which can be infected by bTB but for which no information is available on the role they play in the epidemiology of the disease in sub-Saharan African contexts. Despite recent conceptual [Reference Caron, de Garine-Wichatitsky, Morand, Morand, Beaudeau and Cabaret103] and methodological (e.g. [Reference Hlokwe104]) developments, there is a general lack of longitudinal data (with the exception of South Africa). Longitudinal surveys are needed in order to monitor the (temporal) dynamics of the infection, and also to provide M. bovis isolates from African wildlife populations. These isolates are needed to elucidate the epidemiological role of the various species and the relationship between the wild and domestic host populations. The second knowledge gap relates to the absence of understanding of the ecological and anthropogenic drivers of contacts between wildlife and livestock (see [Reference de Garine-Wichatitsky89]), and humans, potentially leading to bTB transmission. Recent advances in telemetry have improved our understanding of the movements of free-ranging animals, but there is a need for multidisciplinary approaches bringing together ecology, sociology and epidemiology in order to identify the main drivers of contacts, and to investigate how the manipulation of shared resources might mitigate the risks of disease transmission. The third knowledge gap concerns environmental source of M. bovis, and the persistence of infectious material in various African ecosystems. Previous studies have given contrasting results [Reference Michel44, Reference Tanner and Michel45], and there is need for more thorough investigations in the future, as the importance of indirect transmission in the epidemiology of bTB remains elusive.
Improving surveillance and control of bTB at African wildlife–livestock–human interfaces also requires accurate, affordable and reliable diagnostics, as the performance of existing tests is unknown for most wild African species. However, this technical limitation will probably remain a major challenge because the veterinary bTB diagnostic market is very small, and virtually non-existent for (African) wildlife [Reference Chambers105]. In addition, new developments in the field are often not suitable for developing countries, either because they require sophisticated laboratory infrastructure and/or well-trained personnel [Reference Michel, Müller and van Helden28]. In the long term, the development of effective vaccines could significantly contribute to protecting targeted wildlife populations and/or maintaining bTB prevalence below a threshold that would reduce the risk of spillback to livestock. A BCG vaccine has shown some promise in badgers in Europe [Reference Corner106]. BCG vaccine failed to protect African buffalo against bTB in an initial study [Reference de Klerk, Michel, Bengis, Kriek and Godfroid107]. Nevertheless, the potential long-term benefit from reducing the infection pressure through vaccination of maintenance hosts warrants further investigation. BCG vaccination of cattle populations living in contact with wildlife could also be envisaged in order to prevent or reduce the risks of bTB spillback and spillover, but there is also a need to evaluate the efficiency of such strategies.
From a wider mycobacterial perspective, we also acknowledge that M. tuberculosis remains a greater risk to African human populations than M. bovis. In addition, there are several other animal diseases that have more significant direct or indirect detrimental impacts on livestock at wildlife–livestock interfaces in Africa (e.g. corridor disease and foot-and-mouth disease). The effort and resource allocation required to control the threat of bTB at the wildlife–livestock–human interface should therefore be linked to other critical transdisciplinary programmes aiming at improving the health of humans and their livestock and associated wildlife.
ACKNOWLEDGEMENTS
We thank the organizers of the 1st Wildlife Tuberculosis Conference, held 9–12 September 2012 in Kruger National Park, South Africa, for the opportunity to gather first-hand information on the latest developments regarding wildlife bTB. This work was conducted within the framework of the Research Platform ‘Production and Conservation in Partnership’ (RP-PCP) and the Animal and Human Health Environment and Development initiative (AHEAD). M.dG.W and A.C. were supported by the Ministère Français des Affaires Etrangères through the French Embassy in Zimbabwe (RP-PCP grant 2008–2012), and R.T. by the Swiss National Science Foundation.
DECLARATION OF INTEREST
None.




