Birth weight is considered a marker of intrauterine development. It is a strong predictor of infant survival and an indicator of health status later in life(Reference Barker1–Reference Gunnarsdottir, Birgisdottir and Thorsdottir3). Maternal diet and nutritional status during pregnancy is an important determinant of fetal growth(Reference Godfrey, Robinson and Barker4). Seafood is not only a rich source of nutrients such as marine n-3 fatty acids, vitamin D, iodine and Se, but can also be a source of pollutants such as methylmercury, As and polychlorinated biphenyls(Reference Mahaffey, Clickner and Bodurow5, 6). Several observational studies have reported a positive association between maternal seafood consumption and fetal growth(Reference Olsen, Grandjean and Weihe7–Reference Muthayya, Dwarkanath and Thomas11). Some observational studies also associate marine n-3 fatty acid intake with greater growth measures at birth(Reference Olsen, Hansen and Secher12, Reference Elias and Innis13). However, other observational studies have reported negative associations between maternal seafood and n-3 intake and fetal growth. In the Danish national birth cohort, intake of fatty fish, but not lean fish, was negatively associated with fetal growth(Reference Halldorsson, Meltzer and Thorsdottir14), while other studies have reported negative associations between marine n-3 fatty acids from fish(Reference Grandjean, Bjerve and Weihe15, Reference Oken, Kleinman and Olsen16) or fish-oil supplements(Reference Thorsdottir, Birgisdottir and Halldorsdottir10) and fetal growth measures. These results might be compatible with a hypothesis that there is a differential influence by different types and/or constituents of fish and seafood on fetal growth and birth size(Reference Guldner, Monfort and Rouget17–Reference Mendez, Plana and Guxens19). The negative association with birth measures has frequently been attributed to the presence of hazardous pollutants, as fatty fish and marine n-3 supplements are likely to contain more persistent organic pollutants than lean fish(Reference Thorsdottir, Birgisdottir and Halldorsdottir10, Reference Halldorsson, Meltzer and Thorsdottir14, Reference Lee and Jacobs20). The concentrations of pollutants not only vary among different fish and seafood species, but also depend strongly on habitat, life stage and origin(21, Reference Haug, Thomsen and Brantsæter22). Further studies of associations between maternal intake of subtypes of seafood and supplementary marine n-3 fatty acids and infant birth measures are warranted.
Norwegians traditionally have relatively high fish and seafood consumption, especially in the coastal areas, but national surveys have shown decreasing intakes over the past decades(Reference Johansson and Solvoll23). In general, young women have the lowest fish consumption. We have previously reported a wide range of fish and seafood intakes among pregnant Norwegian women(Reference Meltzer, Brantsæter and Ydersbond24, Reference Brantsæter, Haugen and Thomassen25). An additional source of marine n-3 fatty acids is cod-liver oil or fish-oil supplements, which are the most frequently used dietary supplements in pregnant Norwegian women(Reference Haugen, Brantsæter and Alexander26).
The aim of the present study was to address the hypothesis that different seafood subtypes differentially influence pregnancy outcomes by examining how maternal seafood consumption, including intake of lean and fatty fish, shellfish and fish liver, and supplementary marine n-3 fatty acids, was associated with infant birth weight, birth length and head circumference in a large cohort of pregnant Norwegian women.
Materials and methods
Population and study design
The data set is part of the Norwegian Mother and Child Cohort Study (MoBa), initiated by and maintained at the Norwegian Institute of Public Health(Reference Magnus, Irgens and Haug27). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Regional Committee for Ethics in Medical Research and the Norwegian Data Inspectorate. Written informed consent was obtained from all MoBa participants.
In brief, MoBa is a nation-wide pregnancy cohort that has included more than 107 000 pregnancies in the years from 1999 to 2009. Women were recruited to the study through a postal invitation in connection with a routine ultrasound examination offered to all pregnant women in Norway. The participation rate in MoBa was 43 %(Reference Magnus, Irgens and Haug27). The potential bias due to self-selection in MoBa has been evaluated. Despite differences in prevalence estimates between the cohort participants and the total population of pregnant women, no statistically relative differences in association measures were found regarding eight exposure–outcome associations evaluated, e.g. prenatal smoking and low birth weight ( < 2500 g), maternal vitamin use and placental abruption, and parity and pre-eclampsia(Reference Nilsen, Vollset and Gjessing28).
The present study uses the quality-assured data files released for research in 2009 (version 4). Pregnancy and birth records from the Medical Birth Registry of Norway (MBRN) are linked to the MoBa database(Reference Irgens29). When preparing the data set, 76 218 women had answered the MoBa FFQ (Q2), the baseline MoBa questionnaire covering sociodemographic information and general health (Q1) and the data were recorded in MBRN. We included only the first participation for women with multiple participation in MoBa and women with singleton births. This led to the exclusion of 12 240 women. Furthermore, we excluded participants with a pregnancy duration < 28 weeks or >42 weeks (n 628), if the birth weight of the baby had not been recorded or if the birth weight was < 600 g (n 35). We also excluded participants who had not given birth to a live baby (n 153). Lastly, we excluded women having improbable energy intakes, i.e. energy intake < 4·5 MJ or >20 MJ (n 1063), resulting in a final sample of 62 099 women for the present study (Fig. 1). Maternal weight gain in pregnancy was available for a subset of participants who, at the time when the present data files were released, also had answered a questionnaire at 6 months post-partum (n 42 907).

Fig. 1 Sample selection for the present study for inclusion of participants for studying fetal growth in Norwegian Mother and Child Cohort Study (MoBa). MBRN, Medical Birth Registry of Norway; Q1, baseline MoBa questionnaire covering sociodemographic information and general health; Q2, MoBa FFQ.
Dietary assessment
The MoBa FFQ (available at http://www.fhi.no/dokumenter/011fbd699d.pdf) was completed around the 22nd week of gestation and the dietary data were collected from February 2002 and onwards(Reference Meltzer, Brantsæter and Ydersbond24). The MoBa FFQ is a semi-quantitative questionnaire designed to capture dietary habits and intake of dietary supplements during the first 4–5 months of pregnancy. The FFQ included questions about intake of 255 food items with special emphasis on various seafood items. There are ten questions about cold cuts and spreads made from fish or shellfish, sixteen questions about fish or shellfish eaten for dinner and four questions about cod-liver oil, cod-liver oil capsules or fish-oil capsules.
Nutrient calculations were performed with the use of FoodCalc(Reference Lauritsen30) and the Norwegian food composition table(31). The FFQ has been thoroughly validated with regard to nutrients, foods and dietary supplement use(Reference Brantsæter, Haugen and Alexander32, Reference Brantssæter, Haugen and Hagve33).
Definition of fish and seafood variables and n-3 supplement intake
The daily intakes (g/d) of fish and seafood items were grouped into four categories. The first category comprised all items of lean fish eaten as bread spread, dinner fillets or in a mixed item such as fish fingers or fish au gratin. This category included cod, saithe, haddock, pollock, halibut, plaice, flounder, tuna, perch, pike, cat-fish and fish roe (0·3–6·0 % fat). The second category comprised the fatty fish items of mackerel, herring, salmon and trout (10–24 % fat). The third category was cod liver (about 60 % fat) either as dinner or as bread spread (liver and roe pâté). The fourth category comprised shellfish and included shrimp, crab and mussels (0·8–2·5 % fat). Cod liver was kept as a separate category because of its relatively high concentration of persistent organic pollutants such as polychlorinated biphenyls, dioxins, various organochlorine pesticides and polybrominated diphenylethers(Reference Haug, Thomsen and Brantsæter22, Reference Kvalem, Knutsen and Thomsen34, 35). Total seafood was the combined intake of all fish and seafood items.
When evaluating the association between seafood consumption and fetal growth, seafood intake was treated as a continuous variable (g/d) and divided into the following categories: 0–5, >5–20, >20–40, >40–60 and >60 g/d. These categories correspond to the categories used in a recent study from the Danish National Birth Cohort(Reference Halldorsson, Meltzer and Thorsdottir14). Assuming a serving size of 140 g, these categories correspond to; rarely, < 1 serving/week, 1–2 servings/week, 2–3 servings/week and 3 or more servings/week. When seafood was eaten as bread spread the serving size was estimated as 20–25 g.
The intake (mg/d) of marine n-3 fatty acids from supplements (cod-liver oil, cod-liver oil capsules and fish-oil capsules) was estimated from the FFQ. Supplementary n-3 was examined as a continuous variable and divided into three categories, with non-users as one group and consumers ranked into two groups by their estimated total intake of EPA, docosapentaenoic acid and DHA from supplements.
Outcome variables
Birth weight, birth length and head circumference were measured by the midwife who attended the birth and reported these to the MBRN(Reference Irgens29). Data from MBRN also included information on pregnancy duration. Only observations of birth length and head circumference falling within a realistic range were used (35–65 cm for birth length and 25–45 cm for head circumference), resulting in n 59 595 (96 %) for the analysis of birth length and n 60 805 (98 %) for the analysis of head circumference. Infant birth weight was also examined as dichotomous outcomes, first as birth weight < 2500 g and second as birth weight >4500 g. These outcomes were only examined in term pregnancies ( ≥ 37 weeks), resulting in n 59 058 (95 %). Both of these outcomes have been associated with maternal and/or perinatal complications(Reference Wilcox36, Reference Zhang, Decker and Platt37).
Other variables
We included variables previously shown to be associated with fetal growth, which were also associated with the consumption of fish and seafood. Maternal age at delivery was used as a continuous variable except in Table 1 where is was divided into four categories ( < 25, 25–29, 30–34 and 35+ years). BMI was calculated from self-reported height and weight before the pregnancy and categorized according to the WHO classification as normal (18·5–24·9 kg/m2), underweight ( ≤ 18·5 kg/m2), overweight (25·0–29·9 kg/m2) and obese ( ≥ 30·0 kg/m2). Height was divided into quartiles. Parity was based on data from both MoBa and MBRN and categorized into number of previous pregnancies of >22 weeks' duration. Pregnancy duration (MBRN information) was based on ultrasound and used as a continuous variable except in Table 1. Other variables included were total energy intake (continuous), maternal educational attainment ( ≤ 12, 13–16 and 17+ years), first trimester smoking habits (non-smoker, occasional smoker or daily smoker) and whether the respondent or her partner did not have Norwegian as their mother tongue. Participants with unknown/missing values for BMI, height, education or smoking were grouped in a ‘missing data’ category.
Table 1 Seafood intake according to maternal characteristics among 62 099 women in the Norwegian Mother and Child Cohort Study, 2002–2008
(Medians and percentiles)

P5th, 5th percentile; P95th, 95th percentile.
* Total seafood comprises lean fish (including fish in products), fatty fish, fish liver and shellfish.
† P for trend or for differences between groups depending on whether categories are ordinal or nominal.
Statistical methods
We used linear regression to calculate P for trend when examining seafood intake across the ordered categories and Student's t test for dichotomous variables. Associations between seafood consumption and birth weight, birth length and head circumference were analysed using multiple linear regression. For the dichotomous outcomes, we calculated relative risks as OR and controlled for confounding with multiple logistic regression.
All regression models were adjusted for potential confounding by maternal age, height, BMI, parity, education, smoking in pregnancy, pregnancy duration, mother tongue other than Norwegian, total energy intake and marine n-3 intake. The variance inflation factor test for multicollinearity was well within limits, indicating that all confounders in the model could reliably assess their independent contribution.
We examined potential interactions between seafood consumption and other food intakes. No significant interactions were seen. All models were checked for violations from the model assumptions.
All analyses were performed using the statistical software PASW statistics 17 (SPSS, Inc., Chicago, IL, USA) and P < 0·05 was considered significant.
Results
Consumption of fish or seafood was reported by 98 % of the women in this study, with 6 % reporting no consumption of lean fish, 11 % reporting no consumption of fatty fish, 35 % no consumption of shellfish and 94 % no consumption of fish liver. Use of n-3-containing supplements was reported by 66·5 % of the women.
The average intake was 36 g/d (median 33) of total seafood, 20 g/d (median 19) of lean fish, 12 g/d (median 8) of fatty fish, 4 g/d (median 2) of shellfish and < 0·1 g/d of fish liver. Lean fish constituted 58 %, fatty fish 31 % and shellfish 11 % of total seafood consumption.
Seafood contributed on average to 2·9 % of the total energy intake, 7·2 % of the total protein intake and 4·8 % of the total fat intake. For comparison, the average intake of meat was 83 g/d and meat contributed 7·0 % of total energy, 17·8 % of total protein and 12·2 % of total fat intake.
The consumption of seafood, specified as lean or fatty fish, differed across categories of participant characteristics (Table 1). The consumption of seafood increased with increasing maternal age, height, parity, length of education, energy intake and n-3 supplement use. The intakes of lean and fatty fish were higher among women in the normal BMI category than among under- or overweight women, and higher among non-smokers than smokers. Women with mother tongue other than Norwegian, or a partner in this category, had lower intake of seafood, but this only pertained to lean fish (Table 1).
Women in the highest categories of seafood consumption had higher consumption of all seafood subtypes as well as higher intake of supplementary marine n-3 fatty acids (Table 2).
Table 2 Daily intake of various fish and seafood categories by subtypes of total seafood intake among 62 099 women in the Norwegian Mother and Child Cohort Study, 2002–2008
(Mean values and standard deviations)
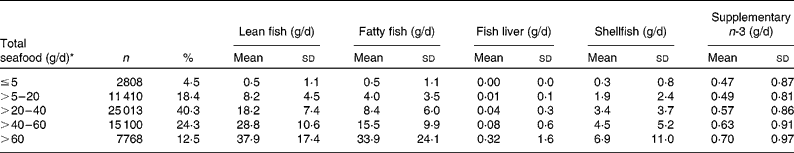
* The categories correspond to rarely, < 1 serving/week, 1–2 servings/week, 2–3 servings/week and 3 or more servings/week.
The average (mean) infant birth size measures were 3590 (sd 540) g for birth weight, 50·36 (sd 2·26) cm for birth length and 35·30 (sd 1·58) cm for head circumference. In the unadjusted analysis, birth weight increased with increasing consumption of seafood (Fig. 2). The unadjusted β coefficients for intake of total seafood and of lean fish were positive for all birth size measures, while supplementary n-3 was negatively associated with birth weight and head circumference (Table 3). When adjusting for confounders, the magnitude of the observed β coefficients decreased for all items except shellfish. However, the significant positive associations between total seafood and lean fish and birth weight and head circumference remained. Likewise, the inverse association between supplementary n-3 and head circumference remained significant (Table 3). The covariates contributing most to attenuating the associations were parity and pregnancy duration, variables with strong independent influence on the infant birth measures.
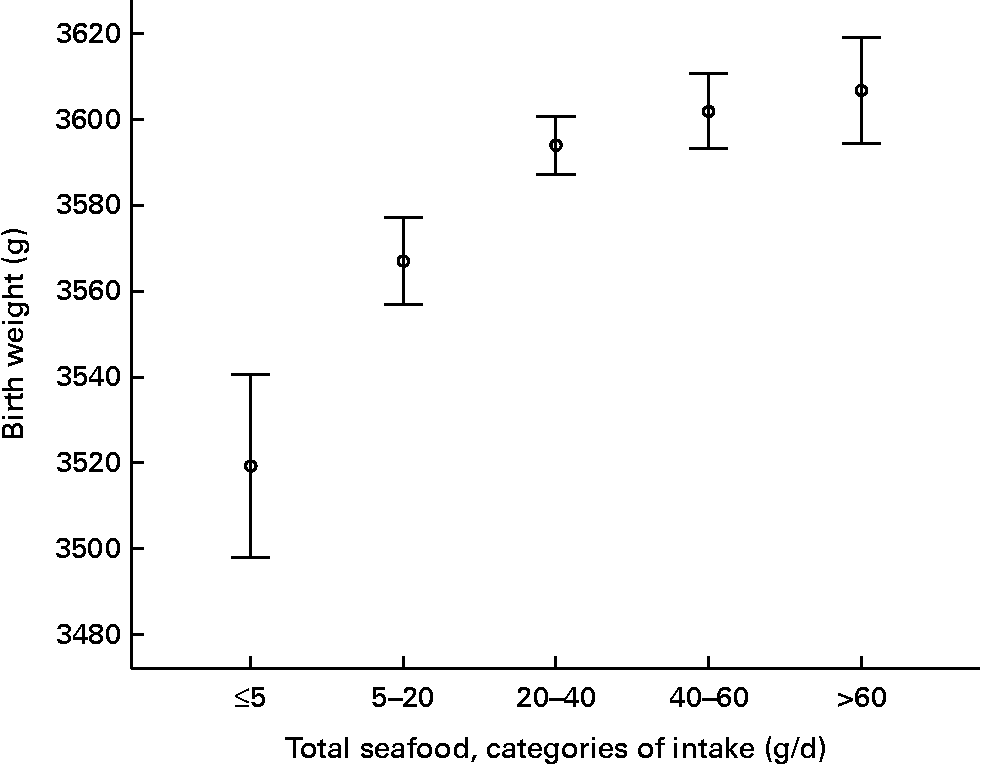
Fig. 2 Birth weight by categories of total seafood intake in 62 099 women in the Norwegian Mother and Child Cohort Study. The values are means and 95 % CI.
Table 3 Impact of seafood consumption on birth weight, birth length and head circumference of infants born to 62 099 women* in the Norwegian Mother and Child Cohort Study, 2002–2008
(β Coefficients and 95 % confidence intervals)

* For birth length n 61 387 and for head circumference n 60 805.
† Adjusted for maternal age, height, pre-pregnant BMI, parity, pregnancy duration, maternal education, smoking status, mother tongue other than Norwegian and total energy intake, and with intakes of seafood/seafood items and supplementary n-3 mutually adjusted.
‡ Total seafood comprises lean fish (including fish in products), fatty fish, shellfish and fish liver.
Maternal weight gain in pregnancy, which was available for a subset of participants, was negatively associated with seafood consumption. Adjusting for gestational weight gain strengthened the association between intakes of seafood and lean fish regarding all birth measures (data not shown).
Examining seafood intake by categories of 20 g/d increase showed that maternal seafood consumption had to be at least 20 g/d (one serving weekly) to be significantly related to birth weight and head circumference (Table 3). Examining n-3 from fish-oil/cod-liver oil supplements by categories showed that the negative association with infant head circumference was evident in both the low ( < 0·4 g/d) and the high ( ≥ 0·4 g/d) intake category (Table 3).
Other dietary variables also influence infant birth size. We examined whether adjusting for intakes of meat, milk, coffee or vegetables attenuated the association between seafood and birth weight. Including these dietary variables in the models increased rather than attenuated the β-value for seafood intake (data not shown). We also examined the association between the total calculated intake of seafood-specific nutrients (e.g. iodine and Se) and birth weight. From the results, it was not possible to attribute the observed influence of lean fish or total seafood to specific nutrients (data not shown).
Because the association between seafood consumption and birth weight may be modified by pregnancy duration, parity, smoking or age covariates, we also examined the association after stratifying by influential covariates. The positive association between maternal seafood intake and infant birth weight was consistent in all strata of pregnancy duration, parity, maternal age, height and BMI, maternal education and total energy intake. However, the strength of the association (β-value) was lower in primparous than in multiparous women and among women with pregnancy duration < 37 weeks compared with full-term pregnancies (data not shown).
On the basis of the positive association between maternal seafood consumption and birth weight, we examined the influence of seafood consumption on the risk of giving birth to small or large babies in full-term pregnancies (pregnancy duration ≥ 37 completed weeks). In total, 437 (0·7 %) infants had birth weights < 2500 g and 2526 (4·3 %) had birth weights >4500 g. The relative risk of giving birth to a small baby decreased with increasing seafood intake, and was significantly lower in women who consumed >60 g/d of seafood than in women who consumed ≤ 5 g/d; adjusted OR = 0·56 (95 % CI 0·35, 0·88). Neither total seafood nor lean fish consumption increased the risk of giving birth to a large baby (data not shown).
Discussion
The main finding in the present study was the positive association between infant birth weight and head circumference with increasing maternal seafood consumption. This association was mainly driven by the consumption of lean fish. Maternal intake of marine n-3 fatty acids through fish-oil/cod-liver oil supplements was associated with a small but significantly smaller head circumference of the baby. Significantly reduced rates of low birth weight babies ( < 2500 g) were found in women who consumed more than 60 g of seafood/d as compared to women with no or very low intakes. The results of the present study did not show negative associations between maternal consumption of any fish or seafood items and infant birth weight, length or head circumference.
The finding of a positive association between maternal fish intake and increase in birth size in the present study is in concordance with other epidemiological studies that reported increased fetal growth measures with increasing fish intake(Reference Olsen, Grandjean and Weihe7–Reference Thorsdottir, Birgisdottir and Halldorsdottir10, Reference Guldner, Monfort and Rouget17). In seemingly contrast to these results, higher rates of birth weights below the 10th percentile were found in women who consumed more than 60 g of fish/d than in women with low fish intake in the Danish national birth cohort(Reference Halldorsson, Meltzer and Thorsdottir14). However, the negative associations between fish intakes and fetal growth measures were only seen for consumption of fatty fish and not for consumption of lean fish. Similar results from a US cohort also found that marine n-3 fatty acid intake, or fish consumption, was associated with reduced fetal growth(Reference Oken, Kleinman and Olsen16). The present study results did not indicate any association between fatty fish and birth size, but a small negative influence was observed for supplementary n-3 in relation to infant head circumference. A significant negative association between intake of fish oil and infant head circumference was previously reported among pregnant women in Iceland(Reference Thorsdottir, Birgisdottir and Halldorsdottir10). Although the clinical importance of the small negative association between supplementary n-3 and head circumference in the present study is uncertain, this finding parallels the negative associations reported for fatty fish or fish oil in the other studies(Reference Thorsdottir, Birgisdottir and Halldorsdottir10, Reference Halldorsson, Meltzer and Thorsdottir14, Reference Oken, Kleinman and Olsen16).
It has been suggested that differences observed regarding maternal seafood consumption and fetal growth might be indirect evidence of harmful contaminants in fish and can therefore reflect a true causal pattern(Reference Lee and Jacobs20). Few studies have examined potential associations between exposure to persistent contaminants and birth size. A Spanish study that showed increased risk of small for gestational age with high intake of crustaceans and tuna adjusted the analysis for known contaminants in maternal blood (polychlorinated biphenyls and dichlorodiphenyldichloroethylene), but this did not change the results(Reference Mendez, Plana and Guxens19). Lean fish is the primary source of dietary exposure to organic Hg and As. In a previous study in MoBa we showed that blood Hg concentrations were low(Reference Brantsæter, Haugen and Thomassen25). Given the consistent positive association between lean fish consumption and birth weight in the present study there is no reason to believe that the relatively low dietary Hg exposure from lean fish consumed in Norway negatively influence infant birth weight.
When two opposing forces, beneficial nutrients important for fetal growth and harmful contaminants, coexist in the same food in varying amount/proportion, the results are bound to be different in different populations(Reference Lee and Jacobs20). Large differences in terms of seafood and supplementary n-3 consumption exist between countries and even within the same country. Despite a decrease in fish intake over the past decades, a higher proportion of women in our study reported intakes >60 g/d (12·5 %) than those in the Danish cohort (5·6 %)(Reference Halldorsson, Meltzer and Thorsdottir14). In addition to differences in patterns of consumption, the concentrations of nutrients and contaminants in various seafood items depend on whether these are farmed or wild(21), and for wild species also with origin and depth(Reference Haug, Thomsen and Brantsæter22). The concentration of organic pollutants in Norwegian farmed fish has been reduced over the last years and are now lower than that in wild fish(21). Even the cooking method has been shown to influence the concentration of persistent organic pollutants in seafood(Reference Del, Tittlemier and Diamond38, Reference Sherer and Price39). The concentrations of organic pollutants in Norwegian commercial cod-liver oil/fish-oil supplements have also been substantially reduced over the last decades, as improved methods to remove contaminants were introduced during the 1990s(Reference Breivik and Thorstad40). However, not all contaminants are or can be fully removed(Reference Bourdon, Bazinet and Arnason41, Reference Jacobs, Santillo and Johnston42).
The main strength of this study is the large sample of pregnant Norwegian women. MoBa is a pregnancy cohort representing women with wide ranges of seafood intake, age, BMI, height and socioeconomic status. Seafood consumption was assessed with a detailed FFQ that was developed and validated for use in pregnancy(Reference Meltzer, Brantsæter and Ydersbond24). A limitation of the present study is that the FFQ was answered in mid pregnancy and covered a time period that for many women has been biased by nausea and may not reflect the dietary intakes later in pregnancy. This may have influenced the reported dietary intakes and is likely to attenuate the observed associations. Furthermore, fish and seafood intakes, as well as n-3 supplement use, were higher in women of older age, non-smokers and those with higher educational attainment (Table 1). We reduced the possibility of confounding by adjusting for a number of relevant factors. Adjusting for parity, maternal age, smoking, and other maternal factors markedly attenuated the associations between seafood consumption and birth size measures (Table 3). Still, the associations between seafood and lean fish remained consistent, but we cannot rule out the possibility that residual or unmeasured confounding may still exist. Many variables influence fetal growth. Gestational weight gain has a strong influence on birth weight(Reference Thorsdottir, Torfadottir and Birgisdottir43). Adjusting for gestational weight gain in a subset of our study population strengthened the positive association between seafood and lean fish on infant birth weight. This finding is in accordance with the results reported from Iceland(Reference Thorsdottir, Birgisdottir and Halldorsdottir10) and France(Reference Guldner, Monfort and Rouget17). We also examined whether other independent dietary variables biased the association between maternal seafood consumption and fetal growth. For comparison, meat intake contributed more to the total intake of protein and energy than seafood, but adjusting for meat, milk or other dietary factors did not attenuate the associations between seafood or lean fish and birth weight. This indicates that the observed associations between maternal seafood intakes and birth weight cannot easily be explained by the overall dietary behaviour in fish consumers.
In observational studies such as this, applying the Hill criteria for assessing disease causation can give further insight into the nature of the relation found(Reference Hill44). The magnitude (strength criteria) of the association between seafood intake and birth weight was consistent in all strata of maternal characteristics. Information about seafood consumption preceded delivery (temporality criteria). A biological mechanism (plausibility criteria) underlying the positive association between seafood and birth weight is not obvious. In the present study, it was not possible to attribute the influence to specific nutrients or constituents. The health benefits of fish consumption documented in human studies (e.g. reduced risk of CHD mortality) are mostly related to consumption of species with a high content of n-3 fatty acids(Reference Mozaffarian and Rimm45). The associations between maternal seafood consumption and infant birth measures observed in the present study are unlikely to be explained by marine n-3 fatty acids because consumption of lean fish seemed to be the driving force of the association (Table 3). One possible explanation may be the composition of protein in fish. Bioactive peptides released from proteins upon intestinal digestion may modulate specific physiological functions in the human body(Reference Erdmann, Cheung and Schroder46). We were not able to calculate concentrations of specific amino acids in this study, but experimental studies have shown that fish proteins fed to pregnant rodents have beneficially influenced insulin resistance and blood pressure in the offspring(Reference Yahia, Madani and Prost47, Reference Tremblay, Lavigne and Jacques48). If the association stands true, and whether the increased birth weight is due to the special composition of fish proteins or other substances or a combination of these, remains to be seen.
In conclusion, this study indicated a consistent increase in infant birth weight and head circumference with increasing maternal seafood consumption and that this association was mainly driven by lean fish. Maternal intake of marine n-3 fatty acids through fish-oil/cod-liver oil supplements was associated with a small decrease in head circumference. These results corroborate the present dietary recommendation to pregnant Norwegian women, which is to include fish and seafood as part of a balanced diet, but to eliminate or avoid highly polluted items such as fish liver. The coexistence of persistent chemical pollutants and essential nutrients in natural foods such as seafood is a serious problem, and more studies are warranted to further disentangle the interplay between nutrients and contaminants in nutritional epidemiology.
Acknowledgements
The study was supported by the Norwegian Ministry of Health, NIH/NIEHS (grant no. N01-ES-85433), NIH/NINDS (grant no. 1 UO1 NS 047537-01), the 6th Research Framework of the European Union and the Norwegian Research Council/FUGE (grant no. 151918/S10). The authors declare that they have no conflicts of interests. A. L. B. and B. E. B. conducted the statistical analyses and wrote the paper. M. H. was in charge of the coordination of the data file and analyses. H. M. M., H. E. K., J. A. and P. M. contributed to the interpretation of the results. All authors reviewed the paper.







