INTRODUCTION
Resistance to currently available antibiotics in nosocomial Gram-negative pathogens has become a significant problem over the past decade; emerging threats include Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBLs) and carbapenemases, and multidrug-resistant Pseudomonas and Acinetobacter isolates. Unfortunately, very few agents with novel mechanisms of action against multidrug-resistant Gram-negative pathogens are in the drug development pipeline, forcing clinical providers to rely on appropriate usage of broad-spectrum agents as a primary strategy to combat multidrug resistance.
Ertapenem is a once-daily intravenous broad-spectrum carbapenem that lacks intrinsic antipseudomonal activity but typically retains activity against ESBL-producing organisms [Reference Livermore, Sefton and Scott1]. It is approved in the USA for the treatment of complicated intra-abdominal infections, complicated skin and skin structure infections (including diabetic foot infections without osteomyelitis), acute pelvic infections (including postpartum endomyometritis, septic abortion, and post-surgical gynaecological infections), community-acquired pneumonia, complicated urinary tract infections (including pyelonephritis), and in preoperative surgical prophylaxis of elective colorectal surgery [2]. One potential concern that has limited the use of ertapenem worldwide is that widespread use would theoretically promote cross-resistance to other carbapenems (imipenem, meropenem, doripenem) in Pseudomonas spp. One in vitro study in which Pseudomonas isolates were exposed to ertapenem did find some selection for broad carbapenem resistance (primarily via loss of porin OprD and/or up-regulation of MexAB-OprM-mediated efflux), but also found that minimum inhibitory concentrations to ertapenem increased four- to eightfold in the presence of 20% serum, probably due to the high protein binding of ertapenem, such that selectivity for carbapenem resistance was predicted to be minimal under most clinical conditions [Reference Livermore, Mushtaq and Warner3]. In the two international trials of ertapenem vs. either piperacillin-tazobactam or ceftriaxone-metronidazole for intra-abdominal infections, rectal carriage of Pseudomonas resistant to imipenem was found in <1% of patients receiving ertapenem at the end of therapy [Reference DiNubile4].
A limited number of single-site clinical studies have not observed carbapenem cross-resistance in Enterobacteriaceae or Pseudomonas clinical isolates with ertapenem use [Reference Goff and Mangino5–Reference Goldstein7]. In particular, Goldstein et al. actually found an increase in Pseudomonas susceptibility to imipenem following the introduction of ertapenem, potentially associated with an observed decrease in overall imipenem use [Reference Goldstein7]. One multicentre study involving a compilation of antibiotic purchase and usage data and antibiogram data from 25 US hospitals also found no association between ertapenem use and imipenem susceptibility in Pseudomonas [Reference Eagye and Nicolau8]. However, all of these studies, except the study by Goldstein et al. [Reference Goldstein7], are limited by their quasi-experimental designs that do not take into account the lack of statistical independence of pre- and post-intervention events that is typically required for the χ2 tests for trend and regression analyses used [Reference Shardell9, Reference Zeger, Irizarry and Peng10]. Interrupted time-series analysis, in which regression models are estimated while relaxing the independence assumption by estimating autocorrelation between observations collected at different times, has been proposed as a way to improve the accuracy of standard error estimates and thus overall statistical inference in pre- and post-intervention studies [Reference Shardell9–Reference Bosso and Mauldin11].
Ertapenem has become widely used at our institution since its introduction onto our formulary in 2005. We hypothesized that its introduction would be associated with a decrease in the usage of other broad-spectrum antibiotics, and that decreased use would result in increased susceptibility to those agents while preserving imipenem susceptibility in nosocomial Enterobacteriaceae and Pseudomonas clinical isolates. These hypotheses were evaluated using interrupted time-series analyses.
METHODS
Ertapenem and the antimicrobial formulary of the VA Greater Los Angeles Healthcare System
This study was conducted at the VA Greater Los Angeles Healthcare System, a teaching hospital and tertiary referral centre with about 300 acute-care beds. In July 2005, ertapenem was added to the hospital formulary, and in March 2006, it was formally approved for unrestricted use for healthcare- and hospital-associated pneumonia and diabetic foot infections, but was also generally available for the treatment of other nosocomial infections including intra-abdominal and urinary-tract infections. Data for this study was collected for the following other broad-spectrum antibiotics in common use during this time-frame: imipenem-cilastatin, meropenem, piperacillin-tazobactam, ampicillin-sulbactam, cefepime, ceftriaxone, intravenous levofloxacin, and intravenous ciprofloxacin. Imipenem-cilastatin use was restricted to empiric treatment of hospital-acquired pneumonia in intensive-care-unit patients, targeted therapy of susceptible Gram-negative pathogens resistant to all available fluoroquinolones, penicillins, cephalosporins, and aminoglycosides, and treatment of susceptible Pseudomonas infections resistant to all other β-lactam antibiotics. Meropenem was restricted for patients with renal insufficiency or history of seizures, but otherwise met the same clinical criteria for imipenem use. Piperacillin-tazobactam and cefepime use was restricted to empiric therapy of hospital-acquired urinary-tract infection, pathogen-directed therapy of susceptible Pseudomonas infections, empiric therapy of necrotizing fasciitis (piperacillin-tazobactam only), and empiric therapy of hospital-acquired and ventilator-associated pneumonia. The use of ampicillin-sulbactam, ceftriaxone, and intravenous ciprofloxacin was unrestricted. Intravenous levofloxacin was restricted to severe community-acquired pneumonia in intensive-care-unit patients and in the setting of allergy to β-lactams.
All restrictions on the usage of the antibiotics studied remained unchanged throughout the study period and could be given for additional indications with the approval of the Infectious Diseases Section. Our infection control department follows the CDC Guideline for Isolation Precautions in Hospitals and places patients colonized or infected with multidrug-resistant organisms in contact isolation. During the time-frame of the study, the VA implemented a nationwide campaign to promote frequent hand-washing to prevent the spread of multi-drug-resistant pathogens as well as a campaign to screen for colonization with methicillin-resistant Staphylococcus aureus upon admission to the hospital or transfer from one acute-care unit to another. No other changes to infection control practices were made during the study period.
Data extraction
Antimicrobial usage
Total grams of usage per day and total numbers of patient-days of use of each antibiotic studied used on acute-care wards from March 2004 to December 2008 were obtained through extraction from the Computerized Patient Record System (CPRS) and converted into defined daily dose (DDD) per month according to World Health Organization recommendations. DDD is the average daily dose in grams of a specific agent given to an average adult patient [12].
Antimicrobial susceptibility in nosocomial Enterobacteriaceae and Pseudomonas clinical isolates
Microbiology data was reviewed through local infection control software available in the Veterans Health Information Systems and Technology Architecture (VISTA) system. Antibiotic susceptibility was collected for the following organisms collected on an acute-care hospital floor:
Enterobacteriaceae: Citrobacter freundii complex, Citrobacter koseri (diversus), Citrobacter spp. not otherwise specified, Enterobacter aerogenes, Enterobacter cloacae, Enterobacter spp. not otherwise specified, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Klebsiella spp. not otherwise specified, Morganella morganii, Proteus mirabilis, Proteus vulgaris, Proteus spp. not otherwise specified, Serratia marcesens, Serratia spp. not otherwise specified.
Pseudomonas: Pseudomonas aeruginosa, Pseudomonas spp. not otherwise specified.
All isolates that were collected less than 48 h following acute-care admission or were collected from the same site from the same patient within 14 days of the original isolate were excluded. Susceptibility methods were performed according to Clinical Laboratory Standards Institute guidelines.
Statistical analysis
Antibiotic usage trends pre- and post-ertapenem introduction
The total monthly DDD of each antibiotic other than ertapenem was measured between March 2004 to December 2008; ertapenem monthly DDD was measured from July 2005 to December 2008. The monthly DDDs of imipenem-cilastatin and meropenem were combined and treated as a single agent in the analysis.
Interrupted time-series analysis was used to determine whether the use of the above agents changed after the introduction of ertapenem into the formulary. Specifically, we used segmented regression analysis [Reference Bosso and Mauldin11] where the model is as follows:
where Y it is the use of drug i at month t. x 1 is a count variable indicating the month order for time t; β1 is the linear trend (slope) of drug usage from month to month without ertapenem in the formulary (i.e. pre-ertapenem trend). x 2 is a binary variable indicating whether or not ertapenem was used concurrently in that month; β2 is the overall change in the use of drug i after introducing ertapenem. β3 is the change in the linear trend for drug usage after introducing ertapenem. Thus, β1+β3 is the linear trend (slope) of drug usage from month to month with ertapenem in the formulary (i.e. post-ertapenem trend). A likelihood ratio test on β3 was performed and the corresponding P value reported evaluates whether the pre- and post-linear trends are different from one another; a Durbin–Watson P value that tests for autocorrelation in the residuals of each respective model, was also reported.
Susceptibility in nosocomial Enterobacteriaceae and Pseudomonas pre- and post-ertapenem introduction
The total number of unique isolates of Enterobacteriaceae and Pseudomonas and percent susceptible to each antibiotic was obtained for March 2004 to December 2008. Susceptibility data were aggregated into yearly quarters (i.e. 3-month periods: March–May, June–August, September–November, December–February). Susceptibility of Enterobacteriaceae was measured against eight antibiotics (ertapenem, imipenem, piperacillin-tazobactam, ampicillin-sulbactam, levofloxacin, ciprofloxacin, cefepime, ceftriaxone); susceptibility of Pseudomonas was measured against five antibiotics (imipenem, piperacillin-tazobactam, levofloxacin, ciprofloxacin, cefepime).
Interrupted time-series analysis was used to determine whether susceptibility to these antibiotics changed after introducing ertapenem into the formulary. Specifically, we used segmented regression analysis [Reference Bosso and Mauldin11] where the model is as follows:
where Y it is the proportion susceptible to drug i at quarter t. The mean proportion of susceptible isolates before introduction of ertapenem into the formulary is given by α (‘pre-ertapenem mean’). x 1 is a count variable indicating the count order for quarter t; β1 is the linear trend (slope) of proportion susceptible to drug i from quarter to quarter without ertapenem in the formulary (i.e. pre-ertapenem trend). x 2 is a binary variable indicating whether or not ertapenem was used concurrently in that month; β2 is the overall change in the proportion susceptible to drug i after introducing ertapenem. The P value for β2 indicates whether the mean difference in proportion susceptible isolates pre- and post-ertapenem is significant. The mean proportion of susceptible isolates after introduction of ertapenem is given by α+β2 (‘post-ertapenem mean’). x 3 is the number of total isolates from quarter t; β3 is the change in susceptibility to drug i with each unit increase in the number of isolates. x 3 was mean-centred. The number of total isolates was adjusted to account for any spurious associations that may arise from differences from quarter to quarter. β4 is the change in the linear trend for proportion susceptible to drug i after introducing ertapenem. The P value for β4 indicates whether there is a significant difference in the linear trend of susceptibility pre- and post-ertapenem. So β1+β4 is the linear trend (slope) of proportion susceptible to drug i from quarter to quarter with ertapenem in the formulary (i.e. post-ertapenem trend). Results for the mean proportion of susceptible isolates pre- and post-ertapenem use (α and α+β2, respectively) are reported. The Wald P value for β2 is reported to assess the significance of this difference. Results for linear trends in proportion susceptible, pre- and post-ertapenem use (β1 and β1+β4, respectively), are reported, along with their respective Wald P values to assess the significance of these trends. A likelihood ratio test P value on β4 is reported, evaluating whether these pre- and post-linear trends are different from one another.
All statistical calculations were performed using the R free software environment (www.r-project.org).
RESULTS
Ertapenem usage following its addition to the hospital formulary
From July 2005 to December 2008, a total of 1202 patients received ertapenem in an acute-care setting. The records of 200 patients who received ertapenem were randomly selected for review for prescribers' indication for treatment and incidence of likely drug allergy to ertapenem. Suspected hospital-acquired and healthcare-associated pneumonia were the most common indications for ertapenem use (42%), followed by skin soft-tissue infections (including diabetic foot ulcers) (29%), urinary-tract infections (10%), intra-abdominal infections (5%), and miscellaneous indications not otherwise specified (14%).
Of the 17 (8·5%) patients in our review who received ertapenem despite reporting a history of β-lactam or cephalosporin allergy, none developed an allergic reaction to ertapenem. Of these 17 patients, three had angioedema, 11 had rash/pruritis, and three had unknown reactions to previous β-lactams or cephalosporins. One patient with no prior history of β-lactam or cephalosporin allergy had a rash that was attributed to ertapenem. No seizures occurred while on ertapenem in the patients whose charts were reviewed.
The number of DDDs of ertapenem used per month is plotted in Figure 1. We had initially anticipated that ertapenem usage would have been minimal between the time it was added to the hospital formulary in July 2005 and when it was formally approved for unrestricted use for healthcare- and hospital-associated pneumonia and diabetic foot infections in March 2006, but significant use did occur in that interim time-frame. Thus, all analyses used July 2005 as the cut-off to compare antimicrobial usage and susceptibility patterns pre- and post-ertapenem introduction.
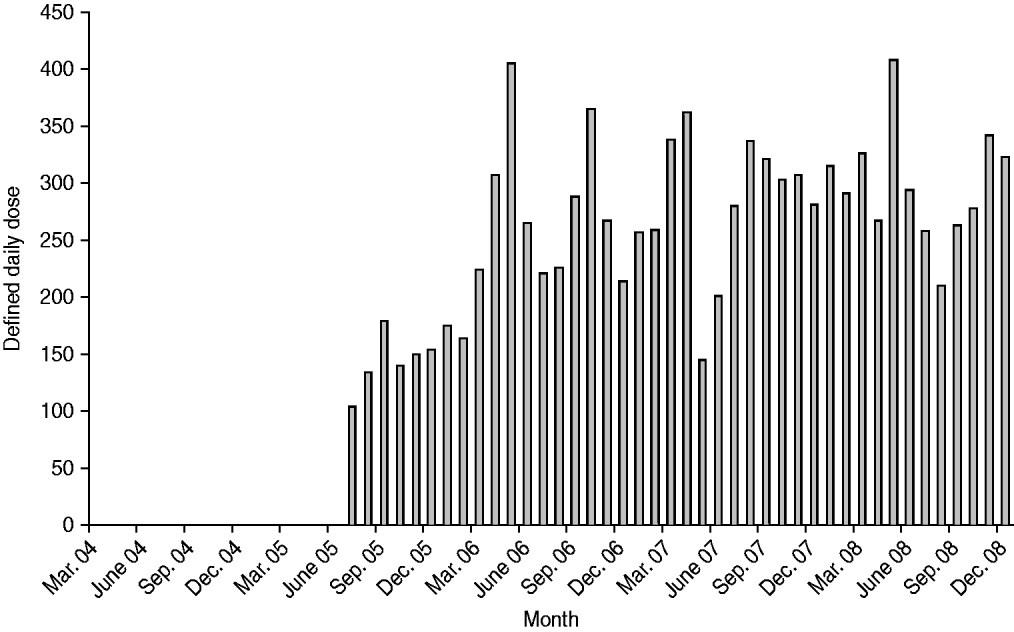
Fig. 1. Monthly ertapenem usage in acute-care wards. Defined daily dose (DDD) for ertapenem is 1 g.
Antimicrobial usage pre- and post-ertapenem introduction
The number of DDDs of each antibiotic studied that were used per month pre- and post-ertapenem introduction are plotted in Figure 2. The segmented regression analysis model coefficients with corresponding Wald P values for pre- and post-ertapenem introduction, likelihood-ratio testing P values, and Durbin–Watson P values are tabulated for each antibiotic in Table 1. Of note, there were significant decreases in the slope of the linear trends pre- and post-ertapenem in the usage of piperacillin-tazobactam (P=0·0013) and ampicillin-sulbactam (P=0·035). All other changes in linear trends of antibiotic usage were not significant.
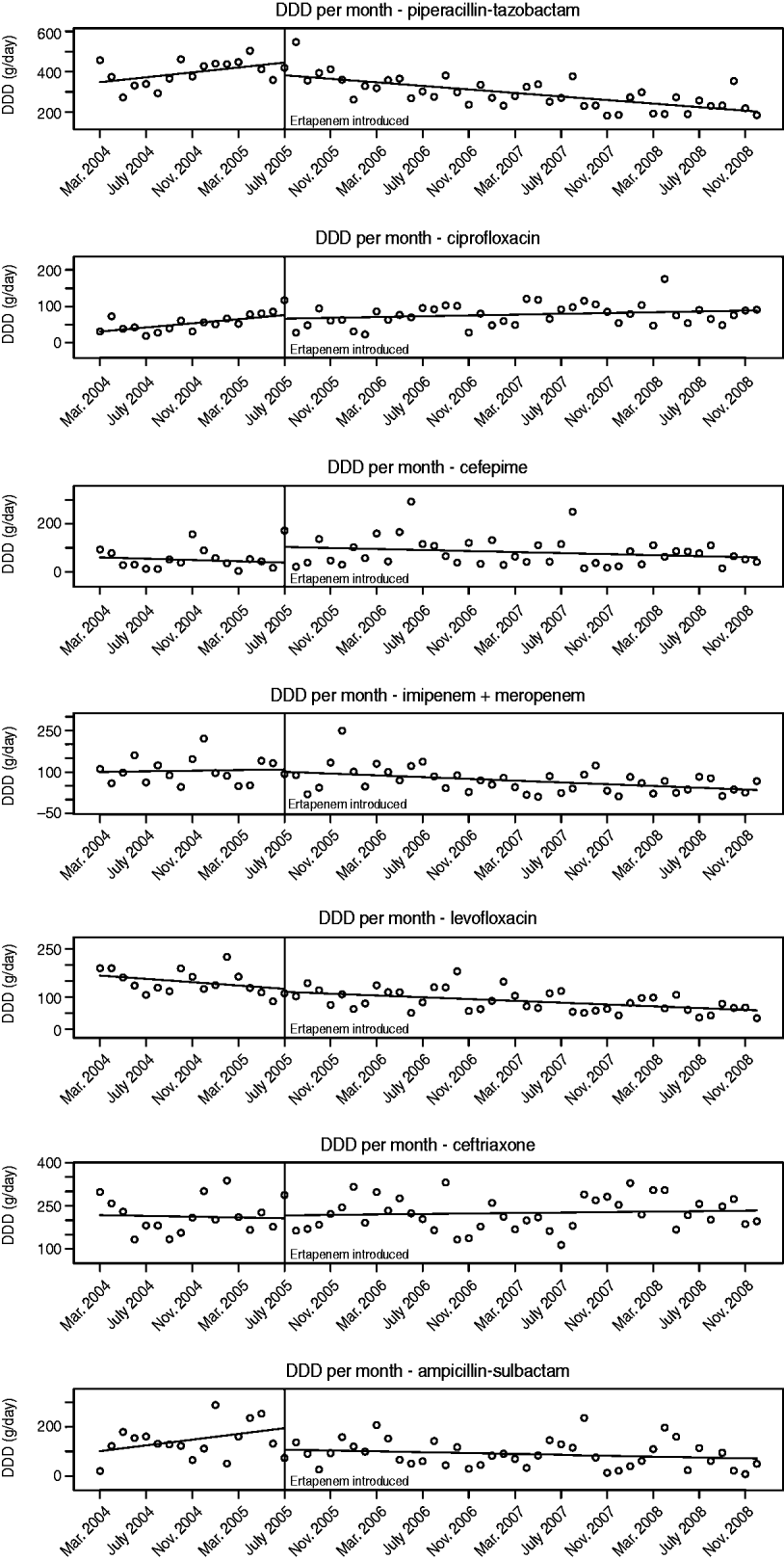
Fig. 2. Monthly antibiotic usage pre- and post-ertapenem introduction. DDD, Defined daily dose.
Table 1. Segmented regression analysis with likelihood ratio testing (LRT) of difference between pre- and post-ertapenem linear trends in antibiotic usage
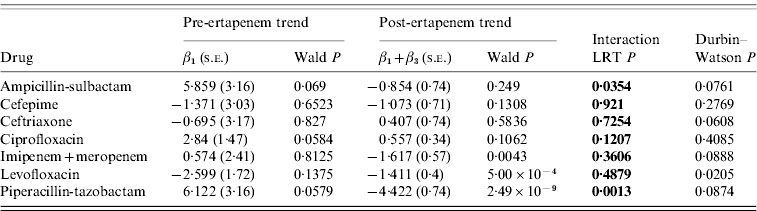
s.e., Standard error.
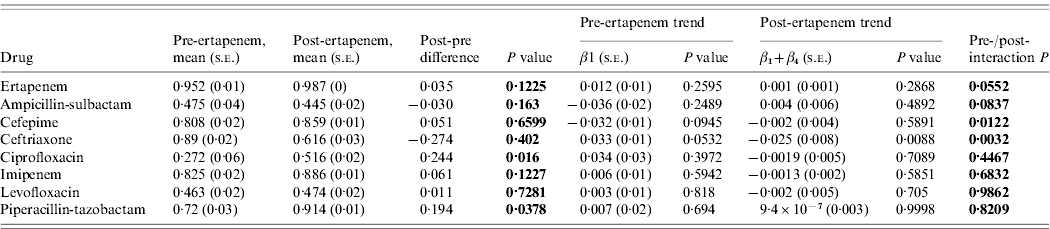
Susceptibility in nosocomial Enterobacteriaceae pre- and post-ertapenem introduction
The susceptibilities of Enterobacteriaceae to each antibiotic studied pre- and post-ertapenem introduction are plotted in Figure 3. The results for the mean proportion susceptible, pre- and post-ertapenem introduction and results in linear trends in proportion of isolates susceptible are tabulated in Table 2. The number of Enterobacteriaceae isolates analysed pre- and post-ertapenem introduction was 477 and 1252, respectively. Of note, in nosocomial Enterobacteriaceae, the mean proportion of Enterobacteriaceae susceptible to ciprofloxacin (P=0·016) and piperacillin-tazobactam (P=0·038) increased following ertapenem's introduction, while the linear trend in susceptibility to ceftriaxone significantly declined (P=0·0032) and the linear trend in susceptibility to cefepime (P=0·012) increased.
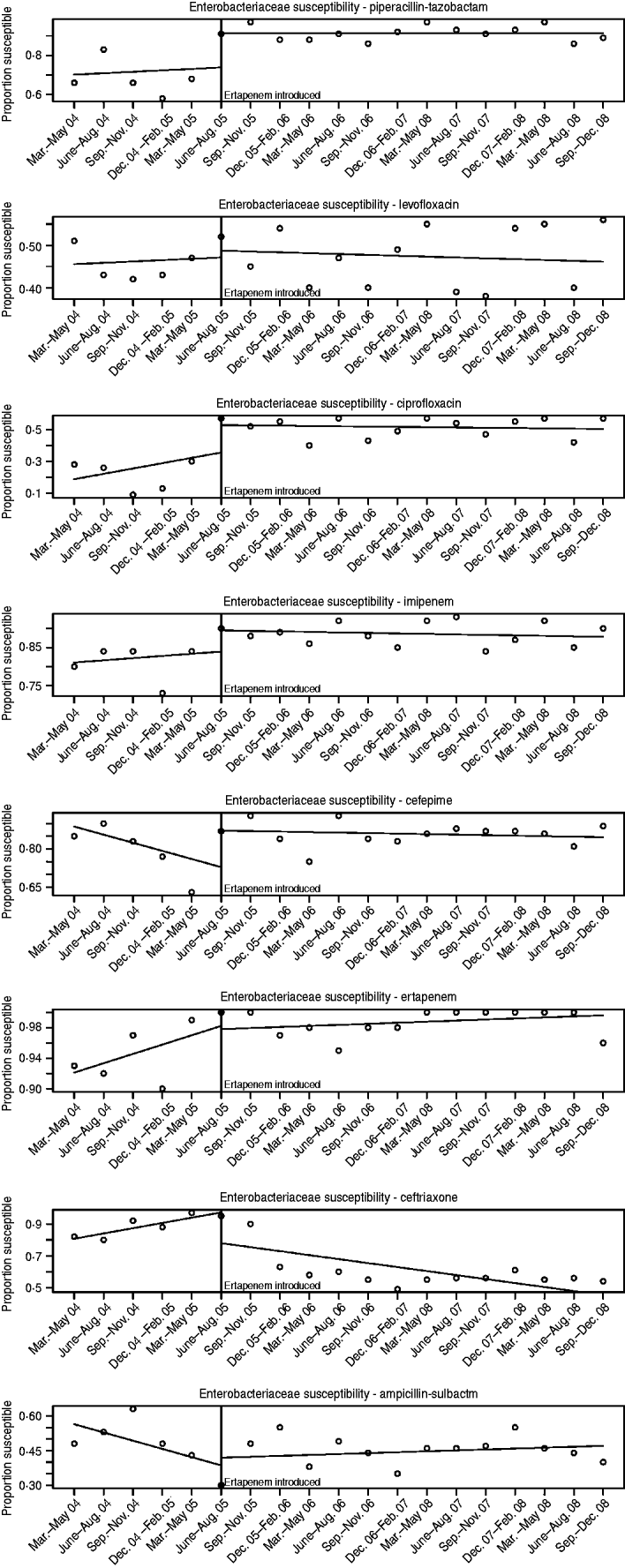
Fig. 3. Enterobacteriaceae susceptibilities pre- and post-ertapenem introduction.
Table 2. Mean proportion of susceptible Enterobacteriaceae, pre- and post-ertapenem introduction and results in linear trends in proportion of Enterobacteriaceae susceptible
s.e., Standard error.
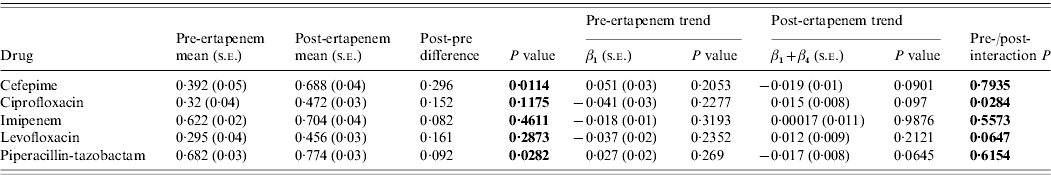
Susceptibility in nosocomial Pseudomonas pre- and post-ertapenem introduction
The susceptibilities of Pseudomonas to each antibiotic studied pre- and post-ertapenem introduction are plotted in Figure 4. The results for the mean proportion susceptible, pre- and post-ertapenem introduction and results in linear trends in proportion of isolates susceptible are tabulated in Table 3. The number of Pseudomonas isolates analysed pre- and post-ertapenem introduction was 167 and 263, respectively. Of note, in nosocomial Pseudomonas, the mean proportion susceptible to cefepime (P=0·011) and piperacillin-tazobactam (P=0·028) increased, as did the linear trend in susceptibility to ciprofloxacin (P=0·028).
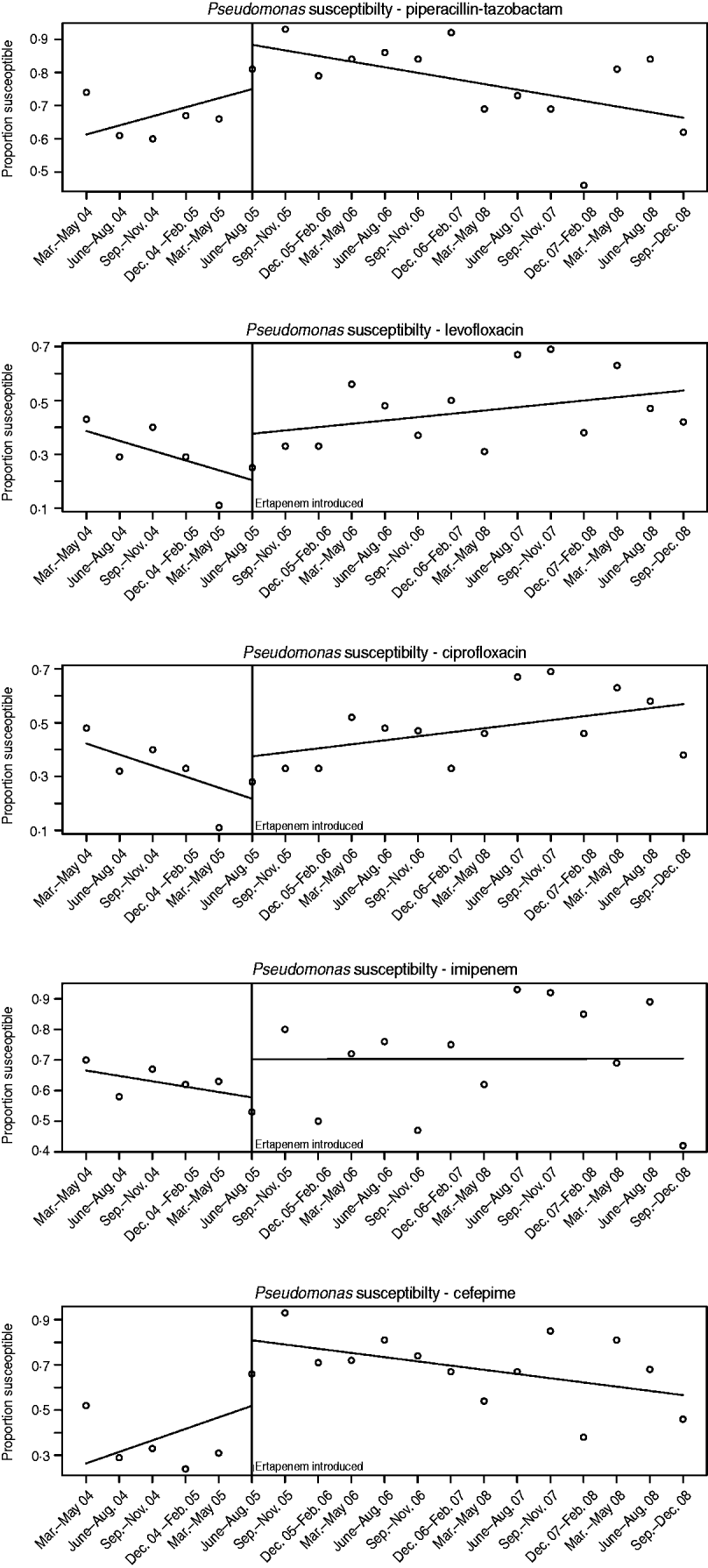
Fig. 4. Pseudomonas susceptibilities pre- and post-ertapenem introduction.
Table 3. Mean proportion of susceptible Pseudomonas, pre- and post-ertapenem introduction and results in linear trends in proportion of Pseudomonas susceptible
s.e., Standard error.
DISCUSSION
Ertapenem has been widely used at our institution since its addition to the formulary in July 2005, becoming one of our most commonly prescribed broad-spectrum antibiotics. Its widespread adoption probably contributed directly to the decrease in linear trend of usage of piperacillin-tazobactam and ampicillin-sulbactam observed in this study. However, ertapenem's adoption did not seem to similarly impact the use of imipenem and meropenem at our institution, in contrast to two prior single-centre studies that did associate adoption of ertapenem with decreased use of imipenem [Reference Lima6, Reference Goldstein7]. Imipenem and meropenem usage is fairly low at our institution (and doripenem is not on formulary). The low use of these agents, which did not appreciably change following ertapenem's introduction, is probably due to strict formulary restrictions and a robust antimicrobial stewardship programme. These factors may explain why one of these two previous studies was able to demonstrate improved susceptibility to imipenem in Pseudomonas following ertapenem introduction [Reference Goldstein7], while we were not. At minimum, although no decrease in susceptibility to imipenem in Pseudomonas or Enterobacteriaceae was seen in our study, the relative roles that ertapenem's potential low cross-resistance and other factors such as our formulary restrictions and antibiotic stewardship programme played in this relative preservation of susceptibility remain unclear. We hypothesized that ertapenem's apparent role as a ‘piperacillin-tazobactam-sparing agent’ at our institution would allow for improved susceptibility of Enterobacteriaceae and Pseudomonas to piperacillin-tazobactam. This was largely observed in that a significantly higher mean susceptibility to piperacillin-tazobactam in Enterobacteriaceae and Pseudomonas was seen post-ertapenem, although no significant changes were seen in linear trends of piperacillin-tazobactam susceptibility. Again, whether the improvement in mean susceptibilities of Enterobacteriaceae and Pseudomonas to piperacillin-tazobactam is directly due to its decreased use or other factors is unclear.
The significant changes seen in mean susceptibility and linear trends of susceptibility to other antibiotics in both Enterobacteriaceae and Pseudomonas are more difficult to interpret, as the use of those agents did not significantly change following the introduction of ertapenem; changes in susceptibility to these agents are probably subject to unmeasured confounding variables. In particular, however, the decrease in linear trend in susceptibility to ceftriaxone in Enterobacteriaceae warrants some concern. The mean susceptibility to ceftriaxone in Enterobacteriaceae also decreased by a large, although not statistically significant amount; the statistical non-significance may have been due to a ‘jackknife’ phenomenon in the data analysis.
Our study's advantages include the relative high rate of ertapenem usage at our institution in comparison to other antibiotics and the statistical methods used to account for autocorrelation between pre- and post-ertapenem introduction time periods. Our study's primary disadvantage is its retrospective nature and inability to account for unknown events that may have confounded relationships between ertapenem usage and other antibiotics and their susceptibility patterns. Furthermore, some authors have suggested that number of days of therapy rather than DDD may be a more accurate measure of antibiotic usage in hospitals serving an adult population [Reference Polk13]. However, DDD should be relatively accurate in comparing antibiotic usage within a single centre compared to between different centres whose patient populations may have varying rates of renal and hepatic disease requiring dose adjustment. Moreover, our usage of ertapenem may be somewhat atypical relative to other institutions, since, throughout the duration of the study, we had a relatively low incidence of Pseudomonas as a causative agent of hospital-acquired and healthcare-associated pneumonia, which allowed us to use ertapenem in the empiric treatment of nosocomial pneumonia in patients who were not critically ill.
Ultimately, we demonstrated that widespread adoption of ertapenem at our institution resulted in decreased usage of piperacillin-tazobactam and ampicillin-sulbactam, with a mostly unchanged to improved antibiotic susceptibility pattern in nosocomial Enterobacteriaceae and Pseudomonas following ertapenem's introduction, with the possible exception of ceftriaxone susceptibility in Enterobacteriaceae. Furthermore, we have demonstrated the usefulness of interrupted time-series analysis as a tool to monitor the impact of antibiotic formulary changes or other hospital epidemiology interventions on antibiotic usage patterns and susceptibility rates.
ACKNOWLEDGEMENTS
This project was supported by a grant from Merck Pharmaceuticals.
DECLARATION OF INTEREST
C.J.G. has received an unrestricted educational grant from Ortho-McNeil-Janssen and has served on an advisory board for Pfizer.









