It is well documented that the ingredients in soft drinks contribute to inflammation-related disorders such as insulin insensitivity and metabolic defects( Reference Gaby 1 , Reference Schulze, Hoffmann and Manson 2 ). For instance, glucose-containing carbonated soft drinks increase carbonyl stress burden( Reference Nakayama, Nakayama and Terawaki 3 ), which, in turn, may result in a decrease in antioxidant concentration in oral saliva( Reference Sculley and Langley-Evans 4 ) and enhance oxidative nitration in association with the inflammatory reaction( Reference Takahama, Imamura and Hirota 5 ).
From the biological viewpoint, the inflammatory reaction caused by such oxidative stress is highly associated with systemic diseases, such as metabolic syndrome, diabetes, hypertension, asthma and periodontal disease (PD)( Reference Southerland, Taylor and Moss 6 – Reference Pietropaoli, Monaco and Del Pinto 16 ). Oxidative stress( Reference Schulze, Hoffmann and Manson 2 , Reference Nakayama, Nakayama and Terawaki 3 , Reference Southerland, Taylor and Moss 6 , Reference Preshaw, Alba and Herrera 8 , Reference D’Aiuto, Nibali and Parkar 9 , Reference Bullon, Morillo and Ramirez-Tortosa 13 – Reference Allen, Matthews and O’Connor 15 ) pertaining to PD may be mediated through advanced glycation end-products that are created through non-enzymatic pathways from monosaccharide substances, dicarbonyls originating from the Maillard reaction, sugar self-oxidation and other molecular pathways( Reference Pietropaoli, Monaco and Del Pinto 16 , Reference Howard and Wylie-Rosett 17 ).
Several studies show a link between soft drinks and systemic disease( Reference Eshak, Iso and Kokubo 18 , Reference Shi, Dal Grande and Taylor 19 ), but the specific association between the intake of soft drinks and PD has not been investigated yet. From the aspect of epidemiology, it is therefore of interest to assess the association between the intake of soft drinks and PD through a large-scale epidemiological study. The main difficulty of conducting such an epidemiological study is that the main consumers of soft drinks are children and young adults, but severe PD is most prevalent in the elderly. The dietary habits observed in the elderly may be altered due to physical function degradation such as severe tooth loss. To shed light on the impact of the intake of certain nutrients on the development of PD, it may not be appropriate to target the elderly. Another reason for not aiming at the elderly is that the WHO has recommended monitoring periodontal conditions in individuals aged 35–44 years( 20 ) for early detection of PD.
A community-based PD screening project, one of the components of the Keelung Community-based Integrated Screening (KCIS) programme( Reference Chen, Chiu and Luh 21 ), was initiated in 2003 and lasted until 2009. The assessment of PD using the community periodontal index (CPI) and loss of attachment (LA) measures was completed among individuals aged 35–44 years( Reference Lai, Lo and Wang 22 ). Because these community-based data also included information on sociodemographic variables (including age, sex and education level), dietary factors (including the intake frequencies of soft drinks, fruits and others), lifestyle factors (including cigarette smoking and teeth-brushing) and variables related to the inflammatory reaction (such as BMI, fasting plasma glucose and white blood cell level), we could assess the association between the intake frequency of soft drinks and the risk for PD making allowance for these established risk factors.
By using these community-based data focusing on a middle-aged population-based cohort, we sought to test a dose–response relationship between the frequency of the intake of soft drinks and PD measured by both PD indices (CPI and LA) after adjusting for other confounding factors.
Materials and methods
Data source
A dental check-up for PD in 2003 was included in the KCIS programme, which is a large population- and community-based screening programme in Keelung, the northernmost city of Taiwan. The KCIS programme was a screening project to detect five types of neoplasm (cervical, breast, colorectal, oral and hepatocellular) and three chronic diseases (hypertension, diabetes and hyperlipidaemia). The KCIS programme was initiated in 1999 and details about its implementation are described in full elsewhere( Reference Chen, Chiu and Luh 21 ). All of the KCIS participants had signed informed consent. The KCIS programme was approved by the local health committee of the Keelung health authority, reviewed by the Ethics Committee of Chang Gung Memorial Hospital, and finally later approved by the Institutional Review Board (issued number 103-3920B).
A total of 10 213 participants among 12 208 invitees aged 35–44 years were enrolled to assess the severity of PD measured by the CPI and LA. The participation rate was 83·7 %. We only used the most recent and accessible data from 2005 to 2009, because some questions related to dietary behaviour in the questionnaire were revised. We used the result of the first dental check-up in the following analysis.
Measurement of community periodontal index and loss of attachment
The present study used two types of measurements, the CPI and the LA, to assess PD. The former shows the current status of PD and the latter reflects the long-term cumulative gum damage due to PD. The screening procedure for PD was described in detail previously( Reference Lai, Lo and Wang 22 ). In brief, the CPI was assessed using a purpose-built CPI (or WHO) probe. This tool has a 0·5 mm diameter ball at its tip, a black band between 3·5 and 5·5 mm, and coloured rings at 8·5 and 11·5 mm from the tip. The scores of CPI reflecting current periodontal status are as follows: 0 for healthy periodontium; 1 for gingival bleeding; 2 for calculus; 3 for a 4–5 mm periodontal pocket; and 4 for a 6 mm or deeper periodontal pocket. LA was assessed using the cementoenamel junction as the reference point for measuring the extent of attachment loss with scores listed as follows: 0 for 0–3 mm; 1 for 4–5 mm; 2 for 6–8 mm; 3 for 9–11 mm; and 4 for 12 mm.
The mouth was divided into sextants, and the highest of the component scores for each sextant was taken to represent the overall sextant score. The highest of the sextant scores for each individual was selected to be representative of his/her overall PD status. All of the thirty-five dentists included in the programme had been trained by a senior periodontist (the second author of the present study). The calibration for both CPI and LA was checked by having a number of dentists perform repeated assessments on the same twenty-five individuals. The weighted kappa values for intra-examiner variability were 0·55–0·61 for CPI and 0·67–0·80 for LA, indicating a moderate to good agreement between dentists. The equivalent figures for inter-examiner variability were 0·42–0·44 for CPI and 0·41–0·57 for LA, which again indicate moderate agreement( Reference Lai, Lo and Wang 22 ).
Measurement of explanatory factors
Information on demographic characteristics, education level, lifestyle, dietary habits and medical history was collected through face-to-face interview using a structured questionnaire administered by public health nurses or trained volunteers. For biochemical variables, 12 h fasting blood samples were drawn at recruitment. Fasting plasma glucose and white blood cell levels were derived from a blood sample after at least an 8 h fast. Hyperglycaemia included impaired fasting glucose and diabetes. The former was defined as having fasting plasma glucose level ≥100 mg/dl but <126 mg/dl, and the latter was defined as fasting plasma glucose ≥126 mg/dl or having a history of diabetes. The question for the intake of soft drinks was ‘How many times have you had the intake of soft drinks (including carbonated beverage, cola, milk tea, and juice or asparagus juice) per week over the past half a year?’ The frequency of the intake of soft drinks was classified into ≤2, 3–4 or ≥5 times/week. The frequency of intake of fruits was classified into ≤2, 3–4 or ≥5 times/week. Cigarette smoking was classified as ever having smoked or never having smoked. The former included current smokers and people who had quit smoking. Having the habit of brushing one’s teeth was defined as brushing teeth both day and night. Height and weight were also collected, and BMI was calculated as weight/height2.
Statistical analysis
The associations between putative risk factors and PD were assessed using a logistic regression model. The estimates of crude and adjusted odds ratios regarding the associations between the intake of soft drinks and PD according to the two indices, CPI≥3 and LA≥1, were obtained from a univariate analysis and a multivariate analysis with adjustment for other covariates, respectively. The statistical significance of each variable was tested by the likelihood ratio test. The original classification of soft drink consumption was five categories, including none or seldom, 1–2 times/week, 3–4 times/week, 5–6 times/week and ≥7 times/week. The descriptive results showed that these five levels could be reduced to three according the distribution of PD prevalence and also considering the sparse numbers in the two tails of the distribution of soft drink consumption. By combining none or seldom with 1–2 times/week, and also 5–6 times/week with ≥7 times/week, three levels were used as follows: ≤2 times/week (including none or seldom, 1–2 times/week), 3–4 times/week and ≥5 times/week (including 5–6 times/week and ≥7 times/week). A trend test was employed to assess whether there was a dose–response relationship between the frequency of the intake of soft drinks treated as an ordinal variable (1 for ≤2 times/week; 2 for 3–4 times/week; 3 for ≥5 times/week) and the risk for PD. Missing values were excluded from the analysis. The number of missing values based on information from Table 1 was 191, about 2 % (191/10 213), for the intake of soft drinks.
Table 1 Prevalence of periodontal disease defined by CPI and LA score among Taiwanese adults aged 35–44 years, Keelung Community-based Integrated Screening programme, 2005–2009
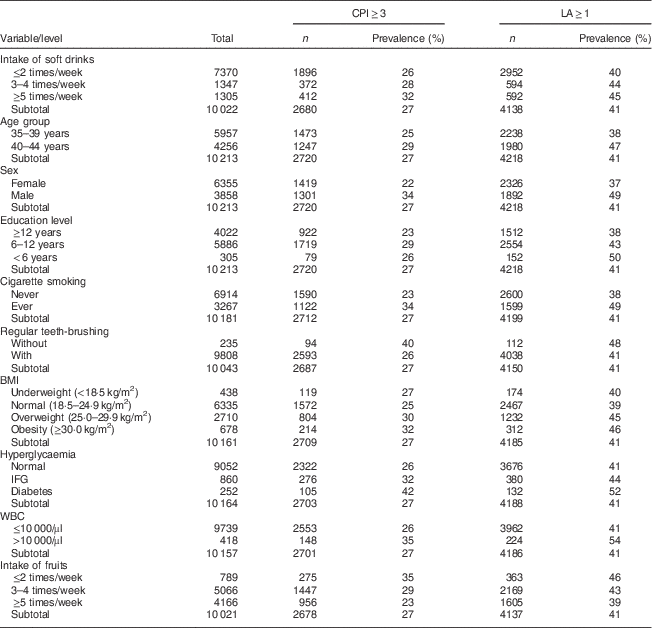
CPI, community periodontal index; LA, loss of attachment; IFG, impaired fasting glucose; WBC, white blood cell.
The statistical significance level was set at P<0·05. All statistical analyses were conducted using the statistical software package SAS version 9·2.
Results
Frequency of covariates by periodontal disease
Table 1 shows the prevalence of CPI and LA by the intake of soft drinks and other explanatory factors. The prevalence of PD increased with the intake frequency of soft drinks, regardless of whether the analysis used CPI≥3 or LA≥1 as the outcome. A higher prevalence was also found in the older age group, males, those with lower education level, ever smokers, those with irregular teeth-brushing, hyperglycaemia, elevated white blood cell level (>10 000/µl) and less intake of fruits.
Association between the intake of soft drinks and periodontal disease (defined as community periodontal index ≥3)
Table 2 shows the associations of the intake of soft drinks and other variables with PD (defined as CPI≥3). Compared with infrequent intake of soft drinks (≤2 times/week), the crude odds indicating the elevated risk for PD resulting from increasing frequency of soft drink intake were 10 % (OR=1·10; 95 % CI 0·97, 1·25) for 3–4 times/week and 33 % (OR=1·33; 95 % CI 1·17, 1·51) for ≥5 times/week, respectively, in the univariate analysis (P<0·01 by trend test). The corresponding adjusted OR in the multivariate analysis increased from 1·05 (95 % CI 0·92, 1·20) for the frequency of 3–4 times/week to 1·17 (95 % CI 1·03, 1·34) for ≥5 times/week compared with infrequent intake of soft drinks (≤2 times/week; P=0·02 by trend test).
Table 2 Association between the intake of soft drinks and periodontal disease (defined as CPI≥3) among Taiwanese adults aged 35–44 years, Keelung Community-based Integrated Screening programme, 2005–2009
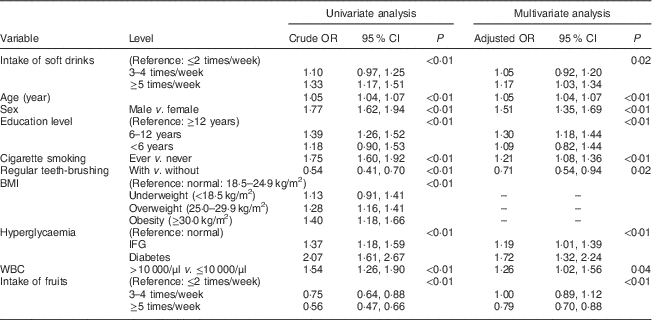
CPI, community periodontal index; WBC, white blood cell; IFG, impaired fasting glucose.
Association between the intake of soft drinks and periodontal disease (defined as loss of attachment ≥1)
Table 3 shows the associations with the intake of soft drinks and other variables responsible for the risk for PD (defined as LA≥1). Compared with the infrequent intake of soft drinks (≤2 times/week), the crude odds of the intake of soft drinks 3–4 times/week and ≥5 times/week led to 18 % (OR=1·18; 95 % CI 1·05, 1·33) and 24 % (OR=1·24; 95 % CI 1·10, 1·40) elevated risk for PD, respectively, in the univariate analysis (P<0·01 by trend test). After adjusting for other covariates, the corresponding adjusted OR in the multivariate analysis were 1·17 (95 % CI 1·03, 1·32) and 1·14 (95 % CI 1·01, 1·29), respectively (P<0·01 by trend test).
Table 3 Association between the intake of soft drinks and periodontal disease (defined as LA≥1) among Taiwanese adults aged 35–44 years, Keelung Community-based Integrated Screening programme, 2005–2009
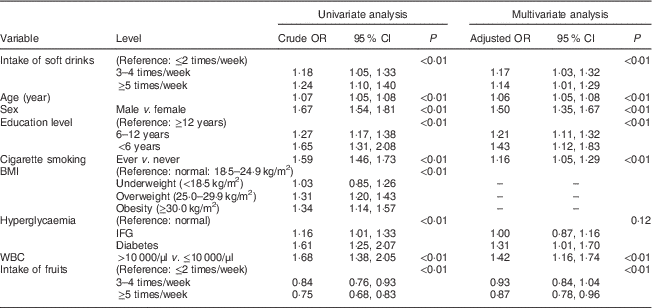
LA, loss of attachment; WBC, white blood cell; IFG, impaired fasting glucose.
Other significant factors related to periodontal disease
Table 2 shows other relevant factors associated with the risk for PD (defined as CPI≥3). Only those variables with statistically significant associations were kept in the final multivariate analysis. An incremental increase in age of one year led to a 5 % elevated risk for PD. Males were more susceptible to PD than females by 51 %. People with a lower education level were more likely to have PD. There was a 30 % increase for those with 6–12 years of education and a 9 % increase for <6 years of education compared with those with ≥12 years of education (P<0·01). Those who had smoked were at increased risk for PD compared with those who had never smoked by 21 %. Regular teeth-brushing reduced the risk for PD by 29 %. Increased risk for PD was noted for those with impaired fasting glucose by 19 % and for those with type 2 diabetes by 72 %. The risk for PD was also elevated for higher white blood cell level by 26 %. There was no difference in the prevalence of PD between those consuming fruit ≤2 times/week and those consuming it 3–4 times/week; however, the prevalence of PD was somewhat lower for people consuming fruit ≥5 times/week in the multivariate analysis.
Similar findings were noted for the LA outcome measure as shown in Table 3 but the effect sizes were smaller compared with the CPI outcome, except for age. Hyperglycaemia (including impaired fasting glucose and diabetes) was not statistically significant but as the 95 % CI of the adjusted OR for diabetes compared with normal did not include 1, we therefore kept this variable in the final multivariate model. Besides, teeth-brushing was not statistically significant (data not shown).
Discussion
Impact of soft drinks on periodontal disease
In the current study, we investigated the association between the intake of soft drinks and the risk for PD using two indices (CPI and LA) among Taiwanese middle-aged adults based on a large population- and community-based screening programme in Keelung City, the northernmost city of Taiwan. The dose–response relationship results support the fact that increasing soft drink intake was associated with the risk for PD, regardless of whether the CPI or LA index was used, after adjusting for hyperglycaemia and other relevant confounding factors, although the causal relationship still needs to be verified.
While the mechanism linking soft drink consumption and PD is not known, it is possible that oxidative stress( Reference Southerland, Taylor and Moss 6 – Reference Preshaw, Alba and Herrera 8 , Reference Sculley and Langley-Evans 4 , Reference Marchetti, Monaco and Procaccini 23 ) or bone mineralization( Reference Inagaki, Kurosu and Yoshinari 24 – Reference Esfahanian, Shamami and Shamami 28 ), either alone or in combination, is involved. More data will be required to verify this.
The influence of other confounding factors on periodontal disease
Hyperglycaemia was the strongest risk factor for PD with outcome of CPI≥3. The association with BMI was confounded by other covariates in the multivariate analysis. However, overweight remains a possible risk factor for systemic disease because adipocytokines, which are produced by fat cells, might modulate the balance between oxidant and antioxidant activities( Reference Sørensen, Raben and Stender 29 ). A previous study also found that overweight subjects with high consumption of sugar-sweetened foods and drinks had an increase in two inflammatory markers: haptoglobin and transferrin( Reference Sørensen, Raben and Stender 29 ).
Smoking was associated with PD in the present study. This finding supported the point proposed by Colombo et al.( Reference Colombo, Dalle-Donne and Orioli 30 ) that oxidative stress caused by smoking would damage the oral cavity tissue. The association with teeth-brushing was significant when the CPI but not LA was the outcome. This finding implies that the association between frequent intake of soft drinks and the current status of PD, indicated by the CPI, may be amenable to treatment, but the long-term, cumulative, adverse effect indicated by the LA measure may not.
Although we had also collected data on other dietary items including the intakes of vegetables, meat, coffee, milk and tea in this community-based cohort, these dietary factors are not shown partly because only the intake of fruits was still associated with PD in the multivariate analysis and partly because the intake of fruits was more relevant owing to their high sugar content compared with other foods. The intake of fruits showed a negative association with PD but it did not confound the effect of soft drinks on PD. In-depth discussions on these non-significant dietary items are beyond the scope the current study.
Application to health education and policy
Consistent with the previous evidence that the negative health outcomes of soft drink consumption play an important role not only in obesity, diabetes and CVD( Reference Howard and Wylie-Rosett 17 , Reference Eshak, Iso and Kokubo 18 , Reference Odegaard, Koh and Arakawa 31 , Reference Ogden, Kit and Carroll 32 ) but also in oral health( Reference Tahmassebi, Duggal and Malik-Kotru 33 ), the current study further demonstrated a significant dose–response relationship between the intake of soft drinks and PD among Taiwanese middle-aged adults.
These epidemiological findings are very meaningful for the health of Taiwanese middle-aged adults who are frequent consumers of soft drinks as they are not aware of such a threat. Therefore, the intake of soft drinks should be reduced through specific health promotion programmes to prevent the progression rates of those related systemic diseases.
Accordingly, appropriate public health strategies that target individual, environmental and policy levels are recommended to increase public awareness of the negative impact of soft drink consumption on health.
Methodological considerations
There are several strengths of the present study. In addition to a large population-based sample, the study was targeted at middle-aged adults. As mentioned before, dietary habits may be altered because of systemic diseases when ageing. Collecting biomarkers, lifestyle characteristics and disease history simultaneously renders the elimination of confounding factors possible. More importantly, two indices, the CPI reflecting current status and the LA reflecting cumulative damage, were measured.
There are also several limitations. First, we used the most common cut-off point of CPI≥3 to classify PD. When treating the CPI as an ordinal outcome with 0 to 4, the association between the intake of soft drinks and CPI was still statistically significant in the univariate analysis with an ordinal logistic regression model, similar to the results from a binary logistic regression model with the dichotomized CPI with <3 and ≥3, with OR of 1·10 and 1·33 for the intake of soft drinks 3–4 times/week and ≥5 times/week, respectively. However, the proportional odds assumption was violated when adjusting for other covariates in the multivariate ordinal logistic regression model treating CPI as an ordinal outcome. Therefore, we reported the results based on the dichotomized classification for CPI. Second, there is no information on whether soft drinks were sweetened with sugar or otherwise and this would have to be one of the possible reasons for an association between soft drinks and various adverse outcomes. In addition, the possibility exists that soft drink consumption is associated with other dietary patterns that contribute to PD but we do not have the data to explore. Third, the current study was based on an epidemiological study and lacked direct evidence, such as the concentration of inflammatory biomarkers, to support whether the association of soft drink intake with PD was mediated through the pathway of increasing the inflammatory reaction. Only the white blood cell level can be considered as an inflammatory biomarker in our study. Having more inflammatory biomarker data would help us determine whether the association observed was mediated through inflammation. Some variables such as hyperglycaemia and BMI have been associated with inflammation in other studies( Reference Southerland, Taylor and Moss 6 , Reference Preshaw, Alba and Herrera 8 , Reference Bullon, Morillo and Ramirez-Tortosa 13 – Reference Pietropaoli, Monaco and Del Pinto 16 , Reference Marchetti, Monaco and Procaccini 23 , Reference Sørensen, Raben and Stender 29 ). The fact that the soft drink–PD relationship persisted after adjustment for these variables may in some part be due to them not accounting completely for inflammation, although there may well be other mechanisms as well. This finding may suggest there is another pathway leading to PD that is independent of the inflammatory reaction.
Regarding the representativeness of our finding, it is unsure whether the study sample can be representative of the general public aged 35–44 years in Taiwan as we do not have the nationwide population data. However, the results are representative of the underlying residents in Keelung City as this is a community-based survey through a community-based screening that almost covers the entire eligible study population in the whole city.
Conclusions
A dose–response relationship between the frequency of the intake of soft drinks and PD was observed in Taiwanese middle-aged adults. Such evidence could be used in health promotion to support reductions in soft drink intake.
Acknowledgements
Acknowledgements: The authors thank all of the personnel of the Health Bureau of Keelung City, Keelung City, Taiwan for contributing to the KCIS programme. This article was identified as KCIS no. 33. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: H.-H.C., J.C.-Y.F. and H.L. designed the research; J.C.-Y.F., S.Y.-H.C., A.M.-F.Y. and S.L.-S.C. conducted the research; H.L. provided essential materials; J.C.-Y.F., S.Y.-H.C., A.M.-F.Y. and S.L.-S.C. analysed the data; J.C.-Y.F. and H.-H.C. wrote the paper; H.-H.C. had primary responsibility for final content. All authors read and approved the final manuscript. Ethics of human subject participation: The KCIS programme was approved by the local health committee of the Keelung health authority, reviewed by the Ethics Committee of Chang Gung Memorial Hospital, and approved by the Institutional Review Board (issued number 103-3920B). All KCIS participants provided signed informed consent.






