INTRODUCTION
Three to five billion cases of diarrhoea occur annually, with about 2·6 million diarrhoea-related deaths worldwide, of which 1·5 million deaths occur in children aged <5 years [Reference Lima and Guerrant1]. Global estimates indicate that each year, more than 120 million cases of gastrointestinal diseases are caused by swimming and bathing in wastewater-polluted coastal waters [Reference Shuval2]. Thus, monitoring aquatic systems for faecal pollution is an important task. Escherichia coli is an abundant and normally harmless representative of the normal mammalian gut microflora. However, certain strains have virulent properties that cause a variety of gastrointestinal infections depending on specific arrays of pathogenic determinants, most of which are believed to be acquired by lateral gene transfer [Reference Pupo3]. It has been suggested that the evolution of E. coli as a gastrointestinal pathogen is comparatively recent [Reference Pupo3] and that it does not originate from a single ancestor. The physicochemical parameters greatly differ between primary (intestine) and secondary (extra-intestinal) habitats, necessitating adaptation of the discharged cells to the extra-intestinal environment. The differences in the selective forces acting in these two different environments have led to the evolution of a dual regulatory system in E. coli, which may also involve the emergence of distinct pathotypes [Reference Shanta4]. Virulent E. coli strains are now considered as major diarrhoeagenic pathogens and are classified into several virulotypes according to their virulence mechanisms [Reference Scott and Kaper5], e.g. enteropathogenic (EPEC), enteroaggregative (EAEC), enterotoxigenic (ETEC), enteroinvasive (EIEC), and enterohaemorrhagic (EHEC) E. coli strains. Diarrhoeagenic E. coli strains were among the first pathogens for which molecular diagnostic methods were developed [Reference Nataro and Kaper6]. Multiplex PCR amplification has been adopted to detect almost all of these virulotypes in a single reaction by using primer pairs that target specific virulence genes and yield PCR products of different sizes that can be distinguished by gel electrophoresis [Reference Aranda, Fagundes-Neto and Scaletsky7]. In this study, we used multiplex PCR for environmental monitoring of E. coli virulotypes in aquatic systems in Bangladesh. The distribution of different E. coli virulotypes in surface water and their temporal fluctuations were observed during three successive seasons, which could help in assessing the seasonal risk for communities that are vulnerable to waterborne diseases as well as in understanding the environmental factors driving these fluctuations.
MATERIALS AND METHODS
Sample collection
Water samples were collected from 46 different natural aquatic locations in Bangladesh between December 2009 and January 2010 (winter season), in April 2010 (summer season) and in July 2010 (rainy season). The sampling sites included 35 freshwater and 11 marine/brackish sites covering a large part of the country (Supplementary Fig. S1). The sampling sites were chosen so that they included major rivers and sea beaches located in regions with higher human activity, economic importance, and tourist interest. The sites were divided into different groups as indicated in Supplementary Table S1, e.g. tourism sites at or near sea beaches or freshwater sites, highly populated commercial areas, industrial city locations, and sites at remote villages at big rivers. Physicochemical parameters, e.g. the temperature of, salinity of, conductivity of, and total dissolved solids (TDS) in the water samples, were measured by a sensION5 conductivity meter (Hach Company, USA), and pH and dissolved O2 were measured by a CyberScan pH/DO Meter (Eutech Instruments, Singapore). The GPS coordinates were recorded by an eTrex Legend HCx personal navigator (Garmin Corporation, Taiwan). All the parameters were measured on site and are listed in Supplementary Table S1.
Enrichment
Sixty millilitres of each water sample were added to 20 ml of concentrated pre-enrichment (PE) broth to achieve the following final concentrations (pH 7): Lab-Lemco powder, 0·5 g/l; yeast extract, 1·0 g/l; peptone, 2·5 g/l; and NaCl, 2·5 g/l. After 8 h incubation at ambient temperature, 1 ml of pre-enriched culture was transferred to 50 ml of two different enrichment broths containing peptone (3 g/l), NaCl (1 g/l), K2HPO4 (1 g/l) and KH2PO4 (1 g/l) at pH 7, supplemented either with 0·15% bile salts (BSE) or with 50 μg/ml streptomycin (SE). The enrichment cultures were incubated for 16 h at 37°C with shaking at 120 rpm.
PCR template preparation
DNA was extracted from pellets collected from direct water filtrate, from PE culture, BSE culture, and SE culture of each sample as described previously [Reference Faruque8]. DNA extracted from direct water filtrate was further purified using the GenElute™ Bacterial Genomic DNA kit (Sigma-Aldrich, Germany).
Multiplex PCR
Detection of virulence marker genes was performed by multiplex PCR [Reference Nguyen9, Reference Toma10] with nine primer pairs (Table 1) targeting the following virulence-related genes: eaeA (intimin of EHEC and EPEC strains), bfpA (bundle-forming pilus of EPEC strains), vt1 and/or vt2 (Shiga toxins 1 and 2 of EHEC strains), eltB and/or estA (enterotoxins of ETEC strains), ial (invasion-associated locus in EIEC and Shigella spp.), ipaH (in the large invasive plasmid and chromosomes in Shigella spp. and EIEC strains), and pCVD (EcoRI–PstI DNA fragment of pCVD432 of EAEC strains). Multiplex PCR was performed in three different sets by choosing primer pairs that generated PCR products that had different sizes and could easily be separated and distinguished by agarose gel electrophoresis [Reference Bier20]. PCR was performed in a 20-μl reaction mixture containing 1 μl template DNA, 0·2 μl of 2 U/μl DyNAzyme™ DNA polymerase, 2 μl of 10 × buffer for DyNAzyme, 0·4 μl of a mixture of deoxynucleoside triphosphates (25 mm each), and 0·5 μl of 25 μ m solution of each primer (Sigma-Aldrich, Germany). The thermocycler conditions used with a PTC 200 Peltier thermal cycler (MJ Research, USA) were as follows: initial denaturation at 96°C for 4 min, 30 cycles at 94°C for 60 s, 55°C for 60 s, and 72°C for 60 s, with a final extension at 72°C for 7 min. PCR products (10 μl) were evaluated by electrophoresis on a 1·5% (w/v) agarose gel at 70 V for 30 min. The DNA bands were visualized and photographed under a UV illuminator (Bio-Rad, USA) after the gel was stained with ethidium bromide (Supplementary Fig. S2).
Table 1. List of oligonucleotide primers used in the multiplex PCR assay

To determine the detection limit of the multiplex PCR, a series of mixed cultures were prepared with cell ratios (determined in terms of c.f.u./ml) ranging from 1:1 to 1:1013 (with tenfold dilutions) of an avirulent laboratory control strain (E. coli DH5α) and two virulent laboratory strains designated as MMLA (possessing vt1 and vt2) and B171 (possessing bfpA). DNA was extracted and multiplex PCR was performed in the same way as for the test DNA samples. The sensitivity of the assay was defined as the lowest ratio of diarrhoeagenic to non-diarrhoeagenic E. coli that yielded a positive result. In the case of bfpA and vt1, a minimum ratio of 1:103 for diarrhoeagenic to non-diarrhoeagenic E. coli was required for a positive PCR result, while the minimum ratio for vt2 was 1:104 (Supplementary Fig. S3).
Data analysis
A χ2 test was used to assess the significance of the data. The relative standard used in the research was P > 0·05.
RESULTS
Prevalence of virulence genes of diarrhoeagenic E. coli
Forty-six sampling sites covering a large part of Bangladesh were monitored for the presence of diarrhoeagenic E. coli in three consecutive seasons. Four different DNA templates, i.e. from direct water filtrate, PE culture, BSE culture, and SE culture of each water sample, were analysed to detect the presence of pathogenic genes. Using three sets of multiplex PCR assays, we analysed the distribution and seasonal fluctuations of nine different pathogenic genes possessed by five different E. coli virulotypes. The detailed results of the analysis have been given in Supplementary Table S2 and summarized in Supplementary Table S3. The use of a number of PE and enrichment media designed for this experiment enabled enrichment of different virulotypes of E. coli. The PE medium was designed to resuscitate stressed or injured environmental bacteria, while the BSE medium was designed to enrich the enteric bacterial population, and streptomycin was added for enrichment of streptomycin-resistant E. coli. The following data were pooled from Supplementary Table S2 and have been compiled in Supplementary Table S4. A total of 138 DNA samples were evaluated per enrichment. No genes could be amplified from DNA extracted from direct water filtrate. Both eltB and vt1 genes were mostly detected in PE cultures; 72 PE cultures were positive for eltB, but only 41 BSE cultures and 26 SE cultures were found to harbour this gene. Similarly, five PE cultures were positive for the vt1 gene, but only three BSE cultures and two SE cultures were positive for this gene. Bile salt enabled enrichment for E. coli harbouring the estA, vt2, and eaeA genes. The estA gene was detected in 18 BSE cultures, 11 PE cultures, and 10 SE cultures. The vt2 gene was detected in nine BSE cultures but only in two PE cultures and one SE culture. The eaeA gene was detected in 45 BSE cultures but only in 20 PE cultures and 11 SE cultures. SE enrichment medium successfully enriched EAEC and EIEC virulotypes in the water samples. The pCVD gene was detected in nine SE cultures, one BSE culture, and none of the PE cultures. The ipaH gene was detected in 24 SE cultures, eight PE cultures, but in none of the BSE cultures, indicating that bile salt suppresses EAEC and EIEC virulotypes. The presence of different virulence-associated genes in the three different enrichments was used to identify virulotypes harboured by a particular sample in a season.
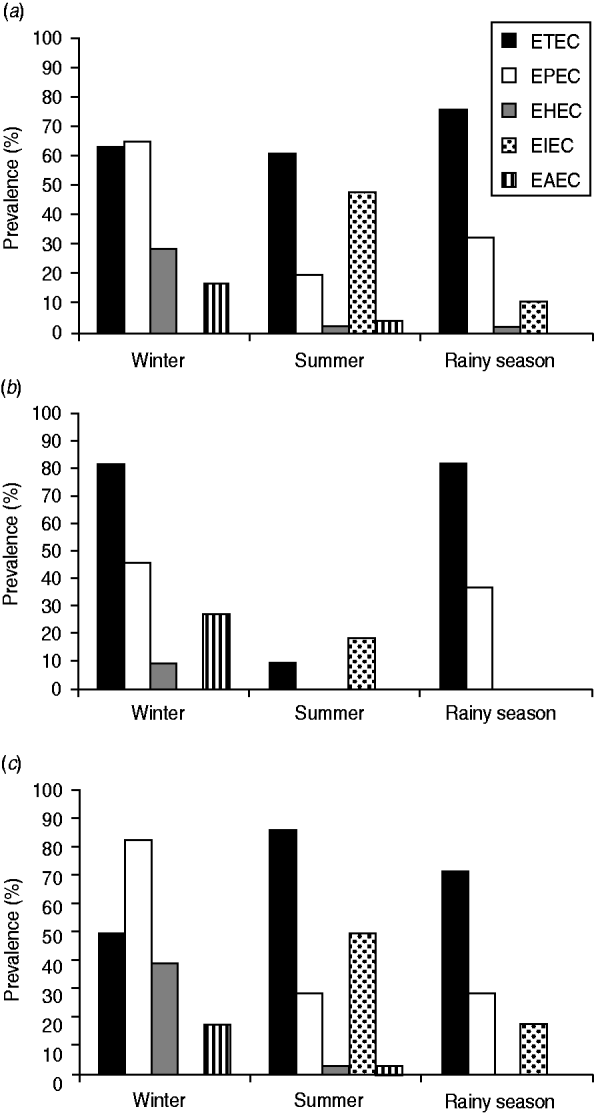
In this investigation, ETEC was found to be the most prevalent virulotype among the five diarrhoeagenic E. coli virulotypes tested (Fig. 1a, Supplementary Table S3) from the sampling sites. The prevalence of the ETEC virulotype in winter, summer, and the rainy season was 63%, 61%, and 76%, respectively. Of the ETEC-containing samples, eltB was present in most cases, i.e. in an average of 60% of the sampling sites (Supplementary Table S3). estA was more prevalent during winter (34%) than during summer (8·6%) and the rainy season (6·5%). In most cases, estA was present along with eltB; only 4% of the sampling sites harboured estA alone (Supplementary Table S3). A total of six sites tested positive for estA alone, but with seasonal variations; sites 4 and 9 in winter, sites 7 and 8 in the rainy season and sites 10 and 24 in summer. Except for site 24, all these sites were adjacent to southern sea beaches with marine or brackish salt concentrations.

Fig. 1. The prevalence (in terms of proportion of positive sampling sites) of five different diarrhoeagenic E. coli virulotypes in the aquatic system of Bangladesh in three successive seasons. (a) Data compiled for all 46 sites, (b) in 11 marine sites, (c) in 35 freshwater sites.
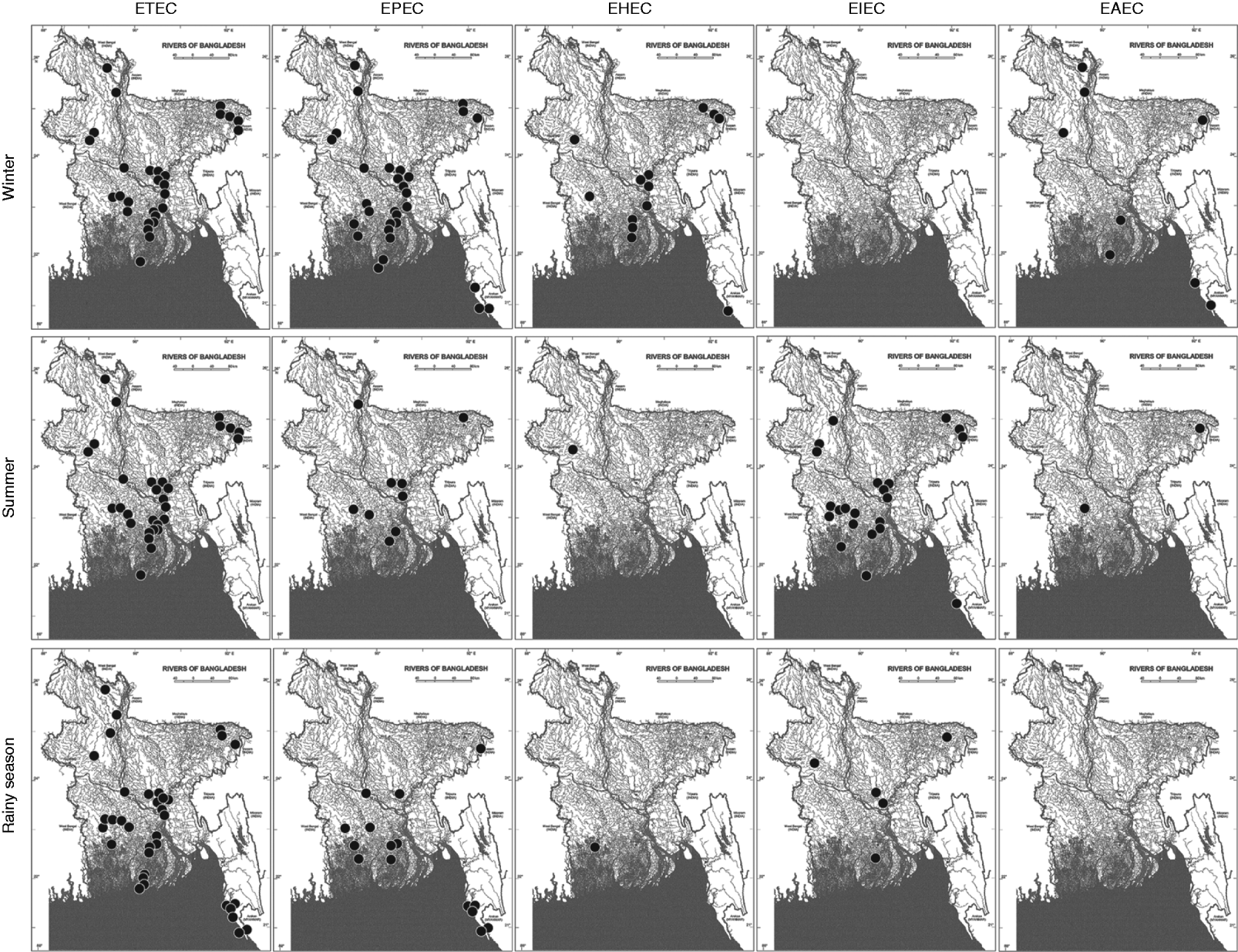
EPEC was the second most prevalent type found. The prevalence of EPEC was 65% in winter, which was greater than that of ETEC in that season. In summer and the rainy season, only 19% and 32% of the sampling sites, respectively, showed positive results for the EPEC virulotype (Fig. 1a, Supplementary Table S3). In all the EPEC-positive sites, only the eaeA (no bfpA) gene was detected. The eaeA signal could also be due to the presence of EHEC strains. To aid interpretation, all eaeA-positive samples were considered as EPEC-positive samples, and vt1- and/or vt2-positive samples were considered as EHEC-positive samples, regardless of the presence of eaeA. Like EPEC, the prevalence of EHEC (28%) and EAEC (17%) was also higher in winter (Fig. 1a). In summer, the prevalence of EHEC and EAEC was 2% and 4%, respectively, and in the rainy season, the prevalence of EHEC was also 2% but EAEC was absent. Unlike the other virulotypes, EIEC was relatively more prevalent in summer (48%). In the rainy season, EIEC was found in 11% of the sampling sites and was absent in winter. Although EPEC and ETEC virulotypes were almost evenly distributed throughout the country, we observed regional distribution patterns for certain virulotypes with distinct temporal variations (Fig. 2).

Fig. 2. The regional and temporal distribution patterns of five different diarrhoeagenic E. coli strains in three seasons in the aquatic system of Bangladesh. The black circles on the map of Bangladesh designate the presence of different diarrhoeagenic E. coli virulotypes in the corresponding geographical location. (Source: Banglapedia database [34], reproduced with permission.)
Significant differences were observed in the virulotype prevalence in freshwater and marine samples (P > 0·05). In marine samples (Fig. 1b), the ETEC virulotype was found to be the most prevalent virulotype both in winter (82%) and in the rainy (82%) season, but in summer its prevalence dropped to 9%. EPEC was the second most prevalent virulotype in winter (46%) and in the rainy (37%) season, but it could not be detected in summer. EHEC and EAEC virulotypes were detected only in winter, in 9% and 28% of the sampling sites, respectively, and EIEC was detected only in summer in 19% of the sites. In freshwater sites (Fig. 1c), the most prevalent virulotype was the ETEC virulotype, both in summer (86%) and the rainy (72%) season, but in winter the EPEC virulotype was the most prevalent virulotype (83%). Similar to the results for the marine samples (Fig. 1b), EIEC was also prevalent in freshwater samples (Fig. 1c) only in summer, but it had higher prevalence in freshwater samples (50%) than in marine samples (19%). Neither the EHEC nor EAEC virulotype was detected in the rainy season in the freshwater samples (Fig. 1c). In summer, the prevalence of EHEC and EAEC virulotypes in freshwater samples was still low (4%), but it increased to 29% and 18%, respectively, in winter.
Effect of seasonal variations on water quality and virulotype prevalence
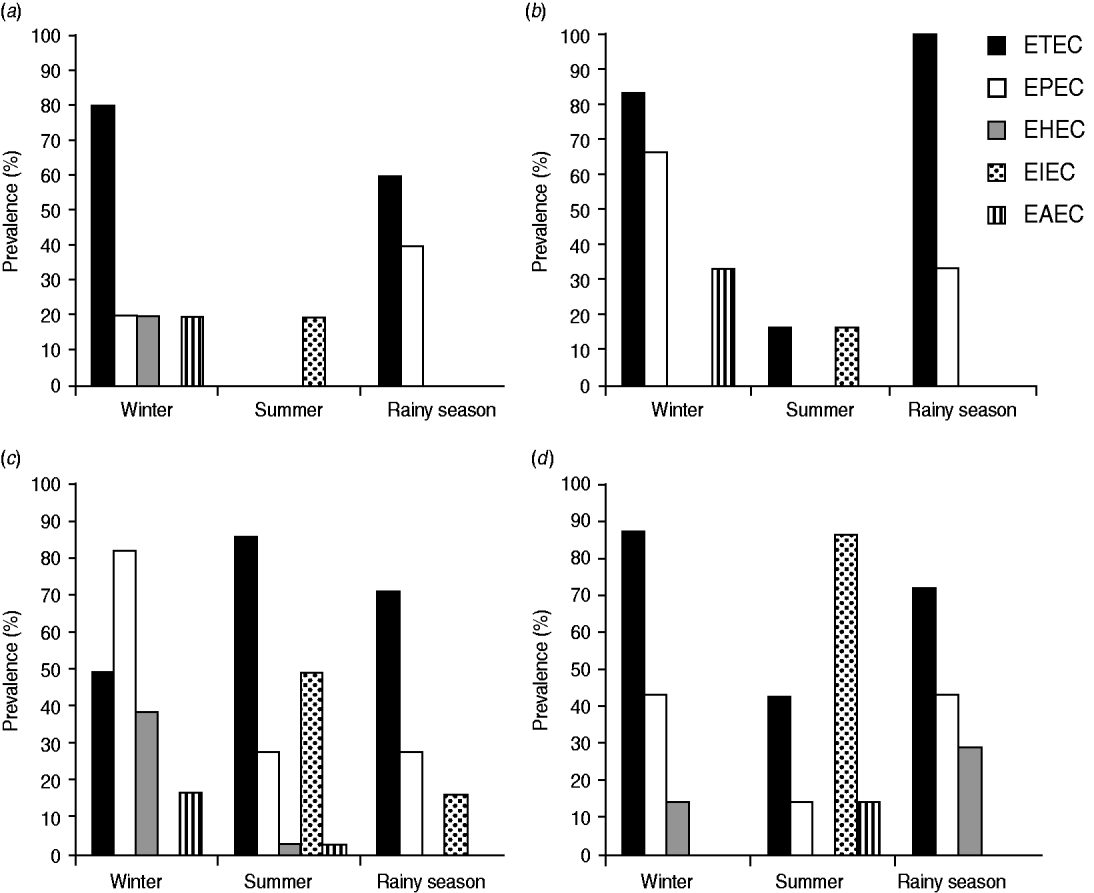
Seasonal variations were found to affect the physicochemical parameters of natural water bodies in Bangladesh (Supplementary Table S1). Water temperature varied between seasons. In winter, the temperature ranged between 16°C and 25°C. Water temperature minutely differed between summer and the rainy season and ranged between 26°C and 32°C. The salinity of the water changed peculiarly with seasons in both fresh and marine sampling sites. In summer, the marine water samples possessed the highest salinity (∼37‰), followed by that in winter (∼34‰). In the rainy season, the salinity decreased due to rainwater runoff in certain sampling sites. From 11 marine sites, marked decreases in salinity were observed at six sites in the rainy season, while the salinities at the rest of the sites were rather stable. Significant (P > 0·05) differences were observed in virulotype prevalence in these two groups of marine sites in the rainy season (Fig. 3a, b). In both groups, only ETEC and EPEC virulotypes were detected in the rainy season, but their prevalence differed between the groups. In the group of marine sites where salinity was rather stable, the prevalence of ETEC and EPEC virulotypes was 60% and 40%, respectively (Fig. 3a). In the marine sites where salinity dropped in the rainy season, the prevalence of ETEC and EPEC was 100% and 26%, respectively (Fig. 3b).

Fig. 3. The effect of seasonal fluctuations in salinity on the prevalence of diarrhoeagenic E. coli virulotypes in marine and freshwater sites. (a) The prevalence pattern of diarrhoeagenic E. coli in five marine sites where salinity did not fluctuate between seasons, (b) the prevalence pattern of diarrhoeagenic E. coli in six marine sites where salinity significantly dropped in the rainy season, (c) the prevalence pattern of diarrhoeagenic E. coli in 28 freshwater sites where salinity did not fluctuate between seasons, and (d) the prevalence pattern of diarrhoeagenic E. coli in seven freshwater sites where salinity significantly increased in summer.
The salinity of freshwater samples (rivers) distant from the sea rarely varied with season. However, the salinity of rivers closer to the sea increased in summer. Because of the lack of rain and blockage of the river flow (Farakka dam and Tista barrage) from the source, the water level decreased in rivers during summer, allowing the seawater to enter. The salinity varied with the distance from the sea. In the rainy season, the situation was reversed because of considerable water both from monsoon rainfall and the opening of the river barrages. Conductivity and TDS were found to be proportional to the salinity of the water.
From 35 freshwater sites, the salinity at 28 sites was mostly stable between seasons. The other seven sites had significantly increased salinity in summer. The pattern of the prevalence of virulotypes differed in these two groups of freshwater sites (Fig. 3c, d). In winter, the prevalence of the EPEC virulotype was the highest in the salinity-stable freshwater sites (Fig. 3c), whereas that of the ETEC virulotype was the highest in the salinity-increased freshwater samples (Fig. 3d). The EAEC virulotype was absent in those samples. In summer and the rainy season, the virulotype prevalence patterns also differed in these two groups of freshwater sites (Fig. 3c, d).
DISCUSSION
Multiplex PCR assays for detecting diarrhoeagenic E. coli have been used earlier for clinical specimens [Reference Nguyen9] and environmental isolates. This work demonstrates the use of multiplex PCR for the environmental monitoring of E. coli virulotypes that evade the traditional isolation procedure and offers a prompt, easy, and reliable method to estimate water-related health risks. DNA extracted from direct water filtrates were assessed for the presence of nine virulence-associated genes of E. coli, but none of the samples were found to be positive. The concentration of pathogenic E. coli in the water samples might be below the detection limit of the PCR method; therefore, three different enrichments were used to enrich the E. coli virulotypes in the water samples. PE culture medium that did not contain any selective agent was more effective for E. coli harbouring eltB and vt1, while bile salt and streptomycin suppressed them. E. coli harbouring estA, vt2, and eaeA genes was selectively enriched in BSE medium. The reduction in the number of positive samples for these three genes in PE cultures could be explained by overgrowth of non-enteric bacteria present in water samples, which reduced the proportion of E. coli in the culture to below the PCR detection limit. In this study, we checked the detection limit of the multiplex PCR assay and found that the ratio of virulent to avirulent E. coli strains must be at least 1:104 to obtain positive PCR results (Supplementary Fig. S3). The pCVD and ipaH genes were mostly detected in SE cultures. It can be assumed that EAEC and EIEC virulotypes (or Shigella spp.) are mostly streptomycin resistant and thus selectively enriched in SE cultures. These E. coli strains might grow slowly and be outcompeted in PE and BSE cultures.
E. coli is the most commonly isolated pathogen in travellers with diarrhoea [Reference Taylor and Echeverria21]. In E. coli virulotypes, the EPEC and ETEC virulotypes are the most important in terms of the total diarrhoeal episodes globally [Reference Schmidt, Henkel and Karch22]. The ETEC virulotype is an important cause of diarrhoea in the developing world, where there is inadequate clean water and poor sanitation [Reference Qadri23], and the ETEC virulotype has already been reported to cause endemic diarrhoeal disease in Bangladesh [Reference Albert24, Reference Qadri25]. Surface water sources in Bangladesh, in both rural and urban areas, are highly contaminated with the ETEC virulotype. In another study, ETEC strains were isolated from clinical samples as well as from 32% of water samples obtained from the ponds, rivers, and lakes around the clinical field sites [Reference Bouzari26]. In our study, we found that more than 60% of the surface water samples were contaminated with the ETEC virulotype. The differences might simply be due to increased contamination in recent years or because rivers and sea beaches are more vulnerable to contamination than isolated ponds and lakes. We can also predict that the enrichment protocol used in our study has successfully resuscitated more targets to the detectable limit. We found that estA-harbouring EPEC strains were mostly found in cities and industrial areas. All five industrial sites harboured both estA and eltB gene of ETEC. Out of nine sea beaches, four sites harboured estA alone and two sites harboured both estA and eltB. estA-harbouring ETEC were also prevalent in highly populated areas as 8/11 sites harboured the estA and eltB gene pair. EAEC is reported to cause sporadic diarrhoea in Bangladesh, particularly in children [Reference Qadri23], as well as in Mexico, Chile, and Iran [Reference Begum27–Reference Nataro30]. However, this virulotype was the least prevalent type found in the experiment, although an increase in the prevalence of EAEC was observed in winter. EAEC was found both in highly populated commercial and tourism sites as well as remote villages. EHEC strains are not common pathogens in Bangladesh in hospitalized patients with diarrhoea. These strains were found in 9/410 children in a hospital study in Bangladesh [Reference Islam31]. Some research findings are available on diarrhoeal epidemics in the country, but most of those are from regional and endemic studies performed in a few specialized hospitals. The information from these studies could help determine some trends in the prevalence of these strains in the country, but that would be a superficial picture.
In Bangladesh, the ETEC virulotype shows a very characteristic biannual seasonality with two separate peaks: one peak at the beginning of summer and the other just after the rainy season, but it remains endemic throughout the year [Reference Qadri25, Reference Khan32–34]. In our study of surface water samples, the ETEC virulotype did not follow any clear seasonal peak variation, but it showed greater prevalence in the rainy season. Prevalence data for other virulotypes in the country are not available, except for some regional epidemic studies. In our study, other virulotypes were found to be more prevalent in winter, except for the EIEC virulotype. The low water temperature might have contributed to the extended survival of these pathogens in water, but the increased prevalence of the EIEC virulotype in summer could not be explained. We found that the aquatic systems in the country harboured different virulotypes of diarrhoeagenic E. coli persistently throughout the year and showed a temporal fluctuation in the prevalence patterns of different virulotypes. ETEC and EPEC virulotypes were distributed throughout the country in three seasons (Fig. 2). The consistent presence and distribution of these virulotypes in the natural waters might be due to their extended survivability in water or the high level of ETEC- and/or EPEC-contaminated faecal discharge throughout the year. The virulotypes maintained regional distribution patterns that had temporal fluctuations. ETEC strains were absent from sea beaches in winter and summer but were present in the rainy season (Fig. 2). The decreased salinity of the water close to the seashore because of large amounts of runoff from the rivers in the rainy season might have an effect on the temporal occurrence of the ETEC virulotype in that region. In summer, the salinity was highest close to the seashore, which could be responsible for the absence of E. coli virulotypes in the region. The EHEC, EIEC, and EAEC virulotypes showed a concrete temporal pattern of occurrence, e.g. the EHEC and EAEC virulotypes were prevalent in winter and the EIEC virulotype in summer. Because of the lack of proper healthcare facilities in remote areas and the occurrence of many unreported home-treated cases where hospitalization would not be useful, a comparative analysis with clinical cases could not be performed in the relevant regions. However, our observations of the distribution and prevalence of E. coli virulotypes may assist with assessing seasonal risk and identifying vulnerable areas of the country prone to E. coli-associated outbreaks.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813000320.
ACKNOWLEDGEMENTS
This work was supported by the Norwegian Programme for Development, Research and Education (NUFU, grant no. 2007/10063).
DECLARATION OF INTEREST
None.