INTRODUCTION
Streptococcus pneumoniae remains the most frequent pathogen in community-acquired pneumonia (CAP) and it is believed that there are more patients with non-bacteraemic pneumococcal pneumonia (BPP) than with bacteraemic episodes [Reference Musher1, Reference Tilghman and Finland2]. With appropriate antibiotic treatment, the fatality rates of BPP were reported as 2·9–9·1% in children aged ⩽14 years [Reference Lagos3], 14% in adults aged ⩾18 years [Reference Lujan4], 44–53% in adults aged ⩾65 years [Reference Martinez5], and 23·4% in critically ill patients [Reference Baddour6]. Furthermore, decreases in the incidence and fatality rate for invasive pneumococcal disease after pneumococcal polysaccharide vaccine and protein-conjugated vaccine immunization have been well documented in recent years [Reference Dominguez7, Reference Tsigrelis8].
In contrast to BPP, there is limited data on the clinical characteristics and outcomes of non-bacteraemic cases primarily because non-bacteraemic cases were more difficult to identify. Diagnosis of non-BPP based on positive culture and Gram stain of sputum is controversial because of the frequent nasopharyngeal carriage of S. pneumoniae [Reference Musher1]. Invasive diagnostic procedures, including protected sheath bronchoalveolar lavage or transthoracic lung aspiration, are difficult to routinely perform before antimicrobial treatment.
Detection of S. pneumoniae antigen in the urine (BinaxNOW®, Binax Inc., USA) by a rapid immunochromatographic membrane test is a valuable tool for the diagnosis of S. pneumoniae infection and is helpful to aid in the diagnosis of non-BPP. Since there is no reference standard for the diagnosis of non-BPP, the sensitivity of the urinary antigen test varied by case definition criteria. Previous studies have reported that the diagnostic sensitivities in patients with confirmed, presumptive, likely and possible non-BPP are 78·3% [Reference Dominguez9], 43·8% [Reference Dominguez9], 24·5% [Reference Smith10] and 20% [Reference Smith10], respectively.
Data on the impact of S. pneumoniae bacteraemia on the outcome of pneumococcal pneumonia are limited. Using the S. pneumoniae urinary antigen test, this hospital-based study of pneumococcal pneumonia in Taiwan during the period 2000–2008 compared the outcome of hospitalized BPP and non-BPP patients.
METHODS
Patients and setting
The study was conducted at National Taiwan University Hospital, a 2000-bed tertiary care hospital in Taiwan. All hospitalized cases of pneumococcal pneumonia diagnosed by a positive blood culture for S. pneumoniae and/or a positive S. pneumoniae urinary antigen test from January 2000 to December 2008 were identified from databases of the microbiology laboratory.
Definitions
The diagnosis of CAP was based on clinical symptoms (fever, respiratory symptoms, typical auscultatory findings), new or progressive infiltrate on chest radiography and laboratory evidences of acute infection. Pneumococcal pneumonia was defined as pneumonia in which S. pneumoniae was identified by blood culture or urinary antigen test. Patients were excluded if they had nosocomial infection, which was defined as a first blood culture or urinary antigen test performed more than 48 h after admission with the absence of any clinical syndrome compatible with pneumococcal infection at admission.
BPP was defined as a diagnosis of pneumonia in combination with the isolation of at least one blood culture positive for S. pneumoniae. Non-BPP was considered when a patient had CAP without bacteraemia (negative blood culture obtained prior to antibiotic administration), and had a positive S. pneumoniae urinary antigen assay without other likely pathogens [Reference Vila-Corcoles11–Reference Charles14].
S. pneumoniae urinary antigen was tested using the BinaxNOW S. pneumoniae urinary antigen test which is an immunochromatographic assay that uses a rabbit anti-S. pneumoniae antibody, conjugated to visualizing particles, to bind any soluble pneumococcal antigen (C polysaccharide) present in the urine sample. The test was performed in accordance with the manufacturer's instructions [15]. All non-BPP cases were recruited after the availability of BinaxNOW S. pneumoniae urinary antigen test in August 2003.
Bacterial isolates
Pneumococcal isolates recovered from blood cultures and sputum specimens were identified by recognition of the typical colony morphology on trypticase soy agar supplemented with 5% sheep blood (BBL Microbiology Systems, USA), Gram staining characteristics, susceptibility to ethylhydrocupreine hydrochloride (optochin; Difco Laboratories, USA), and bile solubility [Reference Ruoff, Murray, Baron, Pfaller, Tenover and Yolken16]. During the study period, all blood culture specimens were inoculated into BACTEC or BACTEC Plus culture bottles using the BACTEC 9240 system (Becton Dickinson, USA).
Antimicrobial susceptibility
Antimicrobial susceptibility testing by the disc diffusion method followed the guidelines established by the Clinical and Laboratory Standards Institute (CLSI) [17]. Minimum inhibitory concentrations (MICs) of penicillin and cefotaxime were determined for available isolates using the agar dilution method and were interpreted according to MIC breakpoints recommended by CLSI [18].
Data collection
Medical records of the hospitalized patients were reviewed and data on age, sex, and comorbid conditions were collected. The following comorbid conditions were recorded: chronic lung disease (chronic obstructive pulmonary disease, asthma, bronchiectasis, pulmonary fibrosis, history of pulmonary tuberculosis), chronic heart disease (congenital heart disease, coronary heart disease, valvular heart disease, congestive heart failure), neurological disease (cerebrovascular disease, dementia, Parkinsonism, epilepsy, cerebral palsy), diabetes, liver cirrhosis, chronic renal disease (chronic renal failure requiring dialysis, nephrotic syndrome), splenectomy, human immunodeficiency virus (HIV) infection, solid organ cancer, haematological cancer, and receiving immunosuppressive therapy (chemotherapy, radiotherapy, transplantation, and long-term use of systemic corticosteroids). Indications for HIV testing included clinical suspicion and screening of contacts of HIV-infected patients. Data on clinical outcomes were collected including all-cause mortality, length of hospital stay, need for intensive-care unit (ICU) admission and extrapulmonary involvement. The 30-day survival status of hospitalized patients were evaluated and that for patients discharged from the hospital within 30 days was investigated using medical records of subsequent outpatient department follow-up.
Statistical analyses
Because the inclusion periods of bacteraemic and non-bacteraemic patients were non-synchronous, the data were analysed for the periods 2004–2008 and 2000–2008, respectively. Continuous variables were expressed as mean±standard deviation and categorical variables were described as percentages. The Student's t test or Mann–Whitney test was used for comparing continuous variables. We used Fisher's exact test or the χ2 test to compare proportions. Survival curves were calculated using Kaplan–Meier analysis and the log-rank test. Univariate and multivariate logistic regressions were used to analyse the association of clinical characteristics with outcome variables of death, need for ICU admission and extrapulmonary involvement. Univariate and multivariate Cox proportional hazards regressions were use to analyse the association of clinical characteristics and the likelihood of discharge. Variables with P<0·1 are included in the multivariate analysis and P values of <0·05 were considered to be statistically significant. All analyses were performed with SPSS version 10.0 (SPSS Inc., USA).
RESULTS
Patent characteristics
During the 9-year study period, 309 hospitalized patients with pneumococcal CAP were identified. After excluding the 13 patients with nosocomial pneumococcal pneumonia with bacteraemia, there were 172 bacteraemic patients and 137 non-bacteraemic patients in the study. While the study period was from January 2000 to December 2008, the BinaxNOW S. pneumoniae urinary antigen test was available in the hospital since August 2003. S. pneumoniae urinary antigen tests identified a total 134 patents with non-bacteraemic pneumococcal CAP from 2004 to 2008 and in the same period, 87 bacteraemic cases were identified by positive blood cultures.
During 2004–2008, several characteristics of BPP patients differed from those with non-BPP (Table 1). BPP patients were significantly older than non-bacteraemic cases. Of BPP patients, 58 (66·7%) had at least one underlying medical disease compared to 45 (33·6%) non-BPP patients (P<0·001). This difference was due to significant greater proportions of liver cirrhosis, solid organ cancer, and immunosuppressive therapy in BPP patients.
Table 1. Demographics and comorbidities of 221 in-patients with bacteraemic and non-bacteraemic pneumococcal community-acquired pneumonia during 2004–2008
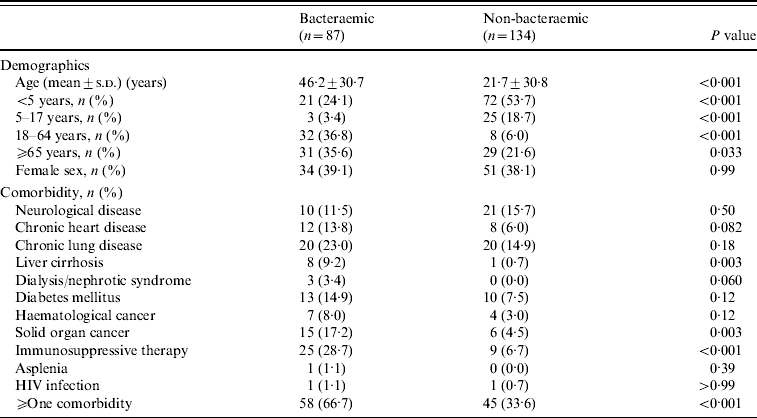
s.d., Standard deviation; HIV, human immunodeficiency virus.
During 2000–2008, more BPP patients had liver cirrhosis (7·0% vs. 0·7%, P=0·015), haematological cancer (9·3% vs. 2·9%, P=0·034), solid organ cancer (16·3% vs. 4·4%, P=0·002), immunosuppressive therapy (27·3% vs. 6·6%, P<0·001) and at least one comorbidity (61·6% vs. 34·3%, P<0·001).
Antimicrobial susceptibilities
During 2000–2008, appropriate sputum samples were obtained from 97 (70·8%) non-bacteraemic patients and S. pneumoniae was isolated from 21 (21·6%) specimens. Susceptible rates of blood isolates in bacteraemic patients and sputum isolates in non-bacteraemic patients by the routine disc diffusion method were 29·2% and 23·8% for penicillin, 96·4% and 100% for cefotaxime, 15·1% and 5% for erythromycin, 18% and 30% for tetracycline, 33·7% and 30% for clindamycin, 76·5% and 65% for chloramphenicol, 97·3% and 100% for levofloxacin, and 98·4% and 100% for moxifloxacin, respectively. None of the antimicrobial agents listed above showed a statistically significant difference of susceptibilities between the isolates recovered from bacteraemic and non-bacteraemic patients. The mortality rates of bacteraemic patients with penicillin-susceptible and non-susceptible pneumococcal isolates were 26% and 20·7% (P=0·57), and all 21 non-bacteraemic cases with pneumococcal isolates recovered from sputum survived. MICs of the 153 available blood isolates from bacteraemic patients were analysed, and the MIC50 and MIC90 for penicillin were 1·0 mg/l and 2·0 mg/l, respectively; and those for cefotaxime were 0·5 mg/l and 1·0 mg/l, respectively. Based on the 2008 CLSI non-meningitis and meningitis criteria of penicillin for S. pneumoniae [Reference Ruoff, Murray, Baron, Pfaller, Tenover and Yolken16], the penicillin-susceptible rates were 98·7% and 36·6%, respectively; and the cefotaxime susceptible rates were 94·8% and 68%, respectively. The MICs of sputum isolates were not determined in this study.
Mortality
During 2004–2008, the overall mortality proportions at 7, 14, and 30 days were significantly higher in BPP than non-BPP patients (12·6% vs. 2·2%, 14·9% vs. 3·7%, 19·5% vs. 5·1%, P<0·01 for all comparisons) (Fig. 1). The in-hospital mortality rate was higher in bacteraemic than non-bacteraemic cases (21·8% vs. 6·0%, P<0·001). The factors associated with in-hospital mortality are shown in Table 2. After adjustment for age and comorbidities in the multivariate logistic regression model, the impact of S. pneumoniae bacteraemia on in-hospital mortality was not statistically significant (P=0·19).
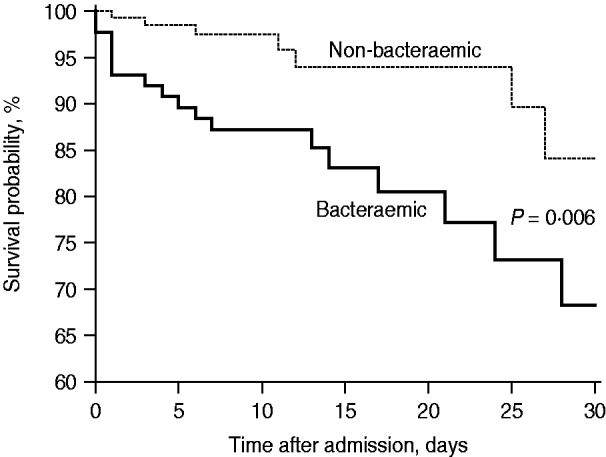
Fig. 1. The 30-day Kaplan–Meier survival curves of bacteraemic and non-bacteraemic pneumococcal community-acquired pneumonia during 2004–2008 (hazard ratio 3·19, 95% confidence interval 1·39–7·07, P=0·006, log-rank test).
Table 2. Univariate and multivariate regression analyses of variables associated with mortality and hospital stay among 221 in-patients with pneumococcal community-acquired pneumonia during 2004–2008Footnote *
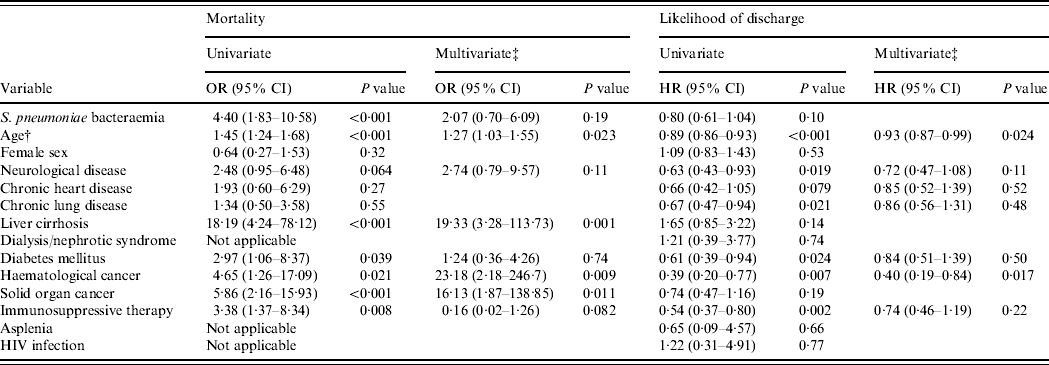
OR, Odds ratio; HR, hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus.
* Logistic regression for mortality and Cox proportional hazards regression for likelihood of discharge.
† Age is treated as a continuous variable and calculated for each 10-year interval.
‡ Variables with P<0.1 in the univariate analysis are included in the multivariate analysis.
During 2000–2008, the presence of S. pneumoniae bacteraemia [odds ratio (OR) 2·70, 95% confidence interval (CI) 1·11–6·57, P=0·028], older age (OR 1·28, 95% CI 1·11–1·46, P<0·001 for each 10-year interval), liver cirrhosis (OR 7·76, 95% CI 2·18–27·60, P=0·002), and solid organ caner (OR 3·37, 95% CI 1·09–10·48, P=0·036) were independent risk factors of in-hospital mortality in the multivariate analysis.
Two bacteraemic patients were infected with penicillin-intermediate (MICs 4·0 mg/l) isolates. One patient, a previously healthy 3-year-old boy who had pneumococcal bacteraemia complicated with septic shock and respiratory failure, was successfully treated with cefotaxime (MIC 1·0 mg/l). Another patient, a 63-year-old male who had lung cancer and underwent chemotherapy, had rapidly fatal outcome (died within 1 day of admission).
Length of hospital stay
During 2004–2008, the length of hospital stay for bacteraemic and non-bacteraemic cases were 17·5±16·6 days and 14·3±14·1 days, respectively (P=0·13). The length of hospital stay was not significantly different between survivors (15·9±15·4 days) and non-survivors (13·1±13·5 days, P=0·36). The Cox proportional hazards model analysis for likelihood of discharge is shown in Table 2. Older age and haematological cancer were associated with increased hospital stay in the multivariate analysis.
During 2000–2008, the factors associated with increased hospital stay were S. pneumoniae bacteraemia (P=0·017), older age (P<0·001), neurological disease (P=0·015), chronic lung disease (P=0·018), diabetes (P=0·003), haematological cancer (P=0·008), and immunosuppressive therapy (P=0·003) in the univariate analysis. The effect of pneumococcal bacteraemia on length of hospital stay was not significant after adjustment for age and coexisting conditions in the multivariate model (P=0·24).
ICU admission
During 2004–2008, rates of ICU admission for BPP and non-BPP patients were 47·1% and 35·8%, respectively (P=0·12). Patients admitted to the ICU had a longer hospital stay (22·8±19·1 days vs. 10·7±9·1 days, P<0·001) and a higher mortality rate (27·0% vs. 2·3%, P<0·001). The estimated ORs from the logistic regression analysis for the risk factors associated with ICU admission are shown in Table 3. Older age and neurological disease were associated with the need for ICU stay in the multivariate analysis.
Table 3. Univariate and multivariate logistic regression analyses of variables associated with ICU admission and extrapulmonary involvement among 221 in-patients with pneumococcal community-acquired pneumonia during 2004–2008
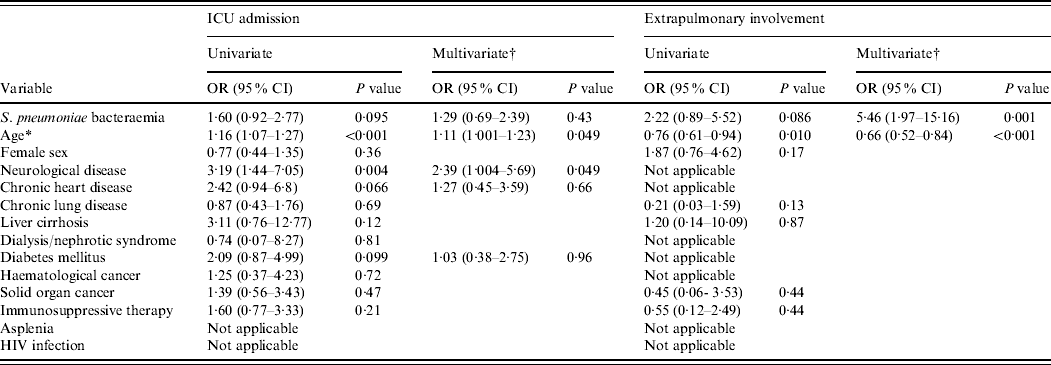
ICU, Intensive-care unit; OR, odds ratio; CI, confidence interval; HIV, human immunodeficiency virus.
* Age is treated as a continuous variable and calculated for each 10-year interval.
† Variables with P<0·1 in the univariate analysis are included in the multivariate analysis.
During 2000–2008, pneumococcal bacteraemia increased the need for ICU admission in the univariate model (OR 1·61, 95% CI 1·02–2·54, P=0·042), and after adjustment for covariates, older age (P=0·015) and neurological disease (P=0·044) were independent predictors of ICU admission while the adjusted effect of pneumococcal bacteraemia on ICU admission was not significant (P=0·35). Both bacteraemic patients infected with isolates that had penicillin MIC ⩾4·0 mg/l were admitted to ICU.
Extrapulmonary involvement
During 2004–2008, extrapulmonary involvement in bacteraemic and non-bacteraemic patients was 13·8% and 6·7%, respectively (P=0·13). The factors associated with extrapulmonary involvement in the logistic regression analysis are shown in Table 3. After adjustment for age, S. pneumoniae bacteraemia was an independent risk factor for extrapulmonary involvement (P=0·001).
During 2000–2008, extrapulmonary involvement was more frequent in bacteraemic than non-bacteraemic patients (16·9% vs. 6·6%, P=0·01). Extrapulmonary involvement developed in 38 patients, including empyema in 35 (92·1%) patients and meningitis in three (7·9%) patients. The age distribution for empyema was 22 (62·8%) patients aged <5 years, six (17·1%) patients aged 5–17 years, six (17·1%) patients aged 18–64 years, and one (2·9%) patient aged ⩾65 years. The age distribution for meningitis was one (33·3%) patient aged 18–64 and two (66·7%) patients aged ⩾65 years; two of the three patients with meningitis died. Pneumococcal bacteraemia (OR 5·0, 95% CI 2·13–11·74, P<0·001), younger age (OR 1·33, 95% CI 1·12–1·56, P=0·001 for each 10-year interval) and asplenia (OR 17·2, 95% CI 1·15–257·3, P=0·039), albeit with a wide CI, independently predicted extrapulmonary involvement in the multivariate analysis.
DISCUSSION
This hospital-based study of pneumococcal pneumonia found that in addition to age and comorbidities, the presence of S. pneumoniae bacteraemia predicted mortality and the development of extrapulmonary involvement in pneumococcal pneumonia. There were associations of S. pneumoniae bacteraemia with a variety of underlying medical conditions.
After the introduction of penicillin, Austrian & Gold [Reference Austrian and Gold19] reported a higher mortality rate (19% vs. 10%) and extrapulmonary involvement (3·5% vs. 0%) in bacteraemic cases. Few recent studies have compared the clinical outcomes of BPP and non-BPP, and the sample sizes of these studies were mostly inadequate to determine the significance of the relationship between S. pneumoniae bacteraemia and clinical outcome of pneumococcal pneumonia. Musher et al. [Reference Musher20] compared 52 veterans of BPP and 48 veterans of non-BPP and found significant higher rates of 7-day mortality rate (19% vs. 4%, P=0·02), ICU admission (44% vs. 25%, P=0·05) and extrapulmonary involvement (19% vs. 0%, P<0·01) in bacteraemic cases. However, there was no significant difference in the 30-day (21% vs. 13%, P=0·25) and 90-day (27% vs. 15%, P=0·11) mortality rate. Brandenburg et al. [Reference Brandenburg21] examined 65 adults with BPP and 93 adults with non-BPP and found no differences in ICU admission (15·4% vs. 10·5–12·2%, P=0·8), length of stay (7·5 days vs. 6·5–7 days), pneumonia-related mortality (7·7% vs. 2·7–5·3%, P=0·4) and the development of a suppurative infection (6·2% vs. 0–2·7%, P=0·49). Watari et al. [Reference Watari22] compared 11 adults with BPP and 26 adults with non-BPP and found a higher mortality rate (27·3% vs. 7·7%) and duration of hospitalization in bacteraemic patients, but these differences were not significant. In a similar study of 82 patients (57 bacteraemic, 25 non-bacteraemic) by Jover et al. [Reference Jover23], in which non-BPP was identified by detection of urinary antigen, a non-significant higher pneumonia-related mortality rate (13·4% vs. 8%, P=0·49) and a significant longer hospital stay was found among bacteraemic cases (7·9 days vs. 6·8 days, P=0·017). The present study has a larger sample size than all of these previous studies [Reference Musher20–Reference Jover23] and adequate power to detect outcome differences between both groups. The use of a multivariate analysis enabled us to compare the factors independently associated with outcome in patients with S. pneumoniae bacteraemic and non-bacteraemic pneumonia.
A previous study of 400 hospitalized CAP patients found that the presence of pneumococcal bacteraemia was associated with a longer time to reach clinical stability among 400 hospitalized CAP patients [Reference Ramirez and Bordon24]. Another study compared 56 patients with BPP and 394 patients with non-bacteraemic CAP and found no significant difference in overall mortality and length of hospital stay [Reference Marrie, Low and De Carolis25]. A multivariate analysis by Bordon et al. [Reference Bordon26] compared 125 subjects with pneumococcal bacteraemic CAP and 1847 subjects with non-bacteraemic CAP and found that the presence of pneumococcal bacteraemia did not increase the time to clinical stability, length of stay and mortality. One possible explanation for the discrepancy in the impact of S. pneumoniae bacteraemia between pneumococcal pneumonia and pneumonia of any aetiology might be the poorer outcome in patients with pneumonia caused by other pathogens [Reference Roson27–Reference Kallen29].
We demonstrated that one of the two patients infected with isolates having penicillin MICs of 4·0 mg/l survived with multiple complications, and another died rapidly. In a meta-analysis involving 3430 hospitalized patients with pneumococcal CAP, a higher mortality rate was noted in patients infected with penicillin intermediate (meningitis criteria, MIC 0·12–1·0 mg/l) and resistant (MIC ⩾2·0 mg/l) isolates [Reference Tleyjeh30]. With a small number of patients (51/3430), it was inconclusive for patients infected with highly penicillin-resistant isolates (MIC ⩾4 mg/l).
This study had several strengths and limitations. Its strengths included large size, enrolment of patients of all ages, adjustment for the effects of covariates on outcome, consideration of important outcome variables including mortality, length of hospital stay, ICU admission and extrapulmonary involvement. Because all patients were admitted to a tertiary care hospital and outpatients were not investigated, the results may not necessarily be representative of the general population. This study was also limited by its retrospective design, and the performance of some investigations, such as blood culture or pneumococcal urinary antigen test, at the discretion of the attending physician. Unlike blood cultures, pneumococcal urinary antigen test was not the routine examination for hospitalized patients with pneumonia in our hospital. A significant bias in the non-bacteraemic study group might be created, although the impact would be diminished after the multivariate analysis.
The presence of false-negative or false-positive pneumococcal urinary antigen tests might affect the quality of the selection process for non-bacteraemic episodes. As mentioned previously, the true sensitivity of pneumococcal urinary antigen test is indeterminate and the number of non-bacteraemic cases is potentially underestimated. The current investigation found 1·5-fold more patients with non-bacteraemic episodes than with bacteraemic episodes during 2004–2008. However, there were probably 3–4 cases of non-bacteraemic episodes for every one case of bacteraemia [Reference Musher1]. The previous investigation demonstrated that urinary antigen test was positive in 43·8% of non-bacteraemic cases having sputum positive for pneumococcus [Reference Dominguez9] and in our study, pneumococcus was isolated from 21·6% of sputum specimens of non-bacteraemic cases. Therefore, further investigations that include non-bacteraemic patients using other microbiological tests are warranted.
Although previous studies indicated a high specificity of pneumococcal urinary antigen tests in adults [Reference Dominguez9, Reference Gutiérrez31], non-infected children with nasopharyngeal S. pneumoniae carriage, pneumococcal vaccination and recent pneumococcal infection might have positive test results [Reference García-Suárez32]. Given that urine pneumococcal C-polysaccharide is inadequate for differentiation between healthy carriers and patients, additional immunoassays or molecular diagnostics may improve the diagnostic accuracy [Reference García-Suárez32]. Finally, the yearly distribution of bacteraemic and non-bacteraemic cases differed in the analysis for the period 2000–2008 because pneumococcal urinary antigen tests were only available in the last 5 years. Because there was no significant change in the mortality rates of pneumococcal diseases from 2000 to 2008 in our recent studies [Reference Liao33, Reference Lin34], the influence on the comparison of mortality would be limited.
Despite the decline in incidence after pneumococcal vaccination and the advances in antimicrobial agents, the presence of pneumococcal bacteraemia increased the risk of mortality and extrapulmonary involvement in patients with pneumococcal CAP. Further study is needed to develop optimal antibiotic and vaccination strategies to improve the outcome of BPP.
DECLARATION OF INTEREST
None.






