Introduction
Alcohol, tobacco use and mood disorders are prominent contributors to the global burden of diseases and are highly comorbid (Anthony, Warner, & Kessler, Reference Anthony, Warner and Kessler1994; Degenhardt et al., Reference Degenhardt, Charlson, Ferrari, Santomauro, Erskine, Mantilla-Herrara and Vos2018; Ezzati, Lopez, Rodgers, Vander Hoorn, & Murray, Reference Ezzati, Lopez, Rodgers, Vander Hoorn and Murray2002; Kessler, Chiu, Demler, Walters, & Walters, Reference Kessler, Chiu, Demler, Walters and Walters2005). Approximately 30% of individuals with major depressive disorder (MDD) have comorbid substance use disorders (SUD) (Davis et al., Reference Davis, Frazier, Husain, Warden, Trivedi, Fava and Rush2006). Vice versa, 30–50% of individuals in treatment for SUD have comorbid mood disorders (European Monitoring Centre for Drugs and Drug Addiction, 2015). Cross-sectional studies indicate a higher prevalence of smoking in people with MDD or with depressive symptoms and that symptoms of depression are more frequent in smokers than never- or former smokers (Anda et al., Reference Anda, Williamson, Escobedo, Mast, Giovino and Remington1990; Glassman et al., Reference Glassman, Helzer, Covey, Cottler, Stetner, Tipp and Johnson1990; Jamal, Willem Van der Does, Cuijpers, & Penninx, Reference Jamal, Willem Van der Does, Cuijpers and Penninx2012). Moreover, the incidence of smoking rises as the level of depressive symptoms increases (Anda et al., Reference Anda, Williamson, Escobedo, Mast, Giovino and Remington1990). Furthermore, alcohol abuse and dependence often coexist with depression (Flensborg-Madsen et al., Reference Flensborg-Madsen, Mortensen, Knop, Becker, Sher and Grønbæk2009; Grant & Harford, Reference Grant and Harford1995; Kessler et al., Reference Kessler, Chiu, Demler, Walters and Walters2005, Reference Kessler, Crum, Warner, Nelson, Schulenberg and Anthony1997).
Previous literature hints at a positive association between alcohol consumption, smoking and symptom levels of depression (Anda et al., Reference Anda, Williamson, Escobedo, Mast, Giovino and Remington1990; Gilman & Abraham, Reference Gilman and Abraham2001; Glassman et al., Reference Glassman, Helzer, Covey, Cottler, Stetner, Tipp and Johnson1990; Grant & Harford, Reference Grant and Harford1995; Pérez-Stable, Marín, Marín, & Katz, Reference Pérez-Stable, Marín, Marín and Katz1990; Pomerleau, Zucker, & Stewart, Reference Pomerleau, Zucker and Stewart2003; Sullivan, Fiellin, & O'Connor, Reference Sullivan, Fiellin and O'Connor2005; Swendsen et al., Reference Swendsen, Merikangas, Canino, Kessler, Rubio-Stipec and Angst1998). Hypotheses explaining this association are based on genetic and environmental interactions, shared aetiology, direct causality or other mechanisms (Khantzian, Reference Khantzian1997; O'Neil, Conner, & Kendall, Reference O'Neil, Conner and Kendall2011). First, smoking and alcohol use might induce depressive symptoms. Previous studies provide evidence that smoking cessation leads to a reduction of depressive symptoms (Jamal et al., Reference Jamal, Willem Van der Does, Cuijpers and Penninx2012; Lechner, Sidhu, Cioe, & Kahler, Reference Lechner, Sidhu, Cioe and Kahler2019; Stepankova et al., Reference Stepankova, Kralikova, Zvolska, Pankova, Ovesna, Blaha and Brose2017; Taylor et al., Reference Taylor, McNeill, Girling, Farley, Lindson-Hawley and Aveyard2014). In addition, results of prospective studies show that alcohol abstinence reduces symptoms of depression (Brown et al., Reference Brown, Inaba, Gillin, Schuckit, Stewart and Irwin1995; Brown & Schuckit, Reference Brown and Schuckit1988; Liappas, Paparrigopoulos, Tzavellas, & Christodoulou, Reference Liappas, Paparrigopoulos, Tzavellas and Christodoulou2002). Second, longitudinal studies suggest that smoking is a potential risk factor for developing depression and that hazardous drinking and alcohol abuse as well as dependence increase the risk of depression (Boden & Fergusson, Reference Boden and Fergusson2011; Boden, Fergusson, & Horwood, Reference Boden, Fergusson and Horwood2010; Breslau, Novak, & Kessler, Reference Breslau, Novak and Kessler2004; Brown, Lewinsohn, Seeley, & Wagner, Reference Brown, Lewinsohn, Seeley and Wagner1996; Fergusson, Boden, & Horwood, Reference Fergusson, Boden and Horwood2009; Gémes et al., Reference Gémes, Forsell, Janszky, László, Lundin, Ponce De Leon and Moller2019; Hasin, Goodwin, Stinson, & Grant, Reference Hasin, Goodwin, Stinson and Grant2005). Other hypotheses for the association between smoking, alcohol use and depressive symptoms include misattribution or self-medication. The latter assumes that patients intend to alleviate certain symptoms (e.g. depression) or adverse effects from medication by using addictive substances (Bolton, Robinson, & Sareen, Reference Bolton, Robinson and Sareen2009; Breslau et al., Reference Breslau, Novak and Kessler2004; Grant & Harford, Reference Grant and Harford1995; Khantzian, Reference Khantzian1997; Kuo, Gardner, Kendler, & Prescott, Reference Kuo, Gardner, Kendler and Prescott2006; McKenzie, Olsson, Jorm, Romaniuk, & Patton, Reference McKenzie, Olsson, Jorm, Romaniuk and Patton2010). Misattribution states that people mislabel relief of withdrawal symptoms by drinking or smoking as positive effects of abuse (McKenzie et al., Reference McKenzie, Olsson, Jorm, Romaniuk and Patton2010; Wootton et al., Reference Wootton, Richmond, Stuijfzand, Lawn, Sallis, Taylor and Munafò2020). Furthermore, other studies have argued that depressive symptoms lead to unhealthy coping such as alcohol abuse and dependence (Grant & Harford, Reference Grant and Harford1995; Kuo et al., Reference Kuo, Gardner, Kendler and Prescott2006). Evidently, the relation of the comorbidity between smoking, alcohol use and depressive symptoms remains unclear, which undermines the development of effective prevention strategies for mental and physical health issues in clinical practice.
Few large, longitudinal prospective studies have examined the associations between smoking, alcohol use and number of symptoms of depression in the general population (Cabello et al., Reference Cabello, Miret, Caballero, Chatterji, Naidoo, Kowal and Ayuso-Mateos2017; Fergusson et al., Reference Fergusson, Boden and Horwood2009; Flensborg-Madsen et al., Reference Flensborg-Madsen, Mortensen, Knop, Becker, Sher and Grønbæk2009). Importantly, previous studies have focused on a diagnosis of depression instead of symptoms of depression as outcome, resulting in a loss of power and of relevant clinical data as subsyndromal depressive symptoms may be associated with functional impairment as well (Cabello et al., Reference Cabello, Miret, Caballero, Chatterji, Naidoo, Kowal and Ayuso-Mateos2017; Fergusson et al., Reference Fergusson, Boden and Horwood2009; Flensborg-Madsen et al., Reference Flensborg-Madsen, Mortensen, Knop, Becker, Sher and Grønbæk2009; Gémes et al., Reference Gémes, Forsell, Janszky, László, Lundin, Ponce De Leon and Moller2019). Furthermore, suffering from an alcohol use disorder can render a clinical diagnosis of MDD less likely given the DSM-IV and DSM-5 exclusion criterion of substance use causing depression. Thus, depressive symptoms may be a relevant and clinically useful outcome measure to study the intricate relationships between nicotine and alcohol on the one hand and depressive symptoms on the other. Other limitations of previous studies investigating the association between tobacco use, drinking behaviour and depressive symptoms include the focus on alcohol use disorder instead of general alcohol drinking behaviour and smaller sample sizes (Fergusson et al., Reference Fergusson, Boden and Horwood2009; Flensborg-Madsen et al., Reference Flensborg-Madsen, Mortensen, Knop, Becker, Sher and Grønbæk2009). Additionally, most studies have investigated the association between alcohol use and depression and between smoking and depression in separate cohorts. In sum, the relationships between quantitative measures of alcohol use and depressive symptoms on the one hand and smoking and depressive symptoms on the other hand have not yet been elucidated.
Based on previous literature, we hypothesized that smoking status and number of cigarettes per day are positively associated with depressive symptoms; and that smoking cessation is negatively associated with depressive symptoms. Similarly, for alcohol use, we hypothesized that alcohol use and severity are positively associated with depressive symptoms; and that alcohol cessation is negatively associated with depressive symptoms. We thus examined the multi-cross-sectional (cross-sectional relationship across multiple time points) and longitudinal associations between (changes in) smoking behaviour, alcohol use and number of depressive symptoms in a unique, large cohort from the general population. We used polygenic liabilities for these traits to corroborate the directionality of our findings.
Methods
Study population and procedure
Data were used from the Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2). This stratified, a-selective study sample of the Dutch population included 6646 people between 18 and 64 years at the time of the baseline interview. The individuals of the sample were randomly selected from households in the Netherlands. Institutionalised individuals living in hostels, hospices or prisons or individuals with insufficient Dutch language proficiency were excluded. Further details concerning selection procedure and study design have been described by de Graaf et al. (de Graaf, ten Have, & van Dorsselaer, Reference De Graaf, Ten Have and van Dorsselaer2010). In the period of 2007–2015, three waves of data were derived from the study population of NEMESIS-2 and several outcome measures were assessed by trained interviewers. Follow-up rates were high with 3- and 6-year response rates of 79.8% (5303 people) and 69.5% (4618 people), respectively (Pries et al., Reference Pries, Guloksuz, Ten Have, de Graaf, van Dorsselaer, Gunther and van Os2018). Approval of an independent Medical Ethics Committee and written informed consent from all participants were obtained. The STROBE and TREND guidelines were followed to report this study (Des Jarlais, Lyles, Crepaz, & TREND Group, Reference Des Jarlais, Lyles and Crepaz2004; von Elm et al., Reference von Elm, Altman, Egger, Pocock, Gøtzsche and Vandenbroucke2007).
Outcome measures
The dependent variable was the number of depressive symptoms using the Mental Health Inventory-3 (MHI-3) as primary outcome. The MHI-3 consists of three depression questions of the Mental Health Inventory (MHI-5), excluding two questions about anxiety symptoms that are included in the MHI-5 (see online Supplementary material for additional information regarding dependent variables). Previous studies have shown that both the MHI-3 and MHI-5 are valid instruments to identify individuals with depressive symptoms (Yamazaki, Fukuhara, & Green, Reference Yamazaki, Fukuhara and Green2005). Considering our focus on numeric depressive symptoms in the general population, and that some anxiety symptoms may be differentially associated with substance use (Groenman, Janssen, & Oosterlaan, Reference Groenman, Janssen and Oosterlaan2017; Kaplow, Curran, Angold, & Costello, Reference Kaplow, Curran, Angold and Costello2001), we chose to use the MHI-3 instead of the MHI-5 as primary outcome. The MHI-3 scores are 3–18 points, with lower scores indicating higher depressive symptoms. Subscales of the Short Form 36 Health Survey (SF-36) were used as sensitivity analysis (online Supplementary material) (Ware & Sherbourne, Reference Ware and Sherbourne1992). The SF-36 is a widely used instrument that assesses the level of mental and physical functioning in the past 4 weeks (Bos et al., Reference Bos, ten Have, van Dorsselaer, Jeronimus, de Graaf and de Jonge2018; McHorney, Ware, Rachel Lu, & Sherbourne, Reference McHorney, Ware, Rachel Lu and Sherbourne1994). This self-report questionnaire consists of nine subscales each ranging from 0 to 100 points, representing poor to good functioning (McHorney et al., Reference McHorney, Ware, Rachel Lu and Sherbourne1994). For our sensitivity analysis, the scores of three subscales of the SF-36 most closely associated with depressive symptoms (i.e. the vitality scale, the mental health scale or the MHI-5, and the emotional role functioning scale) were combined into one scale (total 0–300 points) as an outcome, indicating the number of depressive symptoms in the past 4 weeks, similarly to Bos et al. (Reference Bos, ten Have, van Dorsselaer, Jeronimus, de Graaf and de Jonge2018). The score ranges from poor to good functioning, with low scores representing many depressive symptoms. Both primary (MHI-3) and sensitivity outcomes (SF-36) were measured at baseline (wave one), 3-year (wave two) and 6-year (wave three) follow-up.
Independent variables
Alcohol use as binary trait and severity (drinks per week) and smoking status (smoking v. non-smoking) and severity (cigarettes per day) were the independent variables and measured at baseline, after 3 and 6 years. Smoking behaviour was assessed using self-designed questions, considering smoking status (binary) and daily smoking in the past month (categorized). The average number of cigarettes per day was categorized into six subgroups: <1 cigarette per week, >1 cigarette per week, 1–5, 6–10, 11–20 or >20 cigarettes per day (Cuijpers, Smit, ten Have, & de Graaf, Reference Cuijpers, Smit, ten Have and de Graaf2007; de Graaf et al., Reference De Graaf, Ten Have and van Dorsselaer2010).
Alcohol consumption as a binary trait and severity (drinks per week) was measured using the CIDI 3.0 (American Psychiatric Association, 2000). Alcohol drinks per week were calculated with the frequency of drinking in the past 12 months and the number of glasses when drinking, similarly to Gémes et al. (Reference Gémes, Forsell, Janszky, László, Lundin, Ponce De Leon and Moller2019) and Tuithof, Ten Have, van den Brink, Vollebergh, and de Graaf (Reference Tuithof, Ten Have, van den Brink, Vollebergh and de Graaf2013). Drinking frequency was estimated by coding daily alcohol consumption as a weekly frequency of 7, almost every day as 5.5, 3–4 days per week as 3.5, 1–2 days per week as 1.5, 1–3 days per month as 0.5 and less than once per month as 0.125. The number of glasses per drinking day was multiplied by the estimated drinking frequency. Outliers were removed when above or below three standard deviations from the mean (removal of outliers below three standard deviations resulted in non-existing negative outcomes). Afterwards, drinks per week were categorized in four levels based on interquartile ranges (i.e. 0–1.5, 1.5–3.5, 3.5–10.5, 10.5–84.0 drinks per week). In this study, 10.5–84.0 drinks per week was classified as heavy drinking, 3.5–10.5 drinks per week as moderate drinking and 1.5–3.5 drinks per week as light drinking. In validation analyses, drinks per week were recategorized for women (i.e. 0–1, 1–14, 14–84 drinks per week) and men (i.e. 0–1, 1–21, 21–84 drinks per week), based on the current definitions for excessive alcohol use in the Netherlands (i.e. >14 standard units per week for women and >21 for men) (De Staat van Volksgezondheid en Zorg, 2020).
For longitudinal analyses, changes in smoking and alcohol use behaviour (yes/no) and changes in drinks per week and cigarettes per day [both binary change (increase/decrease) and per level] were determined and calculated between 0–3 and 3–6-year follow-up, similarly to previous studies (Vermeulen et al., Reference Vermeulen, Schirmbeck, Blankers, van Tricht, Bruggeman, van den Brink and van Winkel2018; Vermeulen et al., Reference Vermeulen, Schirmbeck, Blankers, van Tricht, van den Brink, de Haan and van Winkel2019). For example, someone who smoked at baseline and stopped smoking at 3-year follow-up and smoked again at 6-year follow-up was registered as ‘quit smoking’ between 0 and 3-year follow-up and ‘started smoking’ between 3 and 6-year follow-up. A reduction of 10.5–84.0 drinks per week to 1.5–3.5 drinks per week between baseline and 3-year follow-up was described as ‘decrease’ in binary models or ‘decrease of 2 levels’ in models per level. Furthermore, the change in the number of depressive symptoms was calculated by the difference in total scores between 0 and 3-year follow-up and 3 and 6-year follow-up.
Covariates were age, gender, treatment for any psychiatric disorder or negative life events in the previous 12 months, and cannabis dependency/abuse – all measured as binary traits except for age; and education level, which was categorized into either primary, lower secondary, higher secondary or higher professional education. Of these, education level and treatment for a psychiatric disorder were assessed using self-designed questions, while negative life events were measured using questions based on the ‘Brugha Life events section’ and cannabis dependency/abuse was determined by the CIDI 3.0 (Brugha & Cragg, Reference Brugha and Cragg1990). All covariates were determined a priori based on previous studies demonstrating significant associations or using similar covariates with regards to smoking, alcohol use and depressive symptoms (Chaiton, Cohen, Rehm, Abdulle, & O'loughlin, Reference Chaiton, Cohen, Rehm, Abdulle and O'loughlin2014; Fergusson et al., Reference Fergusson, Boden and Horwood2009; Flensborg-Madsen et al., Reference Flensborg-Madsen, Mortensen, Knop, Becker, Sher and Grønbæk2009; Gémes et al., Reference Gémes, Forsell, Janszky, László, Lundin, Ponce De Leon and Moller2019; Hooshmand, Willoughby, & Good, Reference Hooshmand, Willoughby and Good2012; Kotov, Guey, Bromet, & Schwartz, Reference Kotov, Guey, Bromet and Schwartz2010; Troisi, Pasini, Saracco, & Spalletta, Reference Troisi, Pasini, Saracco and Spalletta1998; Wilkinson, Halpern, & Herring, Reference Wilkinson, Halpern and Herring2016). No other substance use than cannabis was included as a covariate given the higher prevalence (European Monitoring Centre for Drugs and Drug Addiction, 2006).
For the purpose of genetic analyses, polygenic risk scores (PRS) were computed in all genotyped participants for three phenotypes – alcohol drinks per week, smoking initiation and cigarettes per day – for 12 p value thresholds (0.5, 0.4, 0.3, 0.2, 0.1, 0.05, 5 × 10−3, 5 × 10−4, 5 × 10−5, 5 × 10−6, 5 × 10−7, 5 × 10−8). PRS estimate the genetic liability of an individual for specific traits or diseases (Choi, Mak, & O'Reilly, Reference Choi, Mak and O'Reilly2020). See online Supplementary material for extensive details regarding PRS analyses (quality control, imputation and principle component analyses).
Statistical analysis
First, cross-sectional baseline comparisons between (1) smoking v. non-smoking and (2) alcohol v. non-alcohol use were conducted using unpaired t test and χ2 test at baseline. Second, linear mixed-effects models corrected for age and gender were applied for multi-cross-sectional analysis followed by longitudinal analysis of the associations between change in alcohol use, smoking and change in the number of depressive symptoms over a period of 6 years. To that end, (change in) smoking status (smoker or non-smoker) or (change in) alcohol status (drinker or non-drinker), wave, age and gender were included as fixed effects. Intercepts for respondents and by-subject random slopes for time were entered as random effects. To gain insight into dose–response associations, the number of cigarettes per day and drinks per week were then entered as predictors. Third, in our first sensitivity analysis, we replaced the MHI-3 with the SF-36 as outcome (see above under ‘Outcome measures’). Fourth, in our second sensitivity analysis, we corrected for all covariates listed under ‘Independent variables’ for both the MHI-3 and SF-36. Fifth, to examine whether associations of smoking and of alcohol use with the number of depressive symptoms were independent of each other, alcohol use and drinks per week were entered as additional covariates to the second sensitivity analysis model for smoking status/cigarettes per day and smoking status and cigarettes per day for alcohol use/drinks per week. At last, we validated our findings for drinks per week and repeated the analyses using different thresholds for women and men (see ‘Independent variables’).
All variables were added in a forward approach. People were included for multi-cross-sectional analysis whenever data regarding smoking or alcohol use and number of depressive symptoms were available for at least one of three measurements (at baseline, after 3 years or after 6 years) due to valid estimation calculation of mixed models. Participants were included for longitudinal analysis whenever data on change in smoking or alcohol use and change in number of depressive symptoms were available for at least two measurements with a minimum follow-up period of 3 years.
Finally, linear mixed-effects models were used for genetic analyses of the associations between PRS for smoking initiation, cigarettes per day and alcohol drinks per week and depressive symptoms. PRS models were adjusted for three ancestry principal components (PC1, PC2 and PC3) (Guloksuz et al., Reference Guloksuz, Pries, Have, Graaf, Dorsselaer, Klingenberg and Os2020). PRS for either smoking initiation, cigarettes per day or alcohol drinks per week using one of the 12 different p value thresholds were inserted as fixed effects in the models in addition to wave, gender, age, ancestry PCs. Similarly, intercepts for respondents and by-subject random slopes for time were entered as random effects.
In line with similar longitudinal studies investigating associations between substance use and depressive symptoms, linear mixed-effects models were used for statistical analyses (Anand, Paquette, Bartuska, & Daughters, Reference Anand, Paquette, Bartuska and Daughters2019; Vermeulen et al., Reference Vermeulen, Schirmbeck, Blankers, van Tricht, van den Brink, de Haan and van Winkel2019). All analyses were performed in R (version 3.6.0) using the lme4 package. Outcomes of the variables of interest were presented as estimates (standard errors) and the corresponding intercept of the complete model. Models were fitted using restricted maximum likelihood (REML). Calculation of p values was done according to the Satterthwaite approach for all analyses with the pbkrtest package, which has the lowest type I error rates together with the Kenward-Roger approach for REML-fitted mixed-effects models (Halekoh & Højsgaard, Reference Halekoh and Højsgaard2015; Luke, Reference Luke2017). The significance threshold was set at p < 0.05.
Results
Descriptive statistics
Data regarding smoking status were available for 6503 (97.8% of the total participants at this time point) individuals at baseline, and 5303 (100%) and 4618 (100%) individuals at the last two waves. In total, 1988 (30.6%) participants out of 6503 smoked at baseline, 1409 (26.6%) out of 5303 participants at the first follow-up assessment and 1076 (23.3%) out of 4618 participants at the second follow-up assessment (online Supplementary Table S2). In the three follow-up periods, respectively, 5443 out of 5763 (94.4%), 4182 out of 4209 (99.4%) and 3627 out of 3646 (99.5%) respondents used alcohol (online Supplementary Table S3). Cross-sectional baseline comparisons revealed that smoking and alcohol use were independent of each other. Additionally, baseline comparisons showed that the mean MHI-3 score was 17 for non-smoking and 16 for smoking people, indicating more depressive symptoms in smoking participants multi-cross-sectionally (p < 0.001; online Supplementary Table S2). At baseline, the mean MHI-3 score was 16 for both non-alcohol using and alcohol using people (p = 0.208; online Supplementary Table S3). Genetic data were available for 3104 participants of whom 1362 were male and 1742 female.
Multi-cross-sectional results
Multi-cross-sectional linear mixed-model analysis showed that smoking was positively associated with depressive symptoms [estimate −0.36, standard error (s.e.) 0.04, p value <0.001; Table 1]. Furthermore, we found a positive association between the mean number of cigarettes per day and depressive symptoms (6–10 cigarettes: estimate −0.22, s.e. 0.07, p value 0.001; 11–20 cigarettes: estimate −0.48, s.e. 0.06, p value <0.001; >20 cigarettes: estimate −1.00, s.e. 0.09, p value <0.01; Table 2). No associations were found between alcohol use as a binary trait and depressive symptoms as well as between heavy drinking (10.5–84 drinks per week) and depressive symptoms (online Supplementary Tables S6 and S7). However, light to moderate drinking (1.5–10.5 drinks per week) was associated with less depressive symptoms (estimate 0.10, s.e. 0.047, p value 0.033; estimate 0.12, s.e. 0.048, p value 0.011, respectively; Table 3). Sensitivity analyses using the SF-36 outcome and correcting for additional covariates in all linear models confirmed our results, except that alcohol use as a binary trait was significantly negatively associated with depressive symptoms on the SF-36 and that light drinking (1.5–3.5 drinks per week) was not significantly associated with depressive symptoms on the MHI-3 (online Supplementary Tables S4–S7, S15, S17). Thus, across models, both smoking and smoking quantity were robustly associated with more depressive symptoms, while for alcohol, moderate drinking was robustly associated with less depressive symptoms.
Table 1. Multi-cross-sectional associations between smoking/non-smoking and symptoms of depression using the MHI-3 score (corrected for age and gender)

Fixed effects in the models were smoking, age, gender and time. Random effects were by-subject random slopes for time and intercepts for respondents. Data are estimates and p values were calculated using the Satterthwaite approach. Significant results (p value < 0.05) are provided in bold.
Table 2. Multi-cross-sectional associations between mean number of cigarettes per day and symptoms of depression using the MHI-3 score (corrected for age and gender)

Fixed effects in the models were cigarettes per day, age, gender and time. Random effects were by-subject random slopes for time and intercepts for respondents. Data are estimates and p values were calculated using the Satterthwaite approach. Significant results (p value < 0.05) are provided in bold.
Table 3. Multi-cross-sectional associations between mean number of drinks per week and symptoms of depression using the MHI-3 score (corrected for age and gender)

Fixed effects in the models were drinks per week, age, gender and time. Random effects were by-subject random slopes for time and intercepts for respondents. Data are estimates and p values were calculated using the Satterthwaite approach. Significant results (p value < 0.05) are provided in bold.
Longitudinal analysis
In total, 978 (18.4%) participants of the total sample changed their smoking behaviour between baseline and 3-year follow-up [increase in the number of cigarettes in n = 384 (7.2%); decrease in the number of cigarettes in n = 594 (11.2%) participants] and 760 (16.5%) individuals between 3 and 6-year follow-up [increase n = 317 (6.9%); decrease n = 443 (9.6%) participants]. In total, 1783 (42.4%) respondents changed their alcohol behaviour between baseline and 3-year follow-up [increase in the number of drinks per week in n = 861 (20.5%); decrease in the number of drinks per week in n = 922 (21.9%) participants], and 1334 (36.6%) between 3 and 6-year follow-up [increase in n = 598 (16.4%); decrease in n = 736 (20.2%) participants]. Additional demographics on changes in independent variables are shown in online Supplementary Table S1.
Longitudinal analysis showed that a reduction of five levels in the number of cigarettes per day was associated with less depressive symptoms (estimate 0.76, s.e. 0.31, p value 0.015; Table 4). Smoking initiation and smoking cessation were not significantly associated with change in depressive symptoms (online Supplementary Table S8). Again, sensitivity analyses confirmed our findings (online Supplementary Table S18–S20).
Table 4. Longitudinal associations between change in cigarettes per day (per level) and symptoms of depression using the MHI-3 score (corrected for age and gender)

Fixed effects in the models were change in cigarettes per day, age, gender and time. Random effects were by-subject random slopes for time and intercepts for respondents. Data are estimates and p values were calculated using the Satterthwaite approach. Significant results (p value < 0.05) are provided in bold.
Alcohol cessation was associated with less depressive symptoms over time (estimate 0.63, standard error 0.29, p value 0.028; Table 5). Additionally, a decrease of two categories of drinks per week was associated with less depressive symptoms (estimate 0.36, standard error 0.11, p value 0.001; Table 6), which was confirmed in sensitivity analyses using additional covariates for a decrease of two and three categories of drinks per week (online Supplementary Table S23).
Table 5. Longitudinal associations between change in alcohol use and symptoms of depression using the MHI-3 score (corrected for age and gender)

Fixed effects in the models were change in alcohol use, age, gender and time. Random effects were by-subject random slopes for time and intercepts for respondents. Data are estimates and p values were calculated using the Satterthwaite approach. Significant results (p value < 0.05) are provided in bold.
Table 6. Longitudinal associations between change in drinks per week (per level) and symptoms of depression using the MHI-3 score (corrected for age and gender)
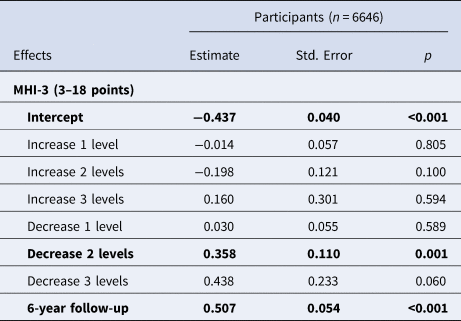
Fixed effects in the models were change in drinks per week, age, gender and time. Random effects were by-subject random slopes for time and intercepts for respondents. Data are estimates and p values were calculated using the Satterthwaite approach. Significant results (p value < 0.05) are provided in bold.
Validation analyses
All multi-cross-sectional and longitudinal results were similar for validation analyses using different thresholds for drinks per week for women and men (online Supplementary Tables S24–S37). Notably, we found additional significant results strengthening the abovementioned findings. For men, heavier drinking (21–84 drinks per week) was associated with more depressive symptoms multi-cross-sectionally (estimate −0.28, standard error 0.107, p value 0.010; online Supplementary Table S29). Decreasing drinks per week (binary) were associated with less depressive symptoms longitudinally for women and men (estimate 0.24, standard error 0.09, p value 0.012; online Supplementary Table S30, and estimate 0.23, standard error 0.09, p value 0.016; online Supplementary Table S27, respectively).
Genetic risk score analyses
Directions of the effect of our PRS analyses of cigarettes per day and drinks per week were consistent with the results of multi-cross-sectional analysis (online Supplementary Tables S38–S39). Moreover, in line with our phenotypic associations, PRS for cigarettes per day were significantly associated with increased depressive symptoms using the SF-36 (PT 5 × 10−3, p = 0.009; PT 0.05, p = 0.017; PT 0.1, p = 0.022; PT 0.3, p = 0.034; PT 0.4, p = 0.038; PT 0.5, p = 0.031; online Supplementary Table S38).
Discussion
In this prospective, longitudinal cohort study in the general population, smoking and number of cigarettes smoked per day were positively associated with depressive symptoms multi-cross-sectionally, whereas moderate drinking was associated with less depressive symptoms. Alcohol cessation and substantial reductions in both cigarette and alcohol consumption were associated with decreases in depressive symptoms. Extensive sensitivity, validation and PRS analyses corroborated the results.
Here, in accordance with previous literature, we found a higher prevalence of depressive symptoms in smoking participants compared to non-smoking participants cross-sectionally (Jamal et al., Reference Jamal, Willem Van der Does, Cuijpers and Penninx2012; Lechner et al., Reference Lechner, Sidhu, Cioe and Kahler2019; Stepankova et al., Reference Stepankova, Kralikova, Zvolska, Pankova, Ovesna, Blaha and Brose2017; Taylor et al., Reference Taylor, McNeill, Girling, Farley, Lindson-Hawley and Aveyard2014). In contrast with several preceding observational studies and a meta-analysis, we found no association between smoking cessation and depressive symptoms over time (Stepankova et al., Reference Stepankova, Kralikova, Zvolska, Pankova, Ovesna, Blaha and Brose2017; Taylor et al., Reference Taylor, McNeill, Girling, Farley, Lindson-Hawley and Aveyard2014). Nevertheless, in line with Taylor et al., a decrease in the number of cigarettes was associated with less depressive symptoms. The latter may indicate that whenever an individual reduces the number of cigarettes smoked per day, effects on the severity of depressive symptoms may be expected. One possible explanation for the non-significant longitudinal results regarding smoking initiation, smoking cessation, alcohol initiation and change in depressive symptoms was the relatively low number of individuals changing binary smoking or alcohol behaviour, resulting in a lack of power.
Our finding that moderate alcohol use was associated with less depressive symptoms in the general population is in line with a recent study (Gémes et al., Reference Gémes, Forsell, Janszky, László, Lundin, Ponce De Leon and Moller2019). In contrast to Gémes et al., however, here heavy drinking was not associated with increases in depressive symptoms, which might be explained by different sample sizes. In the study of Gémes et al., 581 participants were categorized as hazardous drinkers of whom only 55 individuals drank >14 drinks per week, whereas in the current study, 1468 participants drank >10.5 drinks per week (online Supplementary Table S3). However, our validation analyses showed that heavier drinking in men is associated with more depressive symptoms. In a previous study, Lechner et al. reported no effect of alcohol intake reductions on depressive symptoms (Lechner et al., Reference Lechner, Sidhu, Cioe and Kahler2019). Nonetheless, we show that alcohol cessation or a substantial decrease in drinks (i.e. two or three categories) is associated with less depressive symptoms over time. The low number of participants (i.e. 150 heavy-drinking smokers) in the study of Lechner et al. may have restricted the power to detect this effect. Similarly to Haynes et al., heavier drinking was not associated with depressive symptoms but alcohol cessation was associated with a decrease in depressive symptoms (Haynes et al., Reference Haynes, Farrell, Singleton, Meltzer, Araya, Lewis and Wiles2005).
Several hypotheses regarding the association between smoking, alcohol use and depressive symptoms have been described. A frequently mentioned hypothesis is the self-medication theory of smoking and alcohol to treat symptoms of depression (Khantzian, Reference Khantzian1997; O'Neil et al., Reference O'Neil, Conner and Kendall2011). The findings of the current observational, longitudinal study lend no support to the self-medication theory of nicotine and alcohol alleviating feelings of depression in the general population. According to the self-medication theory, smoking and alcohol use initiation and cessation should be negatively and positively associated with symptoms of depression, respectively. However, our observations that smoking was associated with more frequent symptoms of depression multi-cross-sectionally and that alcohol cessation and decreases in cigarettes and drinks per week were associated with less depressive symptoms, argue against the self-medication theory for both smoking and alcohol use. Additionally, conversely to what the self-medication theory assumes, neither smoking cessation, smoking initiation nor alcohol initiation was significantly associated with depressive symptoms. Furthermore, the misattribution hypothesis states that people misinterpret the relief of smoking and drinking withdrawal symptoms as the relief of depressive symptoms and explains why smoking or alcohol cessation leads to less depressive symptoms (McKenzie et al., Reference McKenzie, Olsson, Jorm, Romaniuk and Patton2010; Wootton et al., Reference Wootton, Richmond, Stuijfzand, Lawn, Sallis, Taylor and Munafò2020). In support of this hypothesis, the results of this study indicated that alcohol cessation was associated with decreased depressive symptoms.
Shared genetic vulnerability, environmental interactions or direct causality has also been put forward as hypotheses to explain the association between smoking, alcohol use and depressive symptoms (Khantzian, Reference Khantzian1997; O'Neil et al., Reference O'Neil, Conner and Kendall2011). However, findings in the literature on the temporal relationship between alcohol use, smoking and symptoms of depression are contradictory. Some prior studies suggest that depressive symptoms lead to substance use, while several studies conversely describe that SUD lead to depression or report bidirectional associations (Fergusson, Goodwin, & Horwood, Reference Fergusson, Goodwin and Horwood2003; Gémes et al., Reference Gémes, Forsell, Janszky, László, Lundin, Ponce De Leon and Moller2019; Grant & Harford, Reference Grant and Harford1995; Hasin et al., Reference Hasin, Goodwin, Stinson and Grant2005; Kuo et al., Reference Kuo, Gardner, Kendler and Prescott2006; O'Neil et al., Reference O'Neil, Conner and Kendall2011; Polimanti et al., Reference Polimanti, Peterson, Ong, MacGregor, Edwards, Clarke and Derks2019; Wootton et al., Reference Wootton, Richmond, Stuijfzand, Lawn, Sallis, Taylor and Munafò2020). A recent two-sample bi-directional Mendelian Randomisation (MR) study (Wootton et al., Reference Wootton, Richmond, Stuijfzand, Lawn, Sallis, Taylor and Munafò2020) indicated that smoking is a causal risk factor for a diagnosis of depression, whereas previous MR studies found no evidence for a causal relation (Bjørngaard et al., Reference Bjørngaard, Gunnell, Elvestad, Smith, Skorpen, Krokan and Romundstad2013; Taylor et al., Reference Taylor, McNeill, Girling, Farley, Lindson-Hawley and Aveyard2014; Wium-Andersen, Orsted, & Nordestgaard, Reference Wium-Andersen, Orsted and Nordestgaard2015; Wootton et al., Reference Wootton, Richmond, Stuijfzand, Lawn, Sallis, Taylor and Munafò2020). Similarly, several prospective studies report that alcohol abstinence is associated with reduced depressive symptoms while other studies found no effect on the course of symptoms of depression following alcohol abstinence or any level of intake-reduction (Brown et al., Reference Brown, Inaba, Gillin, Schuckit, Stewart and Irwin1995; Brown & Schuckit, Reference Brown and Schuckit1988; Lechner et al., Reference Lechner, Sidhu, Cioe and Kahler2019; Liappas et al., Reference Liappas, Paparrigopoulos, Tzavellas and Christodoulou2002).
Strengths of this study are the sample size, the prospective design, the range of sensitivity and validation analyses, and the longitudinal follow-up of three quantitative measures of smoking behaviour, alcohol use, depressive symptoms and genetic vulnerability to these traits over a 6-year period. Multiple covariates were added to the models, decreasing the risk of confounding. Naturally, due to the observational design, there was some loss to follow-up and some residual confounding and reverse causation may have occurred. Therefore, no definite conclusions regarding causality may be made. Another limitation was the lack of continuous information and data assessments beyond the past 4 weeks for smoking behaviour and 12 months for alcohol use, precluding conclusions about more acute or longer-term associations. Furthermore, misattribution and self-medication may have a complicated measurement of depressive symptoms (Bolton et al., Reference Bolton, Robinson and Sareen2009; Breslau et al., Reference Breslau, Novak and Kessler2004; Grant & Harford, Reference Grant and Harford1995; Khantzian, Reference Khantzian1997; Kuo et al., Reference Kuo, Gardner, Kendler and Prescott2006; McKenzie et al., Reference McKenzie, Olsson, Jorm, Romaniuk and Patton2010; Wootton et al., Reference Wootton, Richmond, Stuijfzand, Lawn, Sallis, Taylor and Munafò2020). Lastly, the power for PRS analyses was limited.
In summary, the findings of this prospective, 6-year cohort study suggest that smoking and alcohol use are not associated with reduced depressive symptoms over time. Our multi-cross-sectional results provide evidence that smoking and the number of cigarettes smoked per day are positively associated with depressive symptoms. While moderate drinkers experienced fewer depressive symptoms cross-sectionally, heavier drinking in men was associated with more depressive symptoms in our validation analyses, which is in line with previous literature (Gémes et al., Reference Gémes, Forsell, Janszky, László, Lundin, Ponce De Leon and Moller2019). In addition, alcohol cessation or decrease in drinks per week was associated with reduced depressive symptoms longitudinally, supporting the results of several prospective studies (Brown et al., Reference Brown, Inaba, Gillin, Schuckit, Stewart and Irwin1995; Brown & Schuckit, Reference Brown and Schuckit1988; Liappas et al., Reference Liappas, Paparrigopoulos, Tzavellas and Christodoulou2002). Future studies taking genetic risks into account are needed to examine the quantitative, temporal relationship between smoking, alcohol use and depressive symptoms to confirm the results of this study and to explore the underlying mechanisms. As ethical considerations render randomization unlikely for smoking and alcohol behaviours and in light of the observational design of the current study, future MR and (genomic) Structural Equation Modelling studies focused on changes in the variables we investigated may shed light on causality (Grotzinger et al., Reference Grotzinger, Rhemtulla, de Vlaming, Ritchie, Mallard, Hill and Tucker-Drob2019). This study addressed depressive symptoms and alcohol use instead of diagnosis of depression or alcohol use disorder. As such, our results contribute to the elucidation of the associations between smoking, alcohol use and depressive symptoms in the general population and, therefore, inform the development of future effective prevention strategies for public mental health and for clinical psychiatry, where not only depression as a categorical diagnosis but also depressive symptoms are frequently observed.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721002968
Data
Data are available upon reasonable request.
Acknowledgements
Not applicable.
Financial support
This work was supported by the Dutch Ministry of Health, Welfare and Sport (Grant number 310253), with supplement support from the Netherlands Organization for Health Research and Development (ZonMw) and the Genetic Risk and Outcome of Psychosis (GROUP) investigators. The NEMESIS-2 study was conducted by the Trimbos Institute (Utrecht). Financial supporters had no interference in study design, procedures, analysis or interpretation of data. Dr Rutten was funded by a VIDI award number 91718336 from the Netherlands Scientific Organisation. Drs Guloksuz and van Os are supported by the Ophelia research project, ZonMw grant number: 636340001.
Conflict of interest
None. Nini de Boer, Jentien Vermeulen, Bochao Danae Lin, Jim van Os, Margreet ten Have, Ron de Graaf, Saskia van Dorsselaer, Maarten Bak, Bart Rutten, Albert Batalla, Sinan Gülöksüz and Jurjen Luykx have received no funding or compensation from companies.
Ethical standards
Not applicable.









