Dear Editor
Tardive dyskinesia (TD), parkinsonism, akathisia and tardive dystonia are antipsychotic-induced movement disorders that remain a cause for concern in the treatment of patients with psychotic disorder. Movement disorders secondary to antipsychotics constitute a major reason for non-compliance, which results in an increased risk of psychotic relapse (Robinson et al. Reference Robinson, Woerner, Alvir, Bilder, Hinrichsen and Lieberman2002; Lambert et al. Reference Lambert, Conus, Eide, Mass, Karow, Moritz, Golks and Naber2004; Casey, Reference Casey2006).
Although second generation antipsychotics (SGAs) may be associated with a lower incidence of movement disorders, these medications nevertheless still carry risk. We further refer to our previous publication (Bakker et al. Reference Bakker, de Groot, van Os and van Harten2011).
A high-risk group for movement disorders consists of patients with chronic mental illness and therefore chronically exposed to antipsychotic medication, particularly long-stay patients (i.e. patients institutionalized for long periods) with supervised medication regimes (Bakker et al. Reference Bakker, de Groot, van Os and van Harten2011).
Antipsychotic-induced movement disorders (Owens, Reference Owens1999; Factor et al. Reference Factor, Lang and Weiner2005) can be divided into acute syndromes, such as parkinsonism and akathisia, that occur within hours/days or weeks after initiating antipsychotic treatment or increasing the antipsychotic dose (or cessation of anticholinergics), and tardive syndromes, such as TD and tardive dystonia, that develop after months or years of treatment. Given that combinations of acute and chronic movement disorders occur in patients undergoing long-term treatment with antipsychotics, prediction models should include both syndromes, i.e., the four major types of movement disorders (TD, parkinsonism, akathisia and tardive dystonia).
Given the above considerations, the aim of the current prospective study of movement disorders was to provide clinicians with risk information regarding new occurrences of movement disorders for prevention purposes in the population currently most at risk: long-stay patients with chronic mental illness requiring long-term antipsychotic treatment.
Method
Participants
A 4-year prospective naturalistic study (July 2003–May 2007) was conducted with 209 patients with chronic mental illness in order to determine the incidence of and risk factors for the four major types of movement disorders (TD, parkinsonism, akathisia and tardive dystonia). To this end, a cohort was drawn from patients in a general psychiatric hospital (GGZ Centraal, Amersfoort, the Netherlands). Full details of the study design and assessment of movement disorders have been published previously (Bakker et al. Reference Bakker, de Groot, van Os and van Harten2011). The cohort was representative of the population of patients with the most severe chronic mental illness requiring long-stay care, given that the hospital serves an epidemiological catchment area, is the only institute providing this type of care in this area, and patients were randomly selected from a comprehensive list of all inpatients.
Of the patients assessed at baseline (N = 207) 93.7% (n = 194) had one follow-up and 59.4% (n = 123) had two follow-up assessments. Loss to follow-up was due to patients who were difficult to trace after leaving hospital, died or refused assessment after inclusion.
Procedures
The protocol was approved by the standing Institutional Review Board, ‘Medisch-ethische Toetsingscommissie Instellingen Geestelijke Gezondheidszorg’ (Review Board for Human Research in Psychiatry), the Netherlands [protocol number 377].
Written informed consent was obtained from each patient; consent obtained from the next of kin was neither necessary nor recommended by the Review Board for Human Research in Psychiatry.
Measures
Guided by previous literature, variables possibly affecting risk were extracted from patients' case notes including age, sex, BMI, self-reported handedness, diagnosis according to DSM-IV, ethnic group (classified as white and non-white), duration of hospitalization and history of electroconvulsive therapy (ECT). Negative symptoms were rated using the negative symptom subscale of the Positive and Negative Symptom Severity (PANSS) scale (Kay et al. Reference Kay, Fiszbein and Opler1987). The MINI sections for alcohol and drug use were administered, and information on tobacco intake (yes/no, number of cigarettes, cigars, etc; descriptors such as ‘light’, ‘mild’, ‘heavy’ and ‘normal’ use of tobacco) was collected. At baseline and at each follow-up assessment, current use of antipsychotic and anticholinergic medication was collected, and the global symptom rating of the Clinical Global Impression – Schizophrenia severity of illness (CGI-SCH SI) scale was completed. All clinical assessments were carried out by the same psychiatrist (PRB). Information on current use of the above medication was collected from the hospital and outpatient pharmacy databases.
The diagnosis ‘schizophrenia’ hereafter refers to DSM-IV codes 295.30, 295.10, 295.20, 295.90, 295.60, 295.70 and other diagnoses of ‘psychotic disorder’ to 295.40, 297.1, 298.8, 298.9.
Statistical analyses
Incident case definition was based on two consecutive assessments meeting the requirements of Schooler and Kane's criteria for persistent movement disorder (Schooler & Kane, Reference Schooler and Kane1982) (hereafter: persistent movement disorder). Hence, a minimum of one baseline (free of movement disorder) and two follow-up assessments (with evidence of movement disorder at both) were necessary in order to define an incident case by the second follow-up. The yearly incidence rate was estimated by dividing the number of incident cases by the person-years between the first and second follow-up allocated to each patient who at baseline had a score of zero on the scale of a particular movement disorder. Incidence rate is presented as a percentage. Cumulative incidence was defined as the number of the above mentioned incident cases over the follow-up period divided by the number of subjects at risk in the population at baseline.
An alternative analysis was conducted with case definition based on a single occurrence of movement disorder at a single assessment (hereafter: fluctuating movement disorder). The reason for this was that movement disorders constantly fluctuate over time, so that inclusion in the regression of their repeated dichotomous single-occasion measures allowed for calculation of associations between one movement disorder and the other over time.
As the study design consisted of repeated measures nested in the same patient, correlated error of non-independence had to be corrected for. Therefore, we performed multilevel Cox regression models with the measurement occasion (baseline and two follow-ups) as level 1, and subjects as level 2, with the STCOX cluster procedure of the STATA statistical program (StataCorp., 2009). Associations were expressed as hazard ratios and proportional-hazard assumptions were evaluated using the STATA STPHPLOT and STCOXKM graphical procedures, generating log-log plot of survival, and Kaplan-Meier and predicted survival plot, respectively. As these procedures are intended for discrete variables, the distribution of continuous variables was divided by its tertiles, creating tertile groups. For the Cox regression, all 207 patients were included; of the above mentioned variables possibly affecting risk, values missing at random were minimally five times imputed using the STATA ICE procedure within a bootstrap sample to relax normality assumption.
The weighted (for non-missing items) mean score of each movement disorder scale per assessment and of the PANSS negative symptom scale at baseline were included as continuous covariables. Of the CGI-SCH SI, the global score rated at each assessment was used. Continuous variables were mean-centred so that the intercept of the regression line corresponds to the estimated population mean of that variable (Rabe-Hesketh & Skrondal, Reference Rabe-Hesketh and Skrondal2008; Kohler & Kreuter, Reference Kohler and Kreuter2009).
From the full model, including all the above variables, variables with no impact were removed one by one, until only significant variables remained using the criterion of p < 0.05 (final model through backward stepwise regression) (Altman, Reference Altman1999; Steyerberg, Reference Steyerberg2009). In order to assess extra-linearity, quadratic effects were included for continuous variables with p < 0.05 in the initial model (Cleves, Reference Cleves2008). Also, interactions between different variables were included.
Antipsychotic doses were converted to defined daily dose (DDD), for which we refer to our previous publication (Bakker et al. Reference Bakker, de Groot, van Os and van Harten2011). Anticholinergic medication was modelled as a dichotomous variable (yes/no).
Results
Sample characteristics
Over the period of observation (mean = 1.1 years, s.d. = 0.64), of the 209 patients included, 207 participated in the study. One patient developed a brain tumour, another patient died after inclusion. All patients had a history of cumulative antipsychotic intake of minimally 1 year. Attrition was low at 9.8%. Full details of the sample characteristics have been published previously (Bakker et al. Reference Bakker, de Groot, van Os and van Harten2011).
Incidence of persistent movement disorders over period of observation
Yearly incidence rates of persistent movement disorders were 19.6% (95% CI = 10.7%–32.9%) for TD, 21.6% (95% CI = 9.9%–40.9%) for parkinsonism, 3.5% (95% CI = 0.7%–10.1%) for akathisia and 0% for tardive dystonia. Cumulative incidences were 15.9%, 17.7%, 2.7% and 0%, respectively.
Prediction of repeated movement disorders over period of observation
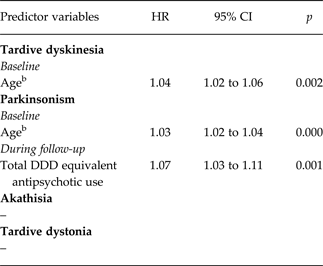
In the final models, after backward elimination, results were as follows (Table 1). Fluctuating TD was positively associated with age (hazard ratio (HR) per year exposure = 1.04, 95% CI = 1.02–1.06). Fluctuating parkinsonism also was positively associated with age (HR = 1.03, 95% CI = 1.02–1.04) and in addition with the total antipsychotic DDD (HR = 1.07, 95% CI = 1.03–1.11). Fluctuating akathisia and tardive dystonia were not associated with any risk factor.
Table 1. Variables related to fluctuating movement disordersa. Final model through backward stepwise Cox regression of repeated measures

a Mean period was 1.1 year (s.d. 0.6).
bAge per year (range = 21.28–85.75).
Discussion
The findings were that (i) long-stay patients with chronic mental illness and long-term antipsychotic treatment have a high risk of incident movement disorder, in particular TD and parkinsonism, (ii) higher age is an important predictor of TD and parkinsonism and (iii) total antipsychotic DDD is an important predictor of parkinsonism. In addition, incidence rates were high for TD and parkinsonism, but, as follow-up data pertain to chronic patients, these may well represent a relapse of an earlier remitted movement disorder. The other variables did not survive the criterion of p < 0.05 into the final model through backward stepwise regression from the full model.
Limitations
This study had some limitations, for which we refer to our previous publication (Bakker et al. Reference Bakker, de Groot, van Os and van Harten2011) as well point to additional limitations. First, it can be hypothesized that age in the current study is not the real risk factor, as older patients in general have been treated over a longer period. Indeed, a post hoc analysis showed a strong and significant correlation between age and years admitted (r = 0.79, p = 0.00), so age in the current study may be a proxy for length of treatment, which is difficult to disentangle further. Second, although in the current study many variables were analyzed in the Cox regression, it could nevertheless be argued that other important variables were not included, for example history of antipsychotic use, which was difficult to retrieve. Third, the cohort in the current study was representative of the population of long-stay patients with the most severe chronic mental illness, hence, results cannot be generalized to chronically ill patients in the community, in whom rates of movement disorder may be different, for example owing to different patient profile, medication regimen or medication compliance.
Strengths
We refer to our previous publication (Bakker et al. Reference Bakker, de Groot, van Os and van Harten2011). In addition, the use of both persistent and fluctuating movement disorders measures, in contrast with many previous studies in which case definition was defined cross-sectionally, may more validly reflect the phenotype, as it more specifically defines the disorder category given the continuously fluctuating nature of the phenotypes under investigation.
Since previous studies, which used a cross-sectional approach and did not focus on the vulnerable subgroup of long-stay hospitalized patients, do not match with the current study, it is not easy to put the current results into context. Although the sample selection and prospective nature of the current study may explain the lack of consistency with some older studies, particularly given that careful meta-analysis indicates that studies of risk factors for movement disorders such as TD show very little consistency (Tenback et al. Reference Tenback, van Harten and van Os2009), other possible explanations for these differences are (i) carryover effects (delayed response effects) after many years of antipsychotic usage in the population studied and/or (ii) the relatively small sample size of the current study.
Given many reported risk factors, but little in terms of consistency or meta-analytic work summarizing the findings, the approach used in the current study was agnostic and explorative, focusing particularly on the most important demographic and illness-related variables.
Although a longitudinal design may offer the possibility to find causal relationships, we think it is premature to interpret the associations found in the current study as causal because (i) the population is using antipsychotics for many years, and (ii) as mentioned before an incident movement disorder may well represent a relapse of an earlier remitted movement disorder. However, the associations found may be helpful in defining high-risk groups for secondary prevention (Szklo & Nieto, Reference Szklo and Nieto2007) which is clinically feasible for implementing prevention in long-stay patients with chronic mental illness requiring long-term antipsychotic treatment. This implies that clinicians should pay special attention to individuals in populations who are older, and/or are on higher doses of antipsychotic medication.
More broadly, prevention starts by systematic screening for movement disorders not only by clinicians but by all care professionals, which takes little time and can be easily implemented in clinical practice.
In conclusion, long-stay patients with chronic mental illness requiring long-term antipsychotic treatment have a disproportionately high risk of incident movement disorders, particularly individuals who are older (TD and parkinsonism), and on higher doses of antipsychotic medication (parkinsonism). Therefore, they deserve special attention. Systematic screening for movement disorders takes little time and can be easily implemented in clinical practice. Future research on movement disorders may be served by the inclusion of (i) all four movement disorders, as done in the current study, since they may represent the pleiotropic effects of (partly) shared genetic factors (Koning et al. Reference Koning, Vehof, Burger, Wilffert, Al Hadithy, Alizadeh, van Harten and Snieder2012), and (ii) scales for subjective well being and quality of life, to better assess patient impact.
Acknowledgements
The authors are grateful to M. Hoornweg-van Beek (MSc), G.V. Boedijn (MSc), M. van Drie (MSc), R. Emons (MSc), A.E. Willems (MSc), Saltro laboratory, hospital and outpatient pharmacies, hospital staff for providing their assistance and to all patients for participating in this study.
Conflict of Interest
P. Roberto Bakker and Izaäk W. de Groot have declared that no competing interests exist.
Jim van Os is/has been an unrestricted research grant holder with, or has received financial compensation as an independent symposium speaker from Eli Lilly, BMS, Lundbeck, Organon, Janssen-Cilag, GSK, AstraZeneca, Pfizer and Servier, companies that have an interest in the treatment of psychosis.
Peter N van Harten has received financial compensation as an independent symposium speaker from Eli Lilly, BMS, Janssen-Cilag and Lundbeck, companies that have an interest in the treatment of psychosis.
This work was supported by grant from the Foundation ‘the Open Ankh’, the Netherlands [grant number SG 21.02]. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.