Introduction
Like many commercial pig populations in the world, the French Large White (LW) breed has been selected for several decades to improve growth efficiency and carcass lean content (Bidanel et al., 2005). Since the early 1990s, litter size has become a major element of the LW overall selection goal and has been successfully selected through ‘hyperprolific’ selection schemes, the use of best linear unbiased prediction (BLUP) animal model and artificial insemination. This improvement has unfortunately been accompanied by an increase in perinatal and, to a lesser extent, birth to weaning mortality.
The adverse effects of selection are often difficult to reveal, as corresponding traits are not routinely recorded in breeding programmes. As suggested by Smith (Reference Smith1977), the use of stored gametes or embryos is an elegant way of measuring genetic trends for a large number of traits. It is complementary to estimations obtained from purely statistical approaches such as the computation of breeding values with BLUP animal models, which are easy to obtain, but are limited to routinely recorded traits. Moreover, contrary to BLUP animal model estimates, results are independent of the genetic parameters of the population. The principle of this methodology is to use frozen material to produce animals that are representative of the population at the beginning of the selection process and compare them with a population sample after selection. This approach was used by Tribout et al. (Reference Tribout, Langant, Caritez, Gogué, Gruand, Guéblez, Labroue and Bidanel2001 and Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003) to estimate genetic trends in the French LW breed from 1977 to 1998. Results showed an increase of 2.9 piglets born per litter, of which 0.8 were additional stillbirths (Tribout et al., Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003). The causes of this deterioration of farrowing survival remain poorly known. It may be due to a higher risk of hypoxia resulting from uterine contractions that reduce oxygenation of the unborn piglets, early placenta detachments, or damage or premature ruptures of the umbilical cords. Such disturbances are more frequent in large litters (Herpin et al., Reference Herpin, Le Dividich, Hulin, Fillaut, De Marco and Bertin1996; Alonso-Spilsbury et al., Reference Alonso-Spilsbury, Mota-Rojas, Villanueva-García, Martínez-Burnes, Orozco, Ramírez-Necoechea, Mayagoitia and Trujillo2005). Several studies have demonstrated the important association between farrowing kinetics (duration and rhythm) and stillbirth (e.g. Fahmy and Flipot, Reference Fahmy and Flipot1981; Fraser et al., Reference Fraser, Phillips and Thompson1997; Canario et al., Reference Canario, Cantoni, Le Bihan, Caritez, Billon, Bidanel and Foulley2006a; Pedersen et al., Reference Pedersen, Jørgensen, Heiskanen and Damm2006). Birth weight is another major component of farrowing survival (Knol et al., Reference Knol, Leenhouwers and VanderLende2002b; Canario et al., Reference Canario, Cantoni, Le Bihan, Caritez, Billon, Bidanel and Foulley2006a). The experiment of Tribout et al. (Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003) was extended to a second generation of sows in order to investigate more thoroughly the different causes of increased pre-weaning piglet mortality and their relationships with sow maternal abilities. The objective of this study was to estimate genetic trends for traits measured at farrowing using the frozen semen methodology proposed by Smith (Reference Smith1977).
Material and methods
Experimental design, animal management and data recording
Two genetic groups of animals (referred to as G77 and G98) have been produced by inseminating French LW sows with semen from boars born either in 1977 or in 1998 (Tribout et al., Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003). Three generations of G77 and G98 pigs were then produced by inter se mating of randomly chosen G77 or G98 boars and gilts. The current experiment used 38 G77 and 40 G98 randomly selected females from the second-generation and their litters produced in the INRA experimental herd of Bourges (Cher). Sows were inseminated twice at a 12-h interval with the semen of boars from the same genetic group. Due to some delay in the production of second-generation boars, gilts were inseminated with frozen semen from first-generation boars for their first parity. Fresh semen was used for second-parity litters as second-generation boars became available and the amount of frozen semen still available was insufficient. Sow and litter characteristics at birth were recorded from August 2003 to September 2004.
Animals were tethered during gestation. Their body composition (body weight and fatness) was measured before entering the farrowing unit (approx. 1 week before the expected date of farrowing). Sows were managed in a batch-farrowing system, with a 3-week interval between successive batches. They were fed 2.5 to 3 kg commercial sow diet twice daily during the whole gestation period. Each farrowing unit contained 20 individual crates but was not always filled. As often as possible, G77 and G98 sows were placed in neighbouring farrowing crates (a G77 female had G98 neighbour sows). Sows were housed on partially slatted floor covered with a thin layer of straw. Crates were separated with a low wall (50 cm high), so that sows could see at least their neighbours. The room was permanently illuminated, and also received natural light. Feed was distributed at 0800 and 1630 h and sows had free access to water with a low-pressure nipple drinker. Farrowing was not induced and birth-assistance treatments (oxytocin and vaginal palpation) were performed only in cases of extreme necessity involving sow survival. Three G77 and four G98 sows were treated during the whole study. From day 111 of gestation, animals were monitored daily to identify signs of impending parturition (milk let down, vulvar swelling and mucous secretion). Another aim of these visits was to reduce their apprehension towards human presence. Sow parturition was observed continuously in 98 of the 137 farrowings. This was facilitated by video supervision from an adjacent room. The onset of farrowing was considered as missing if the first piglet(s) born was (were) dry when seen for the first time and if more than two piglets were observed at the first visit. Piglet birth time and order were individually recorded. Each expelled piglet was immediately caught. Its umbilical cord was cut and a blood sample taken for physiological studies (Canario et al., 2005). The remaining part of the umbilical cord was ligatured with a surgical silk. Subsequently, the piglet was brought to an ‘intervention place’, away from the parturient sow but located inside the maternity, to be dried with straw and drying paper, weighed, sexed and marked on its back with a number corresponding to its birth order. Apart from these manipulations that may stimulate newborn vitality, avoidance of any interference with the natural delivery of the piglets was aimed at. For instance, there was no human intervention to control aggression toward newborns or to avoid crushing, and piglets were not assisted to find a teat. Only piglets blocked in their foetal membranes and about to die from asphyxia were helped. The limitations and non-interventions mentioned above permitted to appreciate the biological phenomenon as objectively as possible. Although restricted, care was provided to the animals when essential to respect the general guidelines outlined in the European animal welfare regulations.
At farrowing, two classes of piglets born dead are commonly defined: pre partum deaths, corresponding to mummified piglets and piglets dying before onset of partum (macerated piglets), and intra-partum deaths associated with intrauterine hypoxia and dystocia (Svendsen et al., Reference Svendsen, Svendsen and Bengtsson1986). These intra-partum deaths will be referred to as ‘stillbirth’ hereafter, in accordance with Christianson (Reference Christianson1992). Thanks to intensive supervision, piglet stillbirth could be precisely defined and determined: a stillborn was an apparently full-term foetus that made no visible movement after birth. All piglets recorded as stillborn were deep frozen for later examination. Two series of post mortem examinations were realised on thawed piglets, including the lung flotation test (Sims and Glastonburry, Reference Sims and Glastonburry1996). Among the 142 piglets that were assumed stillborn, 128 were dissected post mortem. Five piglets were misclassified and then correctly classified as born-alive piglets. Prenatal deaths, including mummified and pre partum stillborn piglets (fully developed piglets, with signs of deterioration) were also counted. Different criteria, reviewed by Alonso-Spilsbury et al. (Reference Alonso-Spilsbury, Mota-Rojas, Villanueva-García, Martínez-Burnes, Orozco, Ramírez-Necoechea, Mayagoitia and Trujillo2005), are associated with stillbirth and birth difficulties. Among those, we chose the criteria described in Table 1: related to death due to hypoxia (meconium staining in the skin, signs of cyanosis), predisposition to farrowing difficulties (stillborn with big head, umbilical cord content and length) and risk of asphyxia (respiratory difficulties, umbilical cord node, membrane wrapping in born-alive piglets). Sow and piglet behaviour was recorded during farrowing using 24 time lapse video (VHS Panasonic video recorder associated with DPX9 multiplexer advanced technology video). The video allowed the birth time of each piglet to be recorded, and more precise and missing values from the direct observations to be retrieved. The birth orientation of each piglet and time to first placenta expulsion were also obtained by video observations.
Table 1 Criteria for piglet birth difficulties and risk of hypoxia

Statistical analyses
A large number of traits potentially involved in stillbirth and differences between the two genetic groups were recorded. Traits describing sow condition at farrowing included age at insemination (AI), gestation length (GEST), sow weight (SWF) and fatness (FAT = mean of six measurements on each side of the spine at the levels of hip joint, last rib and shoulder) at farrowing. Prolificacy traits included the numbers of prenatal deaths (PRE = mummified + macerated piglets), stillborn (NSB) and born-alive piglets (NBA). The number born in total (NBT = NSB + NBA), the number born in global (NBG = NBT + PRE) and the proportion of stillborn piglets per litter (PSB = NSB/NBT) were derived from the previous variables. The piglet individual birth weight (BW) allowed litter birth weight (LBW) as well as the coefficient of variation (CVBW), standard deviation (STDBW), maximum (MAXBW) and minimum (MINBW) of within-litter individual piglet birth weights to be calculated. Similarly, birth interval, defined as the period of time between two successive births, allowed several kinetics traits to be computed:
• the farrowing duration (FD), defined as the interval of time between the birth of the first piglet and that of the last piglet of a litter;
• the time elapsed from the onset of farrowing to piglet birth (ELAPS); farrowings ending more than 10 h after the birth of the first piglet were considered as abnormal and discarded;
• the average (BI), maximum (MAXBI) and standard deviation (SDBI) of birth intervals, as well as the interval before a stillborn piglet birth (IBSB);
• the time of first placenta expulsion (PLA), defined as the time interval between the last piglet born and the first placenta expulsion.
Traits associated with farrowing difficulties were those described in Table 1; they were considered as count data, except umbilical cord length, which was treated as a continuous trait.
Sow traits (including litter size, litter weight and kinetics traits), as well as piglet birth weight and umbilical cord length, were analysed with a mixed linear model including the fixed effects of genetic group (GG: G77 or G98), parity (first or second), a random batch effect plus an additional random effect of the sow, using the MIXED procedure of SAS software (Statistical Analysis Systems Institute, 2001). When the interaction between parity and GG was significant, estimates were obtained for each parity. Least-squares means were estimated with and without adjustment for litter size. The age and weight at measurement were included as additional covariates for the analysis of SWF and FAT, respectively. The analyses of FD, BI and SDBI were performed in the same way but after a Box–Cox transformation in order to normalise their distribution.
Piglets’ probability of stillbirth (SB) and traits associated with farrowing difficulties other than umbilical cord length were analysed using a generalised estimating equation approach applied to a generalised linear mixed model, with the GENMOD procedure of SAS (2001). The model assumed a binomial distribution of the dependent variable and a logit link function, and included the fixed effects of genetic group and dam parity, as well as a random birth litter effect with an exchangeable correlation matrix.
To obtain a better understanding of the relationship of stillbirth risk with the progress of farrowing, additional analyses of piglet’s probability of stillbirth were performed with parity, FD, GG, time of farrowing when birth occurred, recorded as first, second and third part of farrowing, as fixed effects and the litter of birth as a random effect, using the GENMOD procedure of SAS (2001). The three parts of farrowing were established after a first division of the whole farrowing period in 10 equal periods of time (P1 to P10) followed by a grouping of P1 and P2 (first part), P3 to P8 (second part) and P9 and P10 (third part) so as to have a similar number of piglets in each part. Two further sets of factors were considered in two successive analyses by including them as additional independent variables in the model previously described for SB, without the time of farrowing effect. The first set included litter size (NBT, PRE) and piglet weight (BW, CVBW) traits. The second set was performed on a subset of data and included additional traits related to farrowing kinetics (ELAPS, SDBI, PLA) and sow farrowing condition (FAT, SWF) traits. In each of these additional analyses, the contribution of each effect to the variance reduction was evaluated adding them one by one and quantified with the coefficient of determination of Hosmer and Lemeshow (Reference Hosmer and Lemeshow1989).
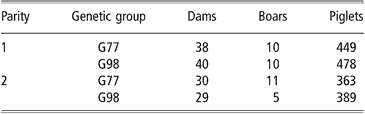
The realised genetic trends from 1977 to 1998 (ΔG) and their standard errors (s.e. ΔG) were estimated for each considered trait as proposed by Smith (Reference Smith1977): ΔG = 2 × (G98 mean–G77 mean) and s.e. ΔG = 2 × s.e. (G98 mean–G77 mean). A total of 137 litters and 1679 piglets were analysed; their distribution according to genetic group and parity are shown in Table 2. All estimates are given on the inverse transformed scale.
Table 2 Number of records according to dam parity and genetic group
Results
Genetic trends for litter size, litter weight and farrowing kinetics traits
Genetic group least-squares means and estimated genetic trends for litter traits, including prolificacy and farrowing mortality, are shown in Table 3. Due to limitations in the experimental facilities, sows were rather old at the first insemination. The higher age at insemination of G98 was due to a random choice of older gilts and did not reflect any difference in sexual maturity. G77 sows had a longer gestation length than G98 sows, and a tendency (P = 0.08) remained when adjusting for litter size. The contrasts between genetic groups for litter size widely differed between the first and second parity. Almost no difference was observed in first-parity litters, whereas second-parity mean litter size of G98 exceeded that of G77 by 2.3 piglets. Yet, both first- and second-parity G98 litters had a higher number and proportion of stillbirths than G77 litters, resulting in a four times greater probability of stillbirth in G98 as compared with G77 piglets.
Table 3 Comparison of sow characteristics at farrowing in two populations produced by use of frozen semen collected on boars born in 1977 or 1998 (G77 and G98): least-squares means, estimated genetic trends (ΔG) and standard errors (s.e. ΔG)†

†ΔG = 2 × (G98 mean–G77 mean) and s.e. ΔG = 2 × s.e. (G98 mean–G77 mean).
‡AI = age at insemination; GEST = gestation length; AGEST = gestation length adjusted for NBT; FAT = sow fatness at farrowing; SWF = sow weight at farrowing; NBG = number born in global; NBT = number born in total; sex ratio = proportion of males in the litter; PRE = number of prenatal deaths; PPRE = proportion of prenatal deaths; NSB = number of stillbirths. PSB = proportion of stillbirths.
Average piglet weights and corresponding genetic trends are presented in Table 4. G98 piglets were heavier at birth than G77 piglets. As a consequence, litter birth weight increased by more than 5 kg over the 1977–98 period of time. Within-litter BW dispersion traits (MINBW, MAXBW, STDBW and CVBW) did not exhibit any significant genetic trend.
Table 4 Comparison of litter and piglet weight characteristics at farrowing between two populations produced by use of frozen semen collected on boars born in 1977 or 1998 (G77 and G98): least-squares means, estimated genetic trends (ΔG) and standard errors (s.e. ΔG)†

†ΔG = 2 × (G98 mean–G77 mean) and s.e. ΔG = 2 × s.e. (G98 mean–G77 mean).
‡BW = birth weight; ABW = birth weight adjusted for NBT; LBW = litter birth weight; MINBW = minimum within-litter birth weight; AMINBW = minimum within-litter birth weight adjusted for NBT; MAXBW = maximum within-litter birth weight; AMAXBW = maximum within-litter birth weight adjusted for NBT; CVBW = coefficient of variation of within-litter piglet weight; ACVBW = coefficient of variation of within-litter piglet weight adjusted for NBT; SDBW = standard deviation of within-litter piglet weight; ASDBW = standard deviation of within-litter piglet weight adjusted for NBT.
Farrowing duration and birth intervals did not differ between the two genetic groups, neither traits describing birth irregularity (SDBI, MAXBI and IBSB, results not shown). IBSB was on average 13 min longer than BI. PLA was similar in both groups and showed a large variability.
Genetic trends in birth difficulties and risk of hypoxia and asphyxia
Estimates associated with stillborn characteristics, risk of hypoxia and asphyxia are shown in Table 5. Birth difficulties were not associated with membrane wrapping, although G98 piglets had a 1.9-times higher risk of getting born in their membrane than G77 piglets. Though very rare (only 10 cases), the risk of having an umbilical node was higher in G98 piglets. The examination of stillborn piglets showed that stillborn G98 piglets tended to suffer more from cyanosis and had longer umbilical cords that were more frequently empty (nearly three-times lower probability of blood presence inside the cord).
Table 5 Comparison of total litter, stillborn and born-alive piglet characteristics at birth between two populations of sows produced by use of frozen semen collected on boars born in 1977 or 1998 (G77 and G98 sows): occurrence and probability of observation (P)

†0 = empty, 1 = full.
‡0 = head first, 1 = hind legs or back first.
Results from modelling probability of stillbirth (Table 6) highlighted between genetic group differences in the main factors of variation of stillbirth, albeit the reduction in the residual sum of squares due to the successive addition of each explanatory variable demonstrated that time of birth was the major determinant of stillbirth in both genetic groups (21.98% and 22.27% variance reduction in G77 and G98, respectively). Gestation length, the presence of prenatal dead piglets and the variability in within-litter birth weight were strongly involved in stillbirth of G77 piglets, whereas sow body condition and, to a much lesser extent, the time of first placenta expulsion as well as the square component of piglet birth weight (i.e. the influence of the lightest and biggest piglets within the litter) resulted in strong deviance reductions in G98 litters. As a result of these differences, only two factors of variation of stillbirth reached significance in the joint analysis of G77 and G98 data: FAT, which was negatively associated with stillbirth and ELAPS, which was positively associated with stillbirth. In both genetic groups, a highly significant increase in piglet’s probability of stillbirth occurred as farrowing proceeded (Figure 1).
Table 6 Reduction (R) of deviance (D) for probability of piglet stillbirth due to the addition of several potentially explanatory factors either globally or for two populations produced by use of frozen semen collected on boars born in 1977 or 1998 (G77 and G98)

†P = sow parity. SEX = sex of the piglet. GEST = gestation length. NBT = number born in total. PRE = number of prenatal deaths. BW = individual birth weight. CVBW = coefficient of variation of within-litter individual birth weight. ELAPS = time of birth, measured as time elapsed from the onset of farrowing. SDBI = standard deviation of within-litter birth interval between successive births. PLA = time of first placenta expulsion. FAT = sow fatness at farrowing. SWF = sow weight at farrowing.
‡Sign of the corresponding estimate indicates whether the association between probability of stillbirth and the explicative variable is negative or positive.

Figure 1 Effect of time of birth (first, second or third part of farrowing) on piglet’s probability of stillbirth in two populations produced by use of frozen semen collected on boars born in 1977 or 1998 (G77 and G98 piglets).
Discussion
This is to our knowledge the first time that genetic trends for a large number of maternal ability traits were estimated in pigs. Although the accuracy of the results is limited by the size of the experiment, much original information is provided regarding the effects of selection and the relative importance of the different components of maternal ability for piglet survival. This information will be of great interest to define more adequate breeding goals and contribute to implementing biologically more sustainable breeding plans.
Prolificacy results showed strong discrepancies between the first and the second parity. Although the random nature of this difference cannot be totally excluded due to the rather limited amount of data available, it may partly be related to the use of frozen semen in the first parity, which could have prevented the genetic differences in prolificacy detected in the previous generation (Tribout et al., Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003) to be expressed. Another potential explanation might be a lower response to selection in the first parity. Indeed, a lower response to selection in the first parity was also obtained in the previous generation (Canario et al., 2006c). Although number born in total did not significantly differ between G77 and G98 litters, presumably because of the limited number of litters produced, the genetic trend in the second parity was in agreement with the results of Tribout et al. (Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003). Moreover, the number of piglets born in global (NBG) differed between G77 and G98 litters, as a consequence of a higher number of both NBT and prenatal deaths in G98 litters. These higher prenatal losses could be due to an increased competition between foetuses in late gestation in relationship with intrauterine crowding, which may result in an insufficient development of some placentas (English and Morrison, Reference English and Morrison1984; Van der Lende et al., 2000). Indeed, attempts to phenotypically increase the number of embryos in the uterus (e.g. Vallet, Reference Vallet2000) resulted in higher losses in late gestation. Conversely, Johnson et al. (Reference Johnson, Nielsen and Casey1999) did not obtain any deterioration of late foetal survival in a line selected for increased litter size. Intrauterine crowding may be accentuated by the higher growth potential of G98 foetuses (Canario et al., 2007).
The deterioration of piglet survival was larger than that found by Tribout et al. (Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003). A similar degradation of piglet survival was observed over the last years in French pig herds (see the large-scale study of Le Cozler et al. (Reference Le Cozler, Guyomarc’h, Pichodo, Quinio and Pellois2002)) and in several other experimental studies (review of Blasco et al., Reference Blasco, Bidanel and Haley1995; Johnson et al., Reference Johnson, Nielsen and Casey1999). This unfavourable trend is generally considered as a response to selection for the number born in total. It is not only due to a scale effect, as G98 first-parity piglets had a higher probability of stillbirth than G77 piglets, albeit litter size was similar in both groups. Johnson et al. (Reference Johnson, Nielsen and Casey1999) suggested that it might be mediated through a negative effect on birth weight. Lighter piglets would be less developed at birth, in association with problems in tissue differentiation and growth (Klemcke et al., Reference Klemcke, Lunstra, Brown-Borg, Borg and Christenson1993) and would have a higher probability of stillbirth. In the current study, stillborn piglets had a 100 g lower weight than piglets born alive, which is in accordance with other recent studies (Leenhouwers et al., Reference Leenhouwers, Van der Lende and Knol1999 and Reference Leenhouwers, Wissink, Van der Lende, Paridaans and Knol2003; Knol et al., Reference Knol, Ducro, Van Arendonk and Van der Lende2002a). This weight difference has remained stable between 1977 and 1998. Yet, things are somewhat more complex in the present study, as piglet birth weight has increased over the last 20 years, presumably as a consequence of selection for post-weaning growth rate. This increase is in agreement with findings from Tribout et al. (Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003), even if somewhat higher values were obtained here: +240 g v. +180 g for BW adjusted for litter size. Moreover, unlike many other studies (e.g. Leenhouwers et al., Reference Leenhouwers, Van der Lende and Knol1999 and Reference Leenhouwers, Wissink, Van der Lende, Paridaans and Knol2003; Knol et al., Reference Knol, Leenhouwers and VanderLende2002b; Canario et al., Reference Canario, Cantoni, Le Bihan, Caritez, Billon, Bidanel and Foulley2006a), individual birth weight did not appear as a major determinant of probability of stillbirth in G77 and G98 litters. This discrepancy may be partly related to the way stillborn piglets were defined. Unlike most other studies, where piglets born alive which die shortly after birth are classified as stillborn piglets, the current study only included piglets born dead. Birth weight becomes a major factor for survival in early lactation (Mesa et al., Reference Mesa, Safranski, Cammack, Weaber and Lamberson2006). Our result was in agreement with Leenhouwers et al. (Reference Leenhouwers, De Almeida Junior, Knol and Van der Lende2001), who found independence between farrowing survival and birth weight in a study where farrowing was carefully supervised. The only noticeable influence of birth weight concerns the quadratic component of birth weight in G98 sows, which is positively associated with stillbirth. This means that modern piglets with extreme weights tend to have a higher probability of stillbirth. On the one hand, heavy piglets would suffer from difficulties to go through the birth canal, especially in first-parity sows (Pejsak, Reference Pejsak1984). On the other hand, England (Reference England1974) suggested that smaller piglets would be propelled less efficiently and remain in the uterine horn longer, overtaken by bigger ones. Thus, they would suffer more from hypoxia. Within-litter variation of birth weight was important for piglet survival in G77 litters. This positive effect of BW homogeneity is in agreement with the results of Tribout et al. (Reference Tribout, Caritez, Gogué, Gruand, Billon, Bouffaud, Lagant, Le Dividich, Thomas, Quesnel, Guéblez and Bidanel2003).
Farrowing duration is known to be a key determinant of intra-partum stillbirth (e.g. Fahmy and Flipot, Reference Fahmy and Flipot1981; Canario et al., Reference Canario, Cantoni, Le Bihan, Caritez, Billon, Bidanel and Foulley2006a). It did not significantly differ between the two genetic groups of sows, even if G98 second-parity farrowings were slightly longer than the G77 ones. However, the time elapsed from the onset of farrowing appeared as the most important contributor to probability of stillbirth in both G77 and G98 litters. The last piglets born in a litter are more likely to suffer from a larger number of uterine contractions, to have a higher risk of premature rupture of the umbilical cord, leading to a higher risk of hypoxia and stillbirth (Randall, Reference Randall1972a and Reference Randall1972b). These time effects were similar in both genetic groups, but the longer umbilical cord of G98 stillborn piglets may have increased the risk of nodes as compared with G77 piglets. The larger occurrence of cyanosis under the body in G98 stillborn piglets is an additional sign of severe hypoxia. Cyanosis is likely due to a concentration of blood around vital organs, particularly the heart, after the rupture of the umbilical cord (Alonso-Spilsbury et al., Reference Alonso-Spilsbury, Mota-Rojas, Villanueva-García, Martínez-Burnes, Orozco, Ramírez-Necoechea, Mayagoitia and Trujillo2005). In this study, the higher proportion of empty cords in stillborn G98 piglets is another cue for a premature rupture of the umbilical cord. This rupture lowers placenta blood pressure, causes a partial collapse of the chorionic villi and thus facilitates placenta detachment (Perry, Reference Perry1954). No differences in time to first placenta expulsion were observed between the two genetic groups, but this simple measurement might not be the best one to reveal differences in premature rupture of the umbilical cord and early decomposition of the farrowing placental tract. No trend in membrane obstruction was found. However, Biensen et al. (Reference Biensen, Wilson and Ford1999) proposed with reference to the Meishan breed that an intense selection for litter size might result in an indirect selection for small, thin but highly vascular placentas. A general thinning of foetal membranes and cords with higher risk of placenta detachment could be involved in the larger stillbirth rate observed in G98 sows.
Sow body fat condition at farrowing strongly influenced probability of stillbirth in G98 litters, in agreement with the results of Herpin et al. (Reference Herpin, Le Dividich and Amaral1993). Selection for litter size has increased the resource demanding processes of pregnancy and lactation, whereas selection for fast lean growth may have indirectly put an increased demand on delivery of nutrients by the placenta and may have put conceptuses at a greater risk of prenatal mortality (Vallet et al., Reference Vallet, Leymaster and Christenson2002) and stillbirth (Grandinson et al., Reference Grandinson, Rydhmer, Strandberg and Solanes2005). Selection for increased lean growth has resulted in higher prenatal mortality in several experiments (Vangen, Reference Vangen1972; Kerr and Cameron, Reference Kerr and Cameron1995). Since animals from both genetic groups were fed the same diet according to the same norm during gestation, G98 sows may have been restricted in their ability to provide enough nutrients to their foetuses in late gestation.
Gestation length decreased by more than 1 day, probably in relationship with the increase in prolificacy. Contrary to previous studies suggesting that despite its low variability, gestation length may be an additional factor involved in piglet maturation at birth and birth survival (Hanenberg et al., Reference Hanenberg, Knol and Merks2001; Rydhmer and Lundeheim, 2005; Canario et al., Reference Canario, Cantoni, Le Bihan, Caritez, Billon, Bidanel and Foulley2006a), its importance for piglet survival was found in G77 piglets only.
Additional parameters have been tested as potential components of the observed difference in probability of stillbirth. Results from studies investigating the influence of birth orientation on stillbirth probability are controversial (Hughes and Varley, Reference Hughes and Varley1980). It does not seem to be involved in the larger number of G98 stillbirths, as no significant genetic trend in birth orientation was observed. Likewise, no significant differences in membrane wrapping or attachment or in the proportion of piglets with big heads were detected. The observed trend on the sex ratio in second parity might be important; but as male piglets show a higher probability of stillbirth than females (Canario et al., Reference Canario, Cantoni, Le Bihan, Caritez, Billon, Bidanel and Foulley2006a), it would rather reduce the difference between G77 and G98 genetic groups. Pedersen et al. (Reference Pedersen, Jørgensen, Heiskanen and Damm2006) reported a more variable farrowing process, i.e. a larger standard deviation in birth interval in litters with stillbirths and a decelerating rhythm of expulsion with progress of farrowing, which may result in a higher risk of hypoxia. No significant trends were observed for these traits in the current study.
Conclusions and implications
The selection of French LW pigs has until recently considerably increased the risk of stillbirth. Previous results have shown that the use of a proper selection criterion such as the number born alive (Johnson et al., Reference Johnson, Nielsen and Casey1999; Canario et al., Reference Canario, Roy, Gruand and Bidanel2006b) or the maternal component of probability of stillbirth (Leenhouwers et al., Reference Leenhouwers, Wissink, Van der Lende, Paridaans and Knol2003) instead of total number born, should prevent further degradation of farrowing mortality. Our results give some insight into the mechanisms associated with this increased mortality and into the traits that may be important to consider when defining future breeding goals. In particular, they confirm that increasing birth weight is unlikely to be a good way to decrease stillbirth probability and suggest that sow body condition at farrowing can be involved in farrowing survival, which is of particular interest, as it is also likely to influence piglet survival during lactation (Grandinson et al., Reference Grandinson, Rydhmer, Strandberg and Solanes2005). Yet, additional studies remain necessary to understand better the complex mechanisms involved in piglet survival at farrowing and during lactation in order to be able to predict better and control the long-term effects of selection. The use of high-throughput genomic technologies is likely to be of high interest for such investigations.
Acknowledgements
The contributions of students (A. Jamois, A. Bonaventure, M. Quiertant and E. Mosnier) who participated to the data collection, helping to keep the first author awake during the long nights on the farm, and those who were involved in preliminary analyses are gratefully acknowledged. We also thank the staff of Bourges experimental unit for its participation in the data collection. This work was supported by the INRA and the French Technical Institute in Pig Production (IFIP).









