Introduction
Attention deficit hyperactivity disorder (ADHD) is a prevalent disorder in adults as well as in children. The central dopamine system may be altered in adults with ADHD. It has been confirmed that the availabilities of the dopamine transporter (DAT) and receptor could be lower.Reference Volkow, Wang and Kollins 1 , Reference Volkow, Wang and Newcorn 2 A recent study with positron emission tomography (PET) demonstrated that the hypodopaminergic tone may be just one of the characteristic of ADHD, the release of dopamine during a challenging task among patients with ADHD, is also elevated than controls.Reference Badgaiyan, Sinha, Sajjad and Wack 3 Also, increased dopamine release induced by amphetamine among treatment-naïve adults with ADHD was also reported.Reference Cherkasova, Faridi and Casey 4 The role of dopamine in ADHD has been confirmed in healthy parents with children with ADHD through gene studies and clinical trials with methylphenidate.Reference Krause, Dresel, Krause, Kung and Tatsch 5 –Reference Tai, Chi and Chu 7 The results showed that altered dopamine function, such as hypodopaminergic tone, with phasic elevated release, can be a characteristic of adult ADHD.
It has been confirmed that poor performance in several domains of executive function is an important characteristic of adult ADHD.Reference Boonstra, Oosterlaan, Sergeant and Buitelaar 8 , Reference Barkley 9 Several researchers have shown that patients with ADHD have a preference for risky behavior, which leads to poor performance in gambling tasks.Reference Garon, Moore and Waschbusch 10 , Reference DeVito, Blackwell and Kent 11 It is worthy to note that the gambling task is not identical to other behavioral tasks. Winning (as the reward) and losing (as the penalty) are the alternative consequences of each trial. Therefore, gambling tasks, such as the Iowa Gambling Test (IGT), could be good instruments by which to probe the nature of the psychological and biological response brought by winning as well as losing.Reference Beitz, Salthouse and Davis 12 Little is known with regard to the physiological response during losing in adults with ADHD. A study of peripheral markers (skin conductance and heart rate) found that patients with ADHD may be less sensitive to the magnitude of the penalty (monetary loss) during a gambling task.Reference Luman, Oosterlaan, Knol and Sergeant 13 An altered event-related potential was also observed among children with ADHD during losing.Reference van Meel CS, Heslenfeld, Oosterlaan, Luman and Sergeant 14 Sonuga-Barke and Fairchild proposed that dopaminergic dysfunction, which is related to the reward system in the ventral frontostriatal network in individuals with ADHD could be associated with their poor evaluation of experienced outcomes, and lead to poor learning of prediction-outcome associations.Reference Sonuga-Barke and Fairchild 15 Whether or not dopaminergic dysfunction is related to the activation of the reward system during winning as well as losing is unclear.
One functional magnetic resonance imaging (fMRI) study demonstrated hypoactivation of the medial orbitofrontal cortex (mOFC) during high-incentive rewarding in patients with ADHD, and such dysfunction of the mOFC is related to the preference for risky behavior observed in patients with ADHD.Reference Wilbertz, Tebartz van Elst and Delgado 16 Another study reported that there is lower activation of the ventral striatum (VS) during anticipation and higher activation of the OFC during rewarding in adults with ADHD.Reference Ströhle, Stoy and Wrase 17 A recent meta-analysis confirmed ventral striatal hyporesponsiveness in patients with ADHD during reward anticipation.Reference Plichta and Scheres 18 As mentioned, little is known with regard to the mechanism related to losing among patients with ADHD. A recent fMRI study indicated a blood oxygen level-dependent (BOLD) response of the medial prefrontal cortex (mPFC) during losing among healthy controls, but this effect was not observed in patients with ADHD.Reference Norman, Carlisi and Christakou 19 In addition, the availability of DAT and dopamine receptors in the reward pathway was found to be associated with the motivation deficiency observed in adults with ADHD.Reference Volkow, Wang and Newcorn 20 Dopamine release in the VS was observed among pathological gamblers, which is also often found in patients with ADHD when losing money.Reference Linnet, Peterson, Doudet, Gjedde and Moller 21 Therefore, it was proposed that these hot cognitive deficits may contribute to poor functioning of the reward system in patients with ADHD, which is related to the dopamine system.Reference Scheres, Tontsch, Thoeny and Kaczkurkin 22
It is well-known that DAT is one of the most important target sites of methylphenidate.Reference Volkow, Fowler, Wang, Ding and Gatley 23 It is also worth noting that expression of the dopamine gene may be correlated with the BOLD signal when a rewarding task is performed by adults with ADHD.Reference Hoogman, Onnink and Cools 24 Although altered reward function and hypodopamine activity have been noted among patients with ADHD, several gaps remain to be filled. First, evidence from studies employing molecular imaging (SPECT or PET) and fMRI is very scarce. Considering the importance of dopaminergic treatment for ADHD, clarification of this association would be helpful in order to clarify the potential effect of pharmacological treatment on risky behavior in clinical practice.Reference Agay, Yechiam, Carmel and Levkovitz 25 , Reference DeVito, Blackwell and Kent 26 Second, little is known regarding the feasibility of the IGTReference Bechara, Damasio, Damasio and Anderson 27 , Reference Dunn, Dalgleish and Lawrence 28 as a reward paradigm for use with fMRI for the study of adults with ADHD. Third, as resting DA tone and phasic release could be important characteristics of ADHD, the cross-sectional evidence on the biological makers during resting and under challenge task, are scarce.
In the present study, we investigated the magnitude of activation of the ventral frontostriatal network induced by reward (monetary gain) and penalty (monetary loss) during a gambling task. The IGTReference Bechara, Damasio, Damasio and Anderson 27 , Reference Dunn, Dalgleish and Lawrence 28 was used to probe neural activity during a reward-related task. It is worth noting that dopamine release in the VS during the IGT is correlated with the excitement level in pathological gamblers.Reference Linnet, Moller, Peterson, Gjedde and Doudet 29 This implies that the IGT could be a useful tool for investigating the neural activity underlying the reward process. In the present study, we used an event-related IGT with fMRI to investigate covariates of the BOLD response induced by different magnitudes of monetary reward and penalty in each IGT trial with parameter estimation.Reference Knutson, Fong, Adams, Varner and Hommer 30 , Reference Jessup and O’Doherty 31 Larger estimations indicated a greater association between the BOLD response in the region of interest (ROI) and the magnitude of reward (or penalty).
The aim of the present study was to probe the association between DAT availability, assessed by SPECT using [99mTc]TRODAT-1, and the BOLD response during winning and losing in the corticostriatal pathway, which varies with the magnitude of monetary reward and penalty, in adults with ADHD and controls using the IGT. We hypothesized that: (a) dysfunction in the magnitude of reward or penalty could be a characteristic of ADHD, patients with ADHD having a lower threshold for engaging in risky gambling as compared with the controls and (b) low dopaminergic tone is associated with altered function in the reward and punishment pathways during decision-making in patients with ADHD. Therefore, we speculated that the BOLD response, estimated by fMRI, is associated with DAT availability, assessed by SPECT.
Methods
Ethics statement
The research protocol was approved by the Ethical Committee for Human Research at the National Cheng Kung University Hospital, and written informed consent was obtained from each subject before any procedures were performed. The methods were carried out in accordance with the relevant guidelines, including any relevant details.
Participants and procedures
Sixteen participants (eight male and eight female, mean age = 26.56 ± 4.07 years) were recruited. The subjects were adults with a clinical diagnosis of ADHD who were referred from psychiatry outpatient clinics of a university hospital between January 2012 and December 2015. The inclusion criteria were: subjects must (a) fulfill the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for ADHD; (b) be aged between 20 and 60 years; (c) have no physical illness and stable vital signs; (d) have no evidence of substance abuse/dependence as assessed during a clinical interview with the research psychiatrist at the time of enrollment; and (e) have never received any antipsychotic or antidepressant treatment and be free of any psychotropic medication for more than 1 week at the time of testing. The exclusion criteria for all participants were as follows: (a) other co-morbid psychiatric illnesses, substance abuse/dependence, or neurological illnesses; (b) mental retardation or an intelligence quotient (IQ) < 70; (c) all female participants of child-bearing age had to take an acceptable form of contraceptive throughout the duration of the study in order to be included. All female participants underwent an instant urine pregnancy test prior to starting the experiment. One participant was taking methylphenidate but stopped 1 week prior to screening, two had received methylphenidate in the past, and 13 had never been medicated.
Sixteen healthy controls (eight female and eight male, mean age = 27.81 ± 6.56 years) were also enrolled from the community. No significant differences were found in age, years of education, smoking, or body mass index (BMI) (t(age) = 0.65, df = 30, P = .52; t(education) = 0.91, df = 30, P = .34, Fisher’s exact test for smoking, P = .60, t(BMI) = 1.08, df = 30, P = .29) between the two groups. All control participants were confirmed by a senior psychiatrist to be free of any mental disorder according to the MINI Neuropsychiatry Interview (MINI), and none had received any medication in the past 3 months.
[99mTc]TRODAT-1 SPECT
The imaging procedure was identical to that used in our previous study.Reference Chen, Yeh and Lee 32 For brain imaging, each subject was intravenously administered 740 MBq (20 mCi) [99mTc]TRODAT-1 (a radio-labeled form of a tropan derivative for the selective labeling of DAT) in a quiet environment about 10 minutes after insertion of an intravenous line. The SPECT data were obtained using an energy window of 15% centered on 140 keV for [99mTc]. Imaging of [99mTc]TRODAT-1 was initiated approximately 240 minutes after injection, and SPECT images were acquired over a circular 360 rotation in 120 steps, 50 seconds per step, in a 128 × 128 × 16 matrix. The images were then reconstructed using Butterworth and Ramp filters (cut-off frequency = 0.3 Nyquist; power factor = 7) with attenuations by Chang’s method, and the reconstructed transverse images were realigned parallel to the canthomeatal line. The slice thickness of each transverse image was 2.89 mm. In addition, all subjects underwent magnetic resonance imaging (Signa CV-I, 1.5 T, GE Medical Systems, Milwaukee, WI). Using the commercial software PMOD (PMOD Technologies, Zurich, Switzerland), each subject’s SPECT image was automatically co-registered with the corresponding T2-weighted magnetic resonance image and was then finely-adjusted manually by an experienced nuclear medicine physician. The MRI image was used as a reference, so the slice thickness of the co-registered images was the thickness of the T2-weighted MRI images (3.3 mm). For co-registration, rigid transformations were defined by six parameters; the rotation angles and translation distances in the three spatial directions. The interpolation method was trilinear. On the co-registered images, the two contiguous transverse slices that contained the most intense striatal radioactivity were further examined in order to ascertain whether the SPECT and MRI images were co-registered accurately and whether the striatum was best seen on the two slices of the MRI images. If that was not the case, further adjustment of co-registration was performed manually until a satisfactory outcome was achieved. ROIs, including the striatum (basal ganglia, caudate nucleus, and putamen) and occipital cortex, were then drawn on the two contiguous MRI transverse slices, and these ROIs were projected onto the co-registered SPECT images. The ratio of the radioactivity [(St-Oc)/Oc ratio] was then derived by dividing the difference between the average activity in the striatum (St) and the average activity in the occipital cortex (Oc) by the average activity in the occipital cortex (Oc).Reference Hwang, Yao, Wey and Ting 33
Task: Iowa Gambling Task (IGT)
The original version of the IGTReference Bechara, Damasio, Damasio and Anderson 27 was applied in this study but was modified for event-related fMRI analysis.Reference Lawrence, Jollant, O'Daly, Zelaya and Phillips 34 , Reference Windmann, Kirsch and Mier 35 All participants were asked to complete 10 runs of the IGT with 10 trials in each run. Each trial started with a 1-second preparation phase, when the response was unavailable, then the sentence “Please select one deck” appeared at the center of the screen and participants were required to choose one deck at their own pace by pressing a button on the pad, which had four buttons corresponding to the four decks (the response phase). The outcome of the selected deck was shown at the center of the screen immediately after participants responded, and the monetary value of the deck could be positive or negative depending on whether the subject earned or lost in the trial. At the same time, the color of the selected deck turned from gray to yellow, and was displayed on the screen for 15 seconds as a feedback phase. The next trial followed at the end of the feedback phase. A blue bar at the top of the screen represented the cumulative monetary reward. As the total gain increased, the length of the bar also increased in proportion. The total monetary value was also displayed at the top-right of the screen and changed with the consequence of each trial. All monetary units were exchanged for New Taiwan dollars (NTD) from the USD used in the original IGTReference Bechara, Damasio, Damasio and Anderson 27 (1USD = 30NTD, 2015).
fMRI procedure
The IGT program was created using E-prime software (version 1.1) and run on a PC with a Windows 7 operating system in this study. To be compatible with the fMRI environment, several technical adaptions were required. First, we divided 100 trials into 10 runs, which allowed participants to rest between each run when they were lying in the scanner. Runs were consistently 3.5 minutes long. After performing pilot scans, we found that 3.5 minutes was sufficient time for participants to complete 10 trials. Second, instead of the original IGT, in which responses were given by mouse-clicking, participants used the MR-compatible response pad with four buttons. They held the pad with both palms and pressed the buttons with their right or left thumb. Prior to the fMRI experiment, participants were instructed that the order of the buttons corresponded to the order of the decks on the screen.
Before scanning, participants were given instructions for the IGT and were allowed some practice trials on a notebook outside the scan room to familiarize them with the interface of the task. The outcomes of the practice trials were identical for all of the decks. Subjects were instructed to pick the deck with the maximum gain or minimum loss in the task to make as much money as possible.
Functional image acquisition and analysis
MRI data were acquired using a GE 3T MR750 scanner with an eight-channel brain array coil in the Mind Research and Imaging Center at National Cheng Kung University. BOLD responses and in-plane anatomical data were recorded for each participant. Anatomical images were obtained using whole-brain sagittal T1-weighted spoiled gradient-recalled scans (flip angle = 12°, FOV = 22.4 cm, matrix size = 256 × 256, slice thickness = 1 mm, gap = 0 mm, slices = 170). Functional images were acquired using a T2*-weighted echo-planar imaging sequence (TR = 2 seconds, TE = 33 ms, FOV = 24 cm, matrix size = 64 × 64, thickness = 3 mm, gap = 0, slices = 40). For each participant, 10 functional runs were performed, which lasted about 40 minutes in total.
Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm) was used to preprocess and analyze the data. The functional images for each participant were slice time corrected to the middle (ie, the 39th interleave images) slice and then spatially realigned using a six-parameter rigid-body spatial transformation. The high-resolution structural image was then co-registered to the mean functional image generated by the realignment phase. The functional images were spatially normalized to the Montreal Neurological Institute (MNI) template with the resulting warping parameters generated by structural image segmentation resampled to voxel size 3 × 3 × 3 mm, then spatially smoothed using a 6-mm FWHM isotropic Gaussian kernel. A high-pass filter with a cutoff of 128 seconds was applied to the data.
In the first-level model, event-related responses were assessed by creating fixed-effects general linear models for each participant. Regressors of interest were the neural activity for three phases (prepare, response, and feedback) of the tasks in each trial and were modeled using the canonical hemodynamic response function. During the response phase, regressors for experimental trials were separated according to the expected value (advantageous deck or disadvantageous deck). During the feedback phase, regressors were separated according to outcome (win or loss) with a parametrical modulator set by the actual feedback in each trial. The neutral trials (outcome of 0), at the feedback phase, were modeled to a regressor without a modulator. In addition, realignment parameters were included as regressors in the first-level model. To achieve the aim of our study, in order to probe the BOLD response in the reward pathway, which varies with the magnitude of monetary reward, following model estimation, parametrical contrasts were built for each subject to assess the BOLD response modulated by monetary outcome during win and loss feedback phases only. For convenience of reading, the parameters were multiplied by 1000. The associations between these parameters and DAT availability were tested.
ROI analysis
We extracted parametrical estimates from parametrical modulator contrasts in each ROI from three studies of the human reward system.Reference Kringelbach and Rolls 36 –Reference Hayes and Northoff 38 The MNI coordinates of these ROIs were [–33, 42, –5] and [33, 41, –5] for the left and right OFC, respectivelyReference Kringelbach and Rolls 36; [–10, 12, –6] and [16, 12, –6] for the VS in the left and right hemispheres, respectively; [0, 46, –10] for the mPFCReference Diekhof, Kaps, Falkai and Gruber 37; [4, 24, 30] for the anterior cingulate cortex; and [–40, 16, 4] and [40, 16, 4] for the insula in the left and right hemispheres, respectively.Reference Hayes and Northoff 38 The eight ROIs were 10-mm spheres centered on the above coordinates. The ROIs are illustrated in Figure 1.

Figure 1. The regions of interest (ROIs) in the present study. Anterior cingulate cortex (red), insula (blue), orbitofrontal cortex (violet), ventral striatum (cyan), and medial prefrontal cortex (green).
Statistical analysis
The data were analyzed using Statistical Package for Social Science software version 17 (SPSS Inc., Chicago, IL). Pearson’s correlation was used to examine the relationships between the parametric estimates from fMRI analysis and DAT availability assessed by SPECT. A supplemental generalized linear model analysis was conducted to probe the group differences in the correlation coefficients. The threshold for statistical significance was set at P < .05. The power for detecting small (r = 0.10), medium (r = 0.30), and large (r = 0.50) effect sizes with our sample size in the group of adults with ADHD was 0.06, 0.19, and 0.51, respectively.
Results
A group difference in DAT availability, that is, a lower DAT availability in adults with ADHD (in the caudate and putamen), was reported in our previous paper.Reference Chu, Lee and Chi39 The BOLD signal parameter is shown in Table 1. A significant association was found between DAT availability and the BOLD signal parameter estimate during losing tasks in the mPFC (r = 0.55, P = .03), right VS (r = 0.69, P = .003), and right OFC (r = 0.53, P = .03). A marginal association was found between DAT availability and the BOLD signal parameter estimate during losing tasks in the right insula (r = 0.49, P = .055). No significant association was found between DAT availability and the parameter estimate for the BOLD response covariate during winning tasks in the patients with ADHD or during winning or losing tasks in the controls, as shown in Table 2. Supplemental generalized linear model analysis indicated a significant group difference in the correlation coefficient of the association between DAT availability and the right VS (F (1, 28) = 4.68, P = .04), as illustrated in Figure 2. All other group differences were nonsignificant.
Table 1. The Parameter Coefficients of the BOLD Signal During the Iowa Gambling Test Among the Patients with ADHD and controls.

Abbreviations: ADHD, attention deficit hyperactivity disorder; BOLD, blood oxygen level-dependent; ROI, region of interest.

Figure 2. The association between DAT availability and the BOLD signal parameter estimate during losing tasks in the right VS. Abbreviations: ADHD, attention deficit hyperactivity disorder; DAT, dopamine transporter; VS, ventral striatum.
Table 2. The associations between DAT availability and parameter coefficients (Pearson’s r) of the BOLD signal during the Iowa Gambling Test.
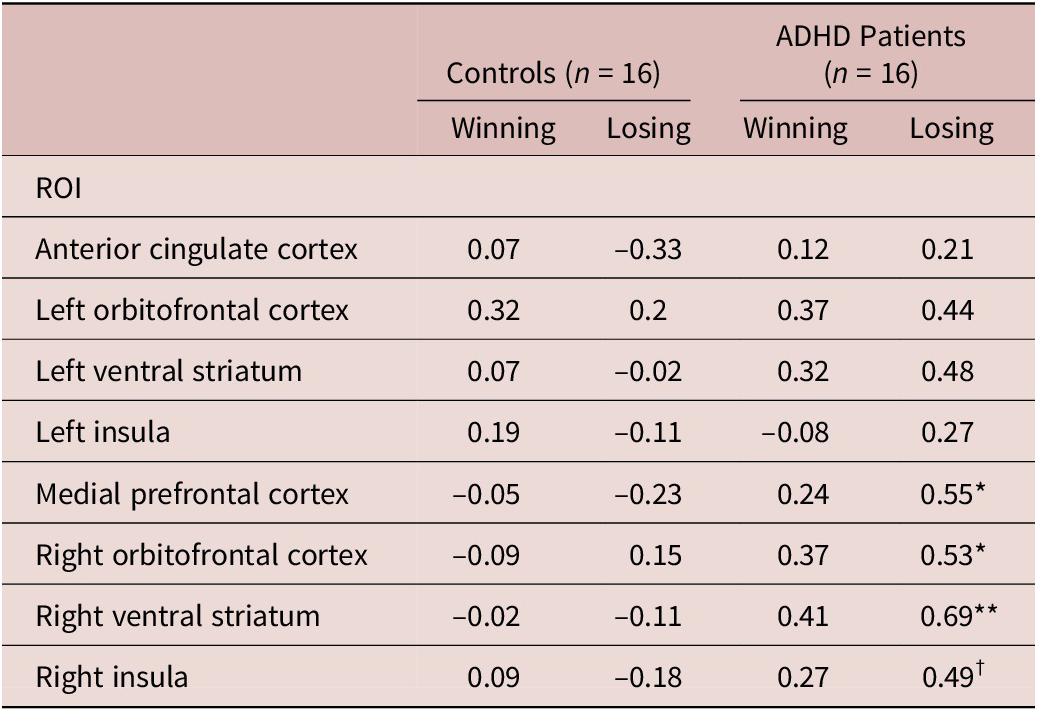
DAT: dopamine transporter; BOLD: blood oxygen level-dependent; ROI: region of interest; ADHD: attention deficit hyperactivity disorder.
†: P < 0.10 *: P < 0.05; **: P < 0.01.
Discussion
The results implied a response in the reward system during losing that is related to the dopamine system. Dopamine system could be the most important etiology of ADHD.Reference Volkow, Wang and Newcorn2, Reference Volkow, Wang and Newcorn20 Also, these effects implied that the response during losing should be addressed.Reference Luman, Oosterlaan, Knol and Sergeant13, Reference van Meel CS, Heslenfeld, Oosterlaan, Luman and Sergeant 14 The results showed that patients with ADHD were not only more sensitive to stimuli, but were also easily blind to the magnitude of penalties.Reference Luman, Oosterlaan and Sergeant 40 This phenomenon was in agreement with the study by Sonuga-Barke and Fairchild, who demonstrated that altered dopaminergic function in the ventral frontostriatal network in patients with ADHD is associated with poor evaluation of reward.Reference Sonuga-Barke and Fairchild 15 We speculated that this could be an important characteristic of adult ADHD. Our finding is also in agree with the finding of Badgaiyan et alReference Badgaiyan, Sinha, Sajjad and Wack 3 Our finding confirmed that hypodopaminergic tone during resting is associated with the response during challenge task. Also, altered response on losing might be one of the mechanisms of poor decision-making under risk among patients with ADHD. However, little is known about the role of a punishment-induced response in decision-making among patients with ADHD. In sum, our finding indicated that hypodopamine tone, as an etiology may linked to other phasic biological response. This association could be an important topic for understanding the nature of ADHD, or even other phenomena with hypodopaminergic tone.Reference Blum, Thanos and Oscar-Berman 41
The current findings also indicated that the monetary-related BOLD response to losing tasks was correlated with DAT availability in several brain areas in the adults with ADHD, particularly during penalty-related tasks, but this was not the case in the controls. The results of a meta-analysis indicated a prefrontal and frontal volume reduction in patients with ADHD.Reference Valera, Faraone, Murray and Seidman 42 It is well-known that the central dopamine system plays an important role in the reward-induced response in healthy controls. As the frontal volume of the controls was not reduced, we speculated that the dopaminergic tone in the control subjects was induced to a lesser extent by the IGT than in the patients with ADHD, meaning that only a relatively small reward stimulation was perceived by the controls in this virtual IGT. Therefore, the correlation between the BOLD signal and DAT availability was insignificant in the controls during the IGT. Seymour et alReference Seymour, Daw, Roiser, Dayan and Dolan 43 proposed that in addition to dopamine, serotonin, and other monoamines may also play roles in the reward system. It is also plausible that subjects with ADHD and controls may use different strategies for decision-making. It was found that different neural mechanisms/circuitries are associated with different strategies in risky decision-making.Reference Venkatraman, Payne, Bettman, Luce and Huettel 44 Although the detailed mechanism remains unclear, our findings supported the idea that there is a qualitative difference in the neural response to the IGT in patients with ADHD in comparison with controls.
Our findings indicated that altered dopaminergic function was associated with brain activity related to losing, which could be considered as a punishment. ADHD has been found to be associated with a reduced sensitivity to punishment in gambling tasks.Reference Groen, Gaastra, Lewis-Evans and Tucha 45 The reduced sensitivity, or insensitivity, to punishment is in agreement with the poor decision-making in the IGT found in this study, and in other behavioral tasks in other studies.Reference Pollak and Shoham 46 , Reference Dekkers, Popma, Agelink van Rentergem, Bexkens and Huizenga 47 Our findings implied that this tendency in adults with ADHD could be associated with low levels of dopaminergic activity.
This study also confirmed that the BOLD response in the right VS was related to the losing response, which was in agreement with the findings of Jessup and O’Doherty,Reference Jessup and O’Doherty 31 who reconfirmed the role of the VS in reward-related processes. We speculated that the pathway between the ventral tegmental area and the nucleus accumbens, and other pathways related to the nucleus accumbens, play roles in this phenomenon, and this has been supported by other studies.Reference Carlezon and Thomas 48 However, our findings did not confirm the role of the left striatum in the reward system. It remains unclear whether there is a lateralization effect. More importantly, our findings showed that the association between dopaminergic tone and brain activity in the VS in the subjects with ADHD and the controls differed significantly. This finding may reconfirm that altered behaviors, which are related to the striatum function, might be rooted in the dopaminergic tone in patients with ADHD. The interplay between dopamine and behavior related to the reward system should be noted in patients with ADHD.
The present results showed that dopamine function in the striatum was associated with reward-related brain activity in the mPFC and OFC in the patients with ADHD, which implied that the frontostriatal network plays an important role in the behavior of those with ADHD. Previous reports also confirmed that the mPFC could play an important role in the learning and evaluation of behavior outcomes,Reference Euston David, Gruber Aaron and McNaughton Bruce 49 especially in the detection of performance errors.Reference Ridderinkhof, van den Wildenberg, Segalowitz and Carter 50 It is worth noting that an animal study indicated that lesions in the mPFC may impair decision-making, and that effect could be reversed by a D1 receptor antagonist.Reference Paine, Asinof, Diehl, Frackman and Leffler 51 Also, it has been proposed that the OFC may be related to functional impairment in patients with ADHD.Reference Soliva, Carmona and Fauquet 52 , Reference Itami and Uno 53 Our findings may provide some evidence that OFC dysfunction among adults with ADHD might be related to dopamine tone. Although our findings were not identical to those of Paine et al,Reference Paine, Asinof, Diehl, Frackman and Leffler 51 taken together, the results implied that the interplay between performance during risky tasks, the dopaminergic system, and medial prefrontal and orbital frontal function could be a complex issue.
In the present study, we disentangled the responses to winning and losing during the gambling task. However, there remains an alternative explanation. It is worth noting that losing in a gambling task, in which players are supposed to win, could be a prediction error. Therefore, the penalty feedback, and its magnitude, could be a kind of prediction error.Reference Li, Lu, D'Argembeau, Ng and Bechara 54 It was found that youth with disruptive behavior disorder were characterized by reduced activation in the insula when the error occurred and increased responsiveness to negative prediction errors within the caudate.Reference White, Pope and Sinclair 55 Our study results implied reduced activity in the right striatum among the patients with ADHD with low DAT availability. Although the findings were not identical, prediction error could be an important underlying factor.
In summary, our findings indicated that the brain activity related to the magnitude of reward could be correlated with resting dopaminergic tone in patients with ADHD. This novel finding may imply that the response related to penalty or losing in patients with ADHD might be more sensitive to dopaminergic tone than in those without ADHD. Meanwhile, as the response during losing may also be related to prediction error (unexpected loss), our findings may also imply that dopamine tone may alter the capacity for better prediction during uncertainty. Recent studies revealed that dopamine neurons in the midbrain could be activated during reward prediction errors.Reference Keiflin and Janak 56 , Reference Schultz 57 This mechanism should be considered as a plausible explanation of our findings. These results provide evidence of interplay between monoamine tone and brain activity induced by tasks in patients with ADHD. Also, our finding may provide addiction evidence on the altered dopamine function during resting, and during challenging task.Reference Badgaiyan, Sinha, Sajjad and Wack 3 , Reference Cherkasova, Faridi and Casey 4 The etiology of ADHD may not only related with hypo-tone resting monoamine tone, but also related with hyper-reaction to stimulus. Although little is known on ADHD, some theories in addiction had also probe postulate that this could be form as a pathological cycle of spiraling dysregulation.Reference Koob and Le Moal 58 Considering the reward deficiency as a common etiology of ADHD and addiction, the activity of DA as a trait or a state, could be complex.
There were several limitations of the present study. First, owing to consideration of the cost of fMRI and the potential risk of SPECT, the sample size was too small; therefore, the statistical power was limited, as mentioned in the “Methods” section. As the effect sizes of the correlations reported were large (r = 0.50), future studies should enroll 28 samples to enhance the strength of the statistical power to 0.80. Multicenter study with a similar imaging technique and fMRI task might be a potential solution. On the other hand, multiple correction for ROI analysis was not conducted in the present study due to the sample size. False-positives cannot therefore be ruled out. The critical P value for Bonferroni correction was .0031, similar to the P value (.0032) of our main finding. Considering this issue, interpretation of other ROIs should be performed cautiously. Second, we used the IGT as a virtual reward task with fMRI, and whether or not our findings can be generalized to daily decision-making tasks in the real world is unclear. Third, whether low dopaminergic tone is a trait or state is as yet unclear. Although the patients with ADHD in our study were methylphenidate-free, and without a diagnosis of substance abuse, the low dopaminergic tone should be interpreted carefully. Fourth, our findings relied on cross-sectional association. The causal relationship between dysfunction in the dopaminergic system and an altered cognitive mechanism was not able to be established. Fifth, although our results showed a group difference in brain activity during winning and losing,Reference Yang, Chi and Chu 59 no significant group difference in brain activity induced by the magnitude of winning or losing was found in this study. The reason for this could be that modeling of the task was not optimal in terms of detecting differences in brain activity between the two groups. Our self-paced procedure employed in the clinical population provided participants with a more natural environment than they would experience within an MRI scanner; however, this may weaken the modeling and decrease the sensitivity of detection of differences. Future studies are needed to confirm this potentially important characteristic among patients with ADHD, and in those with other disorders with a similar pathology. Sixth, potential psychosocial confounding factors could not be completely excluded in the two groups. In particular, some behavior related with reward deficiency syndrome, may play roles. Although there is no significant difference on smoking and obesity in the samples, other potential factors, such as other addictive behaviors, were not probed here. Seventh, the radioligand, [99mTc] TRODAT-1, used in this study is suitable for quantification of the DAT availability in the striatum.Reference Kushner, McElgin and Kung 60 The role of the dopamine tone in the frontal cortex could not be tested in this study.
Conclusions
In this study, we used both a molecular brain imaging technique and fMRI to probe the association between dopaminergic tone and brain activity evoked by monetary feedback in a gambling task. We found that the brain activity in several regions involved in the reward system was correlated with the dopamine transporter availability in the striatum in adults with ADHD. Our findings not only provide evidence for the role of the dopaminergic system in the rewarding process, but also imply that hypodopaminergic tone is related to an insensitivity to punishment. This may help our understanding of the nature of problematic risk preferences in individuals with ADHD.
Acknowledgments
The authors are indebted to the research participants, Ms. Tsai Hua Chang from National Cheng Kung University Hospital, and Professor Yuan-Hwa Chou from Taipei Veterans General Hospital. We thank the Mind Research and Imaging Center (MRIC) at National Cheng Kung University for consultation and instrument availability. MRIC is supported by the Ministry of Science and Technology.
Funding
This study was funded by the National Science Council of Taiwan (NSC 102-2420-H-006-007-MY2), the Ministry of Science and Technology, R.O.C. (MOST 104-2314-B-006-053-MY2) and National Cheng Kung University Hospital (NCKUH-10104019 and NCKUH-10603044).
Disclosures
All authors declare that they have no competing interests. The funding institutions of this study had no further role in the study design, the collection, analysis, and interpretation of data, the writing of this paper, or the decision to submit it for publication.






