A 17- to 19-year follow-up study of patients with depression admitted to the London’s Maudsley Hospital whose index episode marked their first psychiatric contact found that they had a 50% chance of readmission during their lifetime; those with previous admissions had a similar chance of readmission within 3 years (Reference Lee and MurrayLee & Murray, 1988). Less than one-fifth of the patients had remained well, and over one-third suffered severe chronic distress and disability or had died unnaturally. Similar gloomy pictures have been reported in many other long-term follow-up studies reviewed by Reference Piccinelli and WikinsonPiccinelli & Wilkinson in 1994 and in a subsequent case register study carried out in Denmark (Reference Kessing, Andersen and MortensenKessinget al, 1998). Psychiatric in-patients or out-patients (many of them atypical) were the participants in most of this research, but a 2-year survey of individuals drawn from the general adult population of The Netherlands confirmed the recurrent nature of depression (Reference Spijker, de Graff and BijlSpijkeret al, 2002). As a result of such findings, much emphasis is put on the importance of continuation and maintenance treatment for depression.
Continuation treatment is given to help consolidate recovery from an episode of depression and to prevent relapse, whereas maintenance (prophylactic) treatment is given to help prevent a recurrence of depression. A relapse is a worsening of an ongoing or recently treated episode, while a recurrence is a new episode. When there is a long interval between episodes, the distinction is easier to make, but when the interval is short the distinction is arbitrary and may not reflect underlying pathogenic processes. There is a consensus among researchers that 4–6 months’ remission (during which time the individual’s affective state returns to its premorbid level) should occur before a further depressive episode is regarded as a recurrence, although this is not based on sound evidence.
Many trials of continuation and maintenance treatment have been carried out. During recent years, larger numbers of people have been included and methodology has improved. In all of the studies the participants met conventional diagnostic criteria, such as those in DSM–IV, and had predetermined scores on rating scales for depression. Most participants had responded to open treatment with the drug being investigated or had responded during controlled trials of short-term (acute) treatment with the drug. A relapse or recurrence was defined as a worsening or return of depression with a defined increase in a score on a rating scale. Nevertheless, from the results of these studies it is difficult to make meaningful comparisons between older and newer antidepressants (‘newer’ being arbitrarily defined as selective serotonin reuptake inhibitors (SSRIs) and subsequently introduced antidepressants) because of differences between participants and differences of methodology (Box 1).
Box 1 Problems in interpreting the results of studies of long-term treatment
-
• Difficulties in defining remission, relapse and recurrence
-
• Small sample sizes
-
• Matching on all variables, including:
-
• number of previous episodes of depression
-
• effect of previous treatment
-
-
Effect of concomitant treatment
-
Participant’s failure to complete trials
-
Difficulty tracing patients
-
Difficulty obtaining accurate follow-up data
Taking into account these difficulties, in 1997 I reviewed forAPTthe 12 placebo-controlled studies of antidepressants carried out during the decade 1988–1997 (Reference EdwardsEdwards, 1997). My analysis suggested that about 59% (range 22–76%) of those who respond to an antidepressant and are then switched to placebo remain in remission for up to 2 years and possibly longer. If instead they continue with the treatment to which they have responded, they have overall a 23% better chance of maintaining their improvement. Expressed differently, twice as many relapses occur on placebo than on antidepressants – about 41%v. 18%. Some of the studies also revealed advantages of antidepressants over placebo in the time to onset of relapse or recurrence and in the depression scores of those who do not relapse.
Since I carried out that review, the results of other placebo-controlled trials of antidepressants in the long-term treatment of depression have been published. These are included in a systematic review of 3l randomised trials involving 4410 patients (Reference Geddes, Carney and DaviesGeddeset al, 2003). The overall results are identical to those of my less sophisticated report – a 41% relapse rate on placebov. 18% on active treatment. Continuing treatment reduced the risk of a relapse by 50% and, although most trials included in the review were of 12 months’ duration, the effect seemed to last for 36 months. Given a constant relative risk reduction, it was extrapolated mathematically that the absolute treatment benefit would be higher in patients at higher risk of relapse.
The studies cited are important in showing the efficacy of continuation and prophylactic treatment but, apart from the finding that the absolute benefit is greater in those at higher risk of relapse, there is a paucity of knowledge on variables that may predict benefit for individual patients.
Which antidepressant?
It is clear from what has been said so far that depression is a recurrent disorder and that antidepressant drugs help decrease the chances of relapse and recurrence. The rest of this article – an invited update of my earlier publication (Reference EdwardsEdwards, 1997) forAPT’s anniversary series – will focus on the choice of newer or older antidepressants for maintenance and prophylactic treatment.
Therapeutic effects
Meta-analyses have shown that there are no significant differences in efficacy of different types of antidepressant during short-term treatment (e.g. Reference Anderson and TomensonAnderson & Tomenson, 1995; Reference Montgomery and KasperMontgomery & Kasper, 1995; Reference Edwards and AndersonEdwards & Anderson, 1999; Reference Anderson and EdwardsAnderson & Edwards, 200l; Reference Olver, Burrows and NormanOlveret al, 200l; Reference Geddes, Carney and DaviesGeddeset al, 2003; Reference MacGillivay, Arroll and HatcherMacGillivayet al, 2003), although data from some individual trials suggest that SSRIs other than fluvoxamine might be less effective than tricyclic antidepressants (TCAs) in the treatment of severe melancholy in hospital in-patients (Reference Anderson and TomensonAnderson & Tomenson, 1995; Reference AndersonAnderson, 1997). Consistent with this statement are the findings from a naturalistic study carried out in Denmark. These showed that the course of severe depression has remained relatively unaltered since the introduction of new antidepressants (Reference Kessing, Hansen and AndersenKessinget al, 2004). As there are insufficient comparative data on long-term treatment – merely hints of a possible lower relapse rate on SSRIs than on TCAs – there is no strong reason based on efficacy for choosing one antidepressant rather than another for maintenance treatment.
The choice of an antidepressant (or class of antidepressants) for continuation or prophylactic treatment is therefore mostly based on tolerability, adverse effects, toxicity in overdose and cost.
Tolerability and adherence
Failure to adhere to treatment regimens as prescribed is universal and not just related to tolerability. High rates of non-adherence have been demonstrated in the treatment of a wide range of both psychiatric and general medical illnesses, the latter including anaemia, hypertension, tuberculosis and leprosy. Over and above this, however, unwanted effects of medication may increase the problem and this in turn will have an effect on relapse and recurrence of depression. Comments in trials of long-term treatment with TCAs and newer antidepressants imply that both types of drug are well tolerated, although clinicians may be influenced in their choice by the results of meta-analyses of short-term treatment. These show, for example, no difference between SSRIs and tricyclics in losses of participants to trials (‘drop-outs’, ‘discontinuations’) owing to inefficacy, but a small statistically significant difference (4.4– 4.7%) in losses owing to side-effects (Reference Anderson and TomensonAnderson & Tomenson, 1995; Reference Montgomery and KasperMontgomery & Kasper, 1995; Reference AndersonAnderson, 1997; Reference Barbui, Hotopf and FreemantleBarbuiet al, 2000) (Box 2).
Box 2 Advantages and disadvantages of SSRIs compared with older TCAs and related antidepressants
Advantages
-
• Tolerance: 4.4–4.7% fewer participants fail to complete trials because of side-effects
-
• Unwanted effects: less sedation; fewer anti-cholinergic effects; less weight gain
-
• Toxicity in overdose: less likely to be lethal
Disadvantages
-
• Unwanted effects: more gastrointestinal side-effects; very long-term toxicity of newer drugs unknown
-
• Cost: more expensive
Comments
-
• Tolerance: no significant difference in overall rates of failure to complete trials; not known whether advantages exist in routine psychiatric practice and primary care
-
• Unwanted effects: more epidemiological data are needed
-
• Toxicity in overdose: suicide rate by any method among patients treated with newer and older antidepressants is similar
-
• Cost: extra expense means less money available for other areas of healthcare
Much is made of the difference due to side-effects, but more important is the overall discontinuation rate, as it is often difficult to be sure why patients stop their treatment. Drug-induced dysphoria (a side-effect) may be misdiagnosed as lack of efficacy, while intolerance of side-effects or discontinuation for ‘other reasons’ may be determined by depression that has failed to respond to treatment. Overall discontinuation rates in meta-analyses do not show a significant difference between SSRIs and TCAs.
It is possible that overviews of newerv. older drugs obscure differences between individual compounds. For instance, 10% fewer participants failed to complete one comparative trial because of inefficacy and side-effects of paroxetine compared with imipramine (Reference Dunbar, Cohn and FabreDunbaret al, 1991), whereas in a small meta-analysis there were 9.6% fewer losses due to adverse effects of dothiepin compared with SSRIs (Reference Donovan, McGrady and PownallDonovanet al, 1993).
The people included in clinical trials – and thus in meta-analyses of trials – are highly selected. It is therefore not known whether the apparent advantage of SSRIs suggested by trials and meta-analyses also exists in the real world of general practice, where most people with depression are treated. Nor do we know whether there is a lower discontinuation rate during longer-term treatment, when adaptation to adverse effects may occur. It is also not known whether the advantage would be upheld in populations of patients given better information and reassurance about side-effects, which is known to improve adherence to treatment.
Unwanted effects
It is beyond the scope of this article to consider all of the unwanted effects of antidepressants relevant to long-term treatment. I have recently reviewed the most important of these elsewhere (Reference Edwards and KingEdwards, 2004a ). Here I will discuss only some common unwanted effects that occupy a dominant place in the newerv. older antidepressant debate, and others that are less well understood and/or controversial. Conspicuous among the former are autonomic effects and sedation.
Autonomic effects and sedation
There are problems in comparing drugs because of difficulties in defining common effects (which are often identical to the symptoms of depression) and because of differences between studies in the way adverse effects are elicited, recorded, related to treatment and presented. Notwithstanding these problems, the newer antidepressants have been shown to cause fewer autonomic effects and less sedation than the older TCAs. Thus, they produce fewer anticholinergic effects, such as the decreased salivary flow and gastrointestinal mobility that lead to dental caries and constipation, respectively, and a lesser anti-α-adrenoceptor effect, which may result in postural hypotension (causing falls and injuries). Accidents may also be caused by over-sedation. The newer drugs, on the other hand, have other effects such as gastrointestinal symptoms and central nervous system (CNS) excitatory effects in the case of SSRIs (Reference Trindale and MenonTrindale & Menon, 1997). With both older and newer antidepressants, individuals may adapt to unwanted effects such as sedation and nausea and consequently differences observed during short-term treatment may not continue long-term and thereby affect adherence to the extent often assumed.
Cognitive and psychomotor effects
In laboratory tests the older TCAs (and some other antidepressants, e.g. mianserin and trazodone) have been shown to cause more impairment of cognitive and psychomotor function than newer antidepressants, including lofepramine (for references to this and other studies in this section, see Reference EdwardsEdwards, 1997). The older tricyclics have also been shown to cause impairment in driving tests, whereas SSRIs, reversible inhibitors of monoamine oxidase A (RIMAs) and nefazodone cause little or no impairment.
Although these findings support the use of the newer drugs for long-term treatment, the predictive validity of psychomotor tests has been questioned. Many skilled tasks can be performed without undue effort and with spare processing capacity left available, and it has been suggested that information-processing tasks are measures of competence (potential) rather than actual performance.
Furthermore, most of the investigations were carried out after short-term administration of drugs (sometimes in single doses), rather than during long-term treatment, when adaptation may occur. Adaptation to the effects of TCAs on driving has, in fact, been demonstrated. Perhaps these considerations explain why TCAs, despite being widely prescribed, were found in the body fluids of only 0.2% of people who died in traffic accidents, compared with alcohol in 35% and other drugs liable to affect the CNS in 7.4%.
Consistent with the observations that older TCAs cause psychomotor impairment is the finding that elderly drivers treated with these drugs have an increased risk of vehicle crashes in which injuries are sustained and that there is a positive relationship between the risk and dose of drug. This suggests that in elderly people the drugs contribute to the accidents, although inability to control for all potentially confounding variables does not allow for definite conclusions to be reached.
Similar considerations apply to proximal femur (hip) fractures. These occur at a similar frequency in elderly patients receiving SSRIs as in those on TCAs (Reference Liu, Anderson and MittmanLiuet al, 1998), although the possibility of selective prescribing of SSRIs for people at higher risk of falls cannot be ruled out.
The extent to which different antidepressants cause or contribute to road crashes and other accidents is not known. Nevertheless, the above-mentioned concerns should be taken into account in the choice of drug for long-term treatment. Although the risk may be greater when treatment is first introduced, it should also be considered when the dose is increased or when antidepressants are taken with other substances that affect cognition and psychomotor performance. For elderly people and for individuals thought to be at high risk of accidents, including those who experience persistent sedation when taking TCAs or drug combinations, it is best to err on the side of safety and prescribe non-sedating antidepressants.
Suicidal thoughts and behaviour
Depression as a paradoxical side-effect of TCAs was recognised more than 30 years ago, but controversy as to whether antidepressants can provoke suicidal thoughts was sparked by reports of intense, violent, self-destructive preoccupations arising in individuals treated with fluoxetine (Reference Teicher, Glod and ColeTeicheret al, 1990). The controversy was inflamed by subsequent sensational and emotive television programmes of self-injurious (and also violent or homicidal) acts carried out by people while on treatment with fluoxetine. More recently the finger of suspicion has pointed towards paroxetine as another possible provoker of such thoughts.
Critical reviews of the evidence for a causal connection between antidepressants and self-injurious thoughts and behaviour suggest that, on balance, the phenomena are more likely related to the personality of the patient and/or the disorder being treated than to the drug. However, the possibility of a rare idiosyncratic reaction cannot be ruled out. Because of the rarity of the events, there is little or no hard evidence that the thoughts and behaviour occur more often in patients on one SSRI than another, or indeed if they occur more often in patients treated with SSRIs than in those treated with older antidepressants. Furthermore, prescription-event monitoring (PEM) reports have noted similar low rates of occurrence of the events in large cohorts of people treated with fluoxetine, fluvoxamine, paroxetine and sertraline (Reference Edwards, Inman and WiltonEdwardset al, 1997; Reference Mackay, Dunn and WiltonMackay et al, 1997).
Sexual side-effects
Sexual side-effects are less well understood than many other adverse reactions. There are many reasons for this, some of which are listed in Box 3. Clinical trials, including those that are double-blind, randomised and controlled, have reported rates of sexual problems experienced during treatment with various antidepressants. However, most of these trials have methodological flaws and the scales used to assess the dysfunction have mostly been inadequate. For all these reasons, it is not surprising that the results of studies are often inconsistent.
Box 3 Reasons for our poor understanding of sexual side-effects of antidepressants
-
• The high prevalence of sexual dysfunction in the general population and especially in people with depression
-
• Sexual problems may be associated with concomitant psychiatric and physical disorders, the drugs used to treat these, and substance misuse (which may pass undiagnosed)
-
• Sexual side-effects may be related to other adverse reactions, for example sedation or excessive weight gain
-
• Patients may not spontaneously speak about their sexual difficulties and doctors may not ask about them
-
• Reporting in large-scale drug monitoring programmes varies with the knowledge, attitude and interviewing technique of the reporting professional
-
• Extrapolation from laboratory experiments is limited because of species differences and the complexity of the many neurobiological factors that can influence sexual function
Normal sexual function is important in human relationships and it is therefore essential that antidepressant-induced dysfunction be prevented (or reversed) wherever possible. Despite existing uncertainty over the relative risk of sexual side-effects while on various drugs, the best available evidence for choosing an antidepressant for both short- and long-term treatment for individuals who are prone to such problems, or acquire them during treatment, has been provided by Reference BaldwinBaldwin (2004). His review of the results of studies whose design is sufficiently rigorous to allow for valid recommendations suggests that there are probable advantages of maprotiline and moclobemide over amitriptyline and doxepin, respectively; of bupropion (amfebutamone) and reboxetine over fluvoxamine; and of bupropion and nefazodone over sertraline. The worst offenders appeared to be TCAs and SSRIs, as suspected by many practising clinicians.
Drug interactions
Many drug–drug interactions with all types of antidepressant have been reported (British Medical Association & Royal Pharmaceutical Society of Great Britain, 2004). The evidence for some of these is weak, as it is based onin vitro studies, animal experiments (from which we may not be able to extrapolate because of species differences), single case reports or small-scale uncontrolled studies.
Some interactions are more of theoretical interest than of clinical relevance. However, others are potentially hazardous and can be life-threatening. The most serious consequences of these are CNS toxicity; profound sedation; convulsive seizures; ventricular arrhythmias; a large increase or fall in blood pressure; and an increased risk of dangerous side-effects, or decreased therapeutic action, of an important co-prescribed substance (Reference Edwards and KingEdwards, 2004b ) (Table 1).
Table 1 Potentially hazardous interactions with newer and older antidepressants
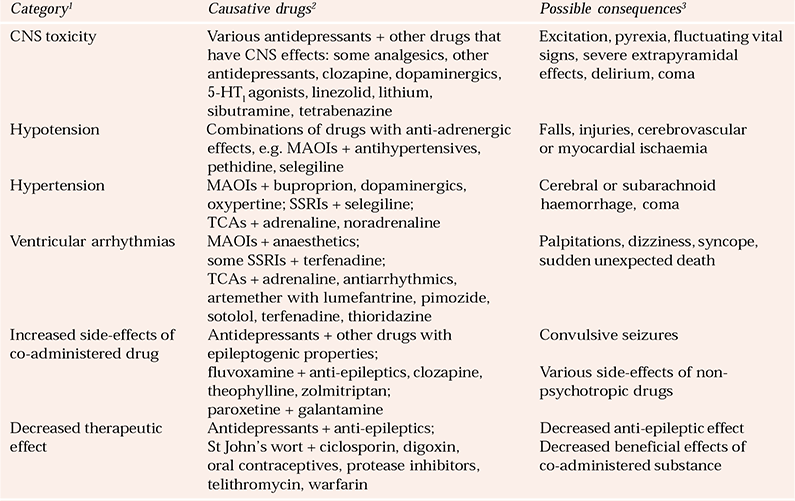
| Category 1 | Causative drugs 2 | Possible consequences 3 |
|---|---|---|
| CNS toxicity | Various antidepressants + other drugs that have CNS effects: some analgesics, other antidepressants, clozapine, dopaminergics, 5-HT1 agonists, linezolid, lithium, sibutramine, tetrabenazine | Excitation, pyrexia, fluctuating vital signs, severe extrapyramidal effects, delirium, coma |
| Hypotension | Combinations of drugs with anti-adrenergic effects, e.g. MAOIs + antihypertensives, pethidine, selegiline | Falls, injuries, cerebrovascular or myocardial ischaemia |
| Hypertension | MAOIs + buproprion, dopaminergics, oxypertine; SSRIs + selegiline; TCAs + adrenaline, noradrenaline | Cerebral or subarachnoid haemorrhage, coma |
| Ventricular arrhythmias | MAOIs + anaesthetics; some SSRIs + terfenadine; TCAs + adrenaline, antiarrhythmics, artemether with lumefantrine, pimozide, sotolol, terfenadine, thioridazine | Palpitations, dizziness, syncope, sudden unexpected death |
| Increased side-effects of co-administered drug | Antidepressants + other drugs with epileptogenic properties; fluvoxamine + anti-epileptics, clozapine, theophylline, zolmitriptan; paroxetine + galantamine | Various side-effects of non-psychotropic drugs |
| Decreased therapeutic effect | Antidepressants + anti-epileptics; St John’s wort + ciclosporin, digoxin, oral contraceptives, protease inhibitors, telithromycin, warfarin | Decreased anti-epileptic effect Decreased beneficial effects of co-administered substance |
Details concerning interactions are beyond the scope of this article but knowledge of them should be taken into account when choosing an anti-depressant for the long-term treatment of individuals with concomitant physical or psychiatric disorders (especially elderly people) who have, or are liable to need, drug combinations.
Knowledge of the mechanisms involved can help predict, and thereby prevent, interactions, but it is also essential that physicians and pharmacists keep up to date with the ever-increasing number of interactions reported and carefully steer a course between effectively treating multiple pathology and avoiding unnecessary risk. This is more important than attempting to generalise as to whether or not newer antidepressants, or particular groups of newer antidepressants, are safer than older compounds.
Lethality in overdose
Antidepressants introduced into clinical practice before 1970 have a higher fatal toxicity index (the number of deaths due to overdose per million prescriptions) than those introduced subsequently (Reference Henry, Alexander and SenerHenry et al, 1995; Reference HenryHenry, 1997). Despite limitations of methodology (especially uncertainty over the cause of death, the quantities of drugs and other substances taken, and the medical condition of the patients), the results show that death due to overdose of antidepressants is more likely to occur if older drugs are taken. This was confirmed by an analysis of deaths from antidepressants between 1993 and 1997 recorded in a new national database (Reference Shah, Uren and BakerShah et al, 2001). It is also consistent with the known cardio-toxic effects of older TCAs and the relative freedom from these effects of the newer antidepressants.
On the strength of these observations, it has been recommended that the newer antidepressants should be used routinely as first-line treatment of depression. However, the risk of death from overdose has to be seen in perspective. Only about 4% of all deaths by suicide are due to overdoses of single anti-depressants (Office of Population Censuses and Surveys, 1975–1992) and it is not known what proportion of these are actually taken during treatment (when the physician’s choice is more relevant).
Furthermore, different suicide rates among people prescribed different antidepressants may be influenced by their doctors’ perception of suicide risk. It has been shown, for instance, that amitriptyline is prescribed more often for individuals with severe depression and depression associated with severe insomnia, which in turn could be associated with an increased propensity for suicide (Reference Isacsson, Redfors and WassermanIsacssonet al, 1994). Also, people treated with TCAs may not be at greater overall risk of suicide (by any method) than those treated with less toxic drugs, because those who have their minds firmly set on killing themselves will choose a method of doing so. In keeping with this are two other findings. First, deaths due to self-poisoning in England and Wales decreased between the mid-1970s and early 1990s, during which time safer newer antidepressants were more widely used, whereas those due to more violent methods increased (Office of Population Censuses and Surveys, 1975–92). Second, the incidence of suicide by any method during treatment with the newer and older antidepressants in general practice has been shown to be similar (Reference Jick, Dean and JickJicket al, 1995).
Citalopram and venlafaxine
Against this background, concern has been expressed during recent years that two of the newer serotonergic antidepressants, citalopram and venlafaxine, may possibly be more lethal in overdose than other newer compounds.
Concern over citalopram arose as a result of a report of six deaths following overdoses of this drug, raising the possibility that it could be intrinsically more toxic than other SSRIs (Reference Ostrom, Eriksson and ThorsonOstromet al, 1996). However, no fatalities occurred among 44 people who took overdoses of citalopram alone in quantities ranging from 70 to 3000 mg (Reference Personne, Sjoberg and PerssonPersonneet al, 1997). Widened QRS complexes in the electrocardiogram (ECG) were observed, and/or convulsions occurred, in about a third of individuals who had taken 600–1800 mg (30–90 × 20 mg tablets) and in all of those who had taken more than 1900 mg (95 × 20 mg tablets). Although there is uncertainty as to the cause of death in the citalopram fatalities, overdoses of other SSRIs were not associated with convulsions or ECG abnormalities to the same extent (Reference Denchant and ClissoldDenchant & Clissold, 1991; Reference HenryHenry, 1991; Reference Borys, Setzer and LingBoryset al, 1992; Reference Klein-Schwartz and AndersonKlein-Schwartz & Anderson, 1996).
The six deaths reported by Reference Ostrom, Eriksson and ThorsonOstromet al (1996) and a later one reported by Reference Barbey and RooseBarbey & Roose (1998) have to be balanced against the extremely small number of fatal overdoses associated with citalopram (Reference Isacsson and BergmanIsacsson & Bergman, 1996). In most lethal cases other anxiolytics were taken together with the citalopram, and the quantity of citalopram taken in one of the deaths reported by Ostromet al was similar to that in the only other well-documented death due to an overdose of an SSRI taken alone – fluoxetine (Reference GlassmanGlassman, 1997).
Concern about the toxicity of venlafaxine in overdose arose following reports of cardiac arrhythmias, convulsive seizures and deaths (Reference SarkoSarko, 2000). It was later found that there was a higher death rate per 1000000 prescriptions associated with this drug than with other newer antidepressants (Reference Shah, Uren and BakerShahet al, 2001) and that the fatal toxicity index was higher than that of other serotonergic antidepressants and similar to that of some of the less toxic older antidepressants such as phenelzine and clomipramine (Buckley & McManus, 2004). A study of mortality data collected from the National Programme of Substance Abuse Deaths and antidepressant prescribing data supported these findings (Reference Cheeta, Schifano and OyefescoCheetaet al, 2004). However, antipsychotics were taken in combination with venlafaxine more often than in deaths associated with other antidepressants. This suggests that the patients treated with this drug may have been more severely depressed or difficult to treat, and thus possibly at higher risk of suicide to begin with.
It is unfortunate that a shadow has been cast over these two efficacious and otherwise safe anti-depressants. Since 1989 citalopram has been prescribed for more than 30 million people in over 70 countries (Reference NemeroffNemeroff, 2003) and its fatal toxicity index is not significantly higher than that of other relatively safe antidepressants (Reference Buckley and McManusBuckley & McManus, 2002). Deaths associated with the drug are extremely rare and could be chance findings. There are fewer data available on venlafaxine, as it was marketed more recently. The possibility that patient selection influences its high fatal toxicity index clearly needs further exploration. In the case of both drugs, and all new antidepressants, more epidemiological research is needed. Until the results of this are available, it is advisable to avoid, or reduce access to, the older, more toxic antidepressants in patients at high risk of suicide and to use citalopram and venlafaxine with greater caution than other, newer antidepressants.
Effects on the unborn and newborn
Safety during pregnancy
Many women become pregnant while receiving long-term treatment with antidepressants. This raises the complex and emotive subject of teratogenicity. A number of studies provide grounds for cautious optimism (e.g. Reference Wilton, Pearce and MartinWiltonet al, 1998; Reference Goldstein and SundellGoldstein & Sundell, 1999), but the numbers of pregnant women studied have been relatively small and the observational designs of the studies cannot definitely prove that the antidepressants are safe. We are therefore faced with a dilemma. On the one hand, many women need to continue drug treatment during pregnancy but, on the other hand, by prescribing drugs we could be inflicting unforeseen harm on their unborn children.
Teratogenicity is much more complicated than often assumed. In the past, a teratogen was defined as a substance that produced congenital structural abnormalities. During recent years, however, the definition has been extended to include all substances that, through a direct or indirect effectin utero, cause functional as well as structural abnormalities in the foetus or in the child after birth. These include abnormalities that do not manifest themselves until late in development.
In view of the potential dangers, here too it is best to err on the side of caution. Until it can be shown beyond all reasonable doubt that an antidepressant does not have harmful effects on the unborn, it should be prescribed during pregnancy – and especially during the first trimester – only if it is absolutely essential for the well-being of the mother. It has been suggested that we will not learn about the teratogenic effects of drugs until a large number of women have been exposed to them during pregnancy. However, to prescribe the drugs in non-essential cases means exposing the most vulnerable of all young humans to unethical uncontrolled experimentation.
If it is absolutely necessary to administer an anti-depressant during pregnancy, older compounds should mostly be chosen because more is known about their teratogenic potential. If there are unequivocal indications for prescribing a newer antidepressant instead of an older compound, the drug on which most safety data are available is fluoxetine.
Safety during breast feeding
Antidepressants are excreted in breast milk and there have been isolated case reports of suspected untoward effects in the breast-fed babies of mothers who have been treated with older and newer antidepressants (e.g. sedation and colic have been associated with doxepin and fluoxetine, respectively). There is little evidence of adverse consequences in the relatively small number of mothers and infants that have been studied in detail (e.g. Reference Yoshida, Smith and KumarYoshidaet al, 1999; Reference Hendrick, Fukuchi and AltshulerHendricket al, 2001), but antidepressants should nevertheless be prescribed for lactating mothers only when they are essential for the well-being of the mother. Here too more is likely to be known about the effects of older antidepressants. Of the newer drugs, most data are available on SSRIs. Of these, fluoxetine has the potential disadvantages of being excreted in breast milk in higher concentrations than paroxetine and sertraline, and of a more prolonged effect because of its long half-life.
Where there is concern over possible adverse effects in the newborn, the clinician should discuss with the mother the possibility of mixed breast and artificial feeding. Slow-release antidepressants should be avoided and, depending on their half-life, consideration should be given to administering the drug immediately after a feed and/or just before the baby’s longest period of sleep. The mother should be reassured that risks are low and she should be involved in decision-making.
Pharmaco-economics
The pharmaco-economics of prescribing drugs are extremely complicated and studies that have been undertaken lack consistency and/or generally accepted methodology (Reference Pirraglia, Rosen and HermannPirragliaet al, 2004). Furthermore, financial considerations, budgets and costs vary from one healthcare system to another. It is therefore not surprising that conflicting results are obtained. The possibility of a publication bias also has to be taken into account, as pharmaco-economic studies sponsored by manufacturers favour their own products more often than non-industry sponsored studies (Reference Baker, Johnsrud and CrismonBakeret al, 2003). For these reasons, it is difficult to make recommendations on the choice of newer or older antidepressants purely on the basis on economic arguments. There are suggestions that the higher initial costs of newer antidepressants are offset by less expenditure on out-patient visits and in-patient care (Reference Simon, VonKorff and HeiligensteinSimonet al, 1996). However, this does not allay the concerns of purse-string holders over the much higher costs of new drugs.
In most healthcare systems, money spent on expensive new products means that there is less money available for other aspects of care. It was shown, for example, that if there were a total shift to prescribing SSRIs for all patients on antidepressants, in England alone the annual cost to the National Health Service at 1995 prices and consumption volumes would be almost £350 million more than treating the same patients with amitriptyline (Reference EdwardsEdwards, 1997). This amount would pay for 4.1 million out-patient attendances per year for people with mental health problems, about 22 million hours of community psychiatric nurse time or, outside psychiatry, tens of thousands of kidney transplants or hundreds of thousands of cataract removals (calculated at 1994/95 costs; Reference EdwardsEdwards, 1995).
The choice of newer or older drug should not, of course, be based solely on economic considerations but costs have to be taken into account, especially in countries and services where there are harsh budgetary restrictions. In these services older antidepressants may be chosen as first-line treatment, with newer compounds reserved for specific sub-categories of patients in whom older drugs may pose a risk (for example, patients with severe cardiovascular disease and those at high risk of deliberate self-poisoning). A compromise on the polarised views that are often held on cost-effectiveness would be to administer less expensive generic SSRIs where appropriate.
Conclusions
Looking at the glass half empty, it is disappointing that after 50 years of research we still do not have a wonder drug to prevent (or treat) depression; the antidepressants that we do have are not free from troublesome and dangerous side-effects; and there are no clinically significant signs of a more effective and safer antidepressant on the horizon. Seeing the glass half full, however, existing antidepressants help prevent relapses and recurrences of depression in many and thereby relieve numerous patients and their families of much suffering. The newer antidepressants have some advantages over the older drugs, but these are only evident in subgroups of patients. By paying meticulous attention to an individual’s needs and selecting the safest alternative, many of the problems caused by the older antidepressants (such as cardiotoxicity and lethality in overdose) can be avoided. Such tailoring of treatment is more consistent with good doctoring than is following fashions and routinely prescribing new compounds as first-choice treatment for all. Such an approach can also prevent dangerous interactions with both newer and older drugs (Box 4).
Box 4 Key points
-
• Patients with major depression who responded to antidepressants in clinical trials and continued to take the same drug for 2–3 years had a 23% greater chance of remaining in remission than those who continued taking placebo. It is not known whether this advantage would be upheld for all patients, particularly those treated in primary care.
-
• Clinicians are influenced in their choice of drugs by differences in side-effects revealed during short-term trials, but many of these differences disappear during long-term treatment, owing to adaptation.
-
• As there are few large-scale, long-term, comparative data on cognitive and psychomotor function, simply avoiding individual antidepressants that cause over-sedation in patients at high risk of accidents is more important than a blanket recommendation to prescribe newer drugs that laboratory tests suggest might produce fewer adverse behavioural effects. Similarly, avoiding drug combinations liable to cause serious interactions is more effective in preventing such interactions than relying on generalities.
-
• Older antidepressants are more lethal in overdose, and newer compounds whose toxic potential is still being investigated should be prescribed with caution. Sexual dysfunction has been reported more often during treatment with tricyclic antidepressants and selective serotonin reuptake inhibitors than with some newer antidepressants.
-
• Little is known about the teratogenic effects of antidepressants. When such drugs are essential for the mother’s well-being, older compounds should be chosen as more is known about their potential effects in utero.
-
• There are insufficient unbiased, generally accepted data on pharmaco-economics to allow for confident recommendations on the choice of antidepressant for long-term treatment. Thus, drug acquisition costs have to be taken into consideration by those with restricted healthcare budgets.
Opinions on the overall advantages and disadvantages of newer and older antidepressants are polarised, but there is little place for dogmatism. If we are to consider the large differences in patients’ needs and in the different healthcare systems and countries in which they are treated, compromise is essential. But whatever is decided, choice should be determined by objective scientific evidence rather than frustration with the relative lack of effectiveness of currently marketed antidepressants, the novelty of wanting to gain personal experience with more recently introduced products or the hype with which they are promoted.
Multiple choice questions
-
1 The following statements are correct:
-
a more than 75% of patients who recover from a depressive episode remain in remission indefinitely
-
b continuation treatment with an antidepressant decreases the risk of relapse in 20% more patients than does continuation treatment with a placebo
-
c the difference in losses to trials owing to side-effects of SSRIs and of TCAs is less than 5%
-
d antidepressant drugs, once started, should be continued indefinitely
-
e newer antidepressants are not more efficacious than TCAs and MAOIs.
-
-
2 With regard to the unwanted effects of anti-depressants:
-
a psychomotor testing can predict untoward events in real-life situations with great accuracy
-
b most research into behavioural effects of anti-depressants has been carried out following long-term treatment
-
c in elderly drivers on antidepressants the risk of a vehicle crash is related to the dose of the drug
-
d people working in dangerous jobs are more liable to accidents if they are receiving drug combinations
-
e sexual dysfunction can be prevented by prescribing SSRIs.
-
-
3 As far as toxicity in overdose is concerned:
-
a newer antidepressants are more cardiotoxic than older antidepressants
-
b increased prescribing of antidepressants has led to a reduction in the number of people who kill themselves by violent means
-
c the incidence of suicide by any method during treatment with SSRIs and TCAs is similar
-
d it is safer to prescribe older antidepressants for patients prone to self-poisoning
-
e venlafaxine has a higher fatal toxicity index than other serotonergic antidepressants.
-
-
4 During prolonged treatment with antidepressants in patients who require concomitant treatment for other illnesses:
-
a we need have no concern over prescribing combinations of drugs that each have safe side-effect profiles
-
b we can be assured that CNS toxicity does not occur when patients on SSRIs require selegiline
-
c it is safe to prescribe lipid-lowering agents for patients on TCAs
-
d individuals with epilepsy taking carbamazepine are not routinely banned from driving if they are receiving an SSRI
-
e ventricular arrhythmias do not occur when SSRIs and terfenadine are co-administered.
-
-
5 When considering long-term treatment:
-
a although antidepressants are effective, there is also a place for psychological treatment in the prevention of depression
-
b we need have no concern over prescribing newer antidepressants for women of childbearing age
-
c prescribing newer drugs for all patients in England who require an antidepressant would cost £¼ billion or more than prescribing an older TCA
-
d taking into account indirect as well as direct costs, there is no doubt that prescribing the newer compounds would be cost-effective
-
e prescribing a generic SSRI offers a practical compromise in the pharmaco-economic debate.
-
MCQ answers
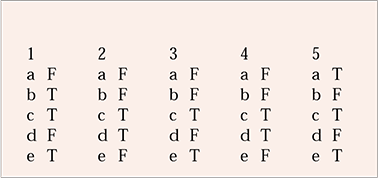
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | F | a | F | a | F | a | T |
| b | T | b | F | b | F | b | F | b | F |
| c | T | c | T | c | T | c | T | c | T |
| d | F | d | T | d | F | d | T | d | F |
| e | T | e | F | e | T | e | F | e | T |




eLetters
No eLetters have been published for this article.