Introduction
During the early 1990s, a significant percentage of the soybean production area in the United States was treated with PRE herbicides, particularly with chlorimuron-ethyl (20%), metribuzin (19%), and imazethapyr (11%; USDA 2020). Between 1996 and 2006, the soybean acreage in the United States planted with glyphosate-resistant (GR) varieties and the area treated with glyphosate increased by approximately 90% and 60%, respectively (Benbrook Reference Benbrook2016; Duke and Powles Reference Duke and Powles2009). The rapid adoption of the GR soybean technology shifted herbicide use patterns from PRE followed by POST herbicides from multiple sites of action (SOAs) to POST only application(s) of glyphosate (Duke Reference Duke2015; Givens et al. Reference Givens, Shaw, Johnson and Stephen2009; Powles Reference Powles2008). The wide adoption of GR soybean varieties and associated reliance on glyphosate use led to drastic reduction in herbicide diversity, induced weed species shifts, and accelerated the evolutionary rate of GR weeds in these production systems (Culpepper Reference Culpepper2006; Green Reference Green2009; Johnson et al. Reference Johnson, Davis, Kruger and Weller2009; Kniss Reference Kniss2018; Owen Reference Owen2008; Owen and Zelaya Reference Owen and Zelaya2005; Webster and Nichols Reference Webster and Nichols2012). Seventeen weed species evolved resistance to glyphosate between 1990 and 2020 in the United States (Heap Reference Heap2020). Due to increased reports of GR weeds throughout the United States, the use of additional herbicide SOAs have become necessary for effective chemical weed management (Hager et al. Reference Hager, Wax, Bollero and Stoller2003; Prince et al. Reference Prince, Shaw, Givens, Owen, Weller, Young, Wilson and Jordan2012; Riggins and Tranel Reference Riggins and Tranel2012; Werle et al. Reference Werle, Oliveira, Jhala, Proctor, Rees and Klein2018). The soybean production area treated with PRE herbicides substantially increased from 2006 through 2017, particularly with sulfentrazone (21%), metribuzin (16%), S-metolachlor (15%), and flumioxazin (10%; USDA 2020), indicating higher herbicide SOA diversity for weed control in soybean cropping systems (Kniss Reference Kniss2018). The integration of PRE herbicides is an effective strategy for management of herbicide-resistant weeds (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barret2012). PRE herbicides can reduce early season weed interference and give growers more flexibility for timely POST applications (Arneson et al. Reference Arneson, Smith, DeWerff and Oliveira2019; Butts et al. Reference Butts, Miller, Pruitt, Vieira, Oliveira, Ramirez and Lindquist2017; Knezevic et al. Reference Knezevic, Pavlovic, Osipitan, Barnes, Beiermann, Oliveira, Lawrence, Scott and Jhala2019; Tursun et al. Reference Tursun, Datta, Sami, Kantarci, Knezevic and Singh2016; Whitaker et al. Reference Whitaker, York, Jordan, Culpepper and Sosnoskie2011). Additionally, PRE herbicides can increase herbicide SOA diversity because there are limited options for effective POST herbicides (Beckie and Reboud Reference Beckie and Reboud2009; Grey et al. Reference Grey, Cuts, Newsome and Newel2014; Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barret2012).
Even though extended soil residual efficacy from PRE herbicides during the growing season is desirable for weed control, certain residual herbicides can persist (carryover) in the soil and negatively affect growth of subsequent crops, including cover crops (Curran Reference Curran2016). The planting of cover crops after cash crop harvest for soil conservation and weed suppression purposes has increased in the United States, but successful cover crop establishment in corn-soybean rotations where PRE herbicides are used remains a concern (Cornelius and Bradley Reference Cornelius and Bradley2017; Oliveira et al. Reference Oliveira, Butts and Werle2019; Whalen et al. Reference Whalen, Bish, Young, Hager, Conley, Reynolds, Steckel, Norsworthy and Bradley2019). For instance, metribuzin + chlorimuron-ethyl applied to soybean reduced the biomass of fall-planted alfalfa (Medicago sativa L.; >55%), indicating that alfalfa planting must be avoided where such herbicide combination has been applied within 4 mo (Walsh et al. Reference Walsh, DeFelice and Sims1993). Similarly, imazapyr + imazapic applied to corn reduced the fresh weight of subsequent pea (Pisum sativum L.), alfalfa, and annual ryegrass (Lolium multiflorum Lam.) by 23%, 75%, and 63%, respectively, 60 d after establishment (Alister and Kogan Reference Alister and Kogan2005). Generally, small-seeded cover crops, including clovers (Trifolium spp.), canola (Brassica napus L.), and annual ryegrass tend to be more sensitive to PRE herbicides than large-seeded species such as cereal rye and oats (Avena sativa L.; Curran Reference Curran2016; Palhano et al. Reference Palhano, Norsworthy and Barber2018).
While both the use of PRE herbicides in response to widespread occurrence of GR weeds and interest in adopting fall-seeded cover crops continue to rise throughout the United States (Heap Reference Heap2020; Oliveira et al. Reference Oliveira, Butts and Werle2019; USDA 2020), evaluations of soil residual efficacy of commonly used PRE herbicides in soybeans on broad spectrum weed control and subsequent cover crop establishment become imperative. The use of plants as bioindicator organisms of herbicide residue in soil (i.e., soil bioassays) has been widely adopted as an alternative technique to chemical extraction analytical methods (e.g., liquid chromatography, gas chromatography, mass spectrometry, capillary electrophoresis, and immunoassays; Geisel et al. Reference Geisel, Schoenau, Holm and Johnson2008; Horowitz Reference Horowitz1976; Mehdizadeh et al. Reference Mehdizadeh, Alebrahim and Roushani2017; Streibig Reference Streibig1988; Wang and Freemark Reference Wang and Freemark1995). Despite being time-consuming and labor-intensive, the adoption of bioassay techniques has advantages compared to analytical methods that include reduced cost, no need for expensive laboratory equipment, biological detection of low herbicide concentrations in soil, and reproducible results (Mehdizadeh et al. Reference Mehdizadeh, Alebrahim and Roushani2017; Riddle et al. Reference Riddle, O’Sullivan, Swanton and Van Acker2013). Thus, the objective of this experiment was to evaluate the length of soil residual weed control from PRE soybean herbicides and the detrimental impact of these herbicides have on cover crop species using field-treated soil in greenhouse bioassays.
Materials and Methods
Field Experiments
Field experiments were conducted in 2018 and 2019 at the University of Wisconsin Arlington (43.30°N, 89.33°W) and Lancaster (42.83°N, 90.76°W) Agricultural Research Stations, near Arlington and Lancaster, WI, respectively, for a total of four experimental site-years. Soil characteristics for each site-year are described in Table 1. The experimental fields had been in a corn-soybean rotation and corn was grown the year before the experiment establishment at all site-years. Before soybean establishment, fields were tilled using a field cultivator. Soybean seeds were planted at a depth of 3 cm, with 76-cm row spacing, and 345,940 seeds ha−1. Soybean cultivars and planting date information are detailed in Table 1. Experimental units were 3 m wide (four soybean rows) by 7.6 m long. Monthly mean air temperature and total rainfall during the soybean growing season were obtained from WatchDog 2700 weather stations (Spectrum Technologies®, Aurora, IL) installed at each site-year (Table 2). The experiments were conducted in a randomized complete block design with four replications. The treatments consisted of 11 PRE herbicides and a nontreated control (Table 3). The PRE herbicides investigated each had a single active ingredient to evaluate their soil residual efficacy independently (e.g., no mixtures or commercial premixes containing multiple active ingredients were evaluated in this research). Herbicides were applied within 3 d after soybean planting (Table 1). The herbicides were applied using a CO2-pressurized backpack sprayer equipped with four Teejet XR11002 (Teejet, Springfield, IL) nozzles spaced 50.8 cm apart, at a height of 45 cm from the soil surface, 248 kPa operating pressure, at a speed of 4.8 km h−1, calibrated to deliver 140 L ha−1 of spray solution. All sites received more than 20 mm of rainfall within 3 d of application. For reference, the half-lives of the PRE herbicides evaluated were obtained from the WSSA Herbicide Handbook (Shaner Reference Shaner2014), Camargo et al. (Reference Camargo, Senseman, Haney, Guiced and McCauleye2013), and Jablonkai (Reference Jablonkai2000) and are reported in Table 3.
Table 1. Soil description, soybean cultivars, and planting and herbicide application dates for the field experiments in Wisconsin.
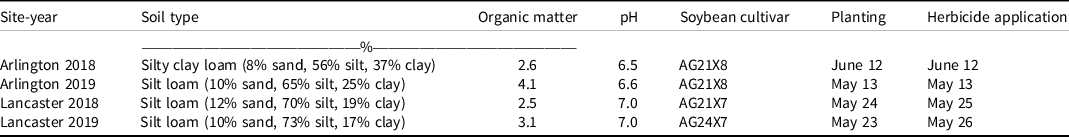
Table 2. Monthly mean air temperature and total rainfall from May through September.
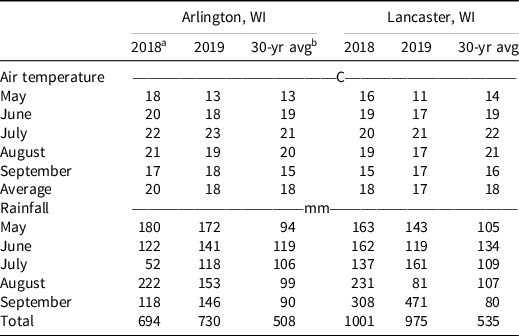
a 2018 and 2019 weather data obtained from in situ weather stations.
b 30-yr avg data (1981–2010) were obtained from the Wisconsin State Climatology Office (~http://www.aos.wisc.edu/~sco/).
Table 3. PRE herbicide, trade names, companies, site of action group, herbicide families, half-lives, and rates used in the field experiments.

a Abbreviations: ALS, acetolactate synthase; PS II, photosystem II; PPO, protoporphyrinogen oxidase; SOA#, site of action; VLCFA, very long chain fatty acid.
b Half-life values were obtained from the WSSA Herbicide Handbook (10th ed.; Shaner Reference Shaner2014) other than saflufenacil and acetochlor, which were obtained from Camargo et al. (Reference Camargo, Senseman, Haney, Guiced and McCauleye2013) and Jablonkai (Reference Jablonkai2000), respectively.
To investigate the residual performance of the aforementioned PRE herbicides over time, soil samples from a depth of 0 to 10 cm were collected from the field experiments at 0, 10, 20, 30, 40, and 50 DAT using a 6-cm-diameter handheld soil sampler (Fiskars®, Middleton, WI). At each sampling time, five soil cores were collected adjacent to the two central soybean rows from each plot and combined into one composite sample inside a sealable plastic bag (˜1,000 g). Soil samples were stored in a freezer (−20 C) until the onset of the greenhouse bioassays. Other than the PRE herbicides, no additional herbicides were applied to the field experiments.
Greenhouse Bioassays
Bioassays were conducted in the fall of 2018 (with the aforementioned soil samples collected in 2018) and in the fall of 2019 (with soil samples collected in 2019) in a greenhouse on the University of Wisconsin-Madison campus (43.07°N, 89.42°W). The bioassay experimental unit consisted of a 158 cm3 seed tray cell (4.9 cm width × 5.7 cm length × 5.7 cm depth; 3601 Series T.O Plastics Inc., Clearwater, MN) filled with the soil samples from the field experiments. Composite soil samples within a site-year were thawed and combined across replications from the same PRE herbicide by sampling time, thoroughly mixed, and then split into the bioassay experimental units (seed tray cells). Four bioindicator species were used: two small-seeded weed species, Palmer amaranth (population Kei 3; Oliveira et al. Reference Oliveira, Giacomini, Arsenijevic, Vieira, Tranel and Werle2020) and giant foxtail, which were collected in 2017 in Keith County, NE, and in 2018 in Columbia County, WI, respectively; and two cover crops, radish (‘Tillage Radish’®; La Crosse Seed, La Crosse, WI) and cereal rye (‘Guardian’® Winter Rye; La Crosse Seed). These species were selected given their commonality as either weeds or cover crops across cropping systems in the United States and to represent a range of seed sizes and plant families (Oliveira et al. Reference Oliveira, Butts and Werle2019; WSSA 2019). To maintain consistent seeding rates for the weed species, the same volume of seeds was planted, and it averaged 60 and 20 seeds of Palmer amaranth and giant foxtail, respectively. Five seeds of each cover crop species were planted. Each species was grown in separate experimental units. The bioassays were conducted as a randomized complete block design with four replications. The bioassay was replicated twice in time with the soil collected per PRE herbicide over sampling time for each site-year. Temperature (14 C minimum, 27 C average, 34 C maximum) and relative humidity (57% average) were monitored throughout the greenhouse experiments using a WatchDog A150 Temp/RH logger (Spectrum Technologies®, Aurora, IL). Plant biomass was collected at 28 d after planting (DAP). Biomass samples were cut at the soil surface, placed in paper bags, and dried (70 C) until a constant weight was achieved. The biomass of plants grown in soil treated with herbicides from each sampling time were compared with that of the average nontreated control from each sampling time for each site-year and expressed as percent biomass compared to the nontreated control using the following equation:
where Z is percent biomass compared to that of the nontreated control (the closer to 100% the lower the herbicide impacted plant growth), B is the observed biomass for the respective experimental unit (g), and C is the average biomass of the nontreated control (g).
Accumulated growing degree day (GDD) units at the field soil sampling times were estimated and used as the explanatory variable to standardize the differences in planting dates and growing conditions across site-years (Tables 1 and 2). GDD was estimated based on recorded field soil temperature (0 to 2 cm) collected with a Watchdog 1650 Micro Station (Spectrum Technologies®, Aurora, IL). Daily soil GDD was calculated according to the equation described by McMaster and Wilhelm (Reference McMaster and Wilhelm1997):
where Tmax is the daily maximum soil temperature (C), Tmin is the daily minimum soil temperature, Tbase is the base temperature (5 C, which indicates the minimum temperature necessary for herbicide degradation in soil; Cupples et al. Reference Cupples, Sims, Hultgren and Hart2000), and n is the number of days after treatment. The first soil sampling at each site-year occurred immediately after PRE herbicide application thus representing the onset of GDD accumulation (0 DAT = 0 GDD).
Statistical Analyses
Linear regression models were fitted to the percent biomass compared to the nontreated control (Z; response variable) and regressed against GDD (explanatory variable) using the lm function of the lm4 package (Bates et al. Reference Bates, Maechler, Bolker and Walker2015) with R statistical software (version 4.0.2; R Core Team 2020). To enable stronger inferences, models were fitted to the data pooled across site-years for each PRE herbicide by bioindicator species combination. The intercept and slope of each model were used to assist with interpreting bioindicator species response to each herbicide, where the intercept indicates the injury expected at the highest herbicide concentration in soil (i.e., day of herbicide application [0 DAT = 0 GDD]), and the slope represents the biological response to herbicide dissipation over time for each species tested (Walker and Thompson Reference Walker and Thompson1977). The percent biomass at 100, 500, and 900 accumulated GDD (GDD accumulation range representative of the soil sampling interval across site-years; 0 to 50 DAT) was estimated for each bioindicator species from the linear regression models using the predict function of the lm4 package (Bates et al. Reference Bates, Maechler, Bolker and Walker2015) to aid in the interpretation of the residual efficacy through the season.
The percent biomass of each bioindicator species across soil sampling time within each site-year were used to calculate the Area Under Biomass Production Curve (AUBPC). AUBPC used the audpc function of the agricolae package (Mendiburu Reference Mendiburu2019). The Shapiro-Wilk test was performed using the shapiro.test function of the stats package to test for normality (Royston Reference Royston1995), and the Levene test was performed using the leveneTest function of the car package to test the homogeneity of residual variance of the AUBPC data (Fox and Weisberg Reference Fox and Weisberg2011) using R statistical software. AUBPC estimates were subjected to ANOVA using a mixed-effect model with the lmer function of the lm4 package (Bates et al. Reference Bates, Maechler, Bolker and Walker2015). In the model, herbicide and bioindicator species were included as fixed effects and experimental runs nested within site-years were considered as random effects (assuming soil samples were collected from random sites in southern Wisconsin). If ANOVA indicated herbicide × bioindicator species interaction or main effects to be significant (P < 0.05), means were separated accordingly using Fisher’s protected LSD test. AUDPC is a valuable tool commonly used in the field of plant pathology to estimate disease progress over time (Madden et al. Reference Madden, Hughes and Van Den Bosch2007) and has been previously adopted by weed scientists to estimate crop injury from POST herbicides across distance or over time (Striegel et al. Reference Striegel, Oliveira, Arneson, Conley, Stoltenberg and Werle2020; VanGessel et al. Reference VanGessel, Johnson and Scott2016). The AUBPC allowed estimation of a single response variable, and to thus rank the overall PRE herbicide impact on biomass of each bioindicator species over the period evaluated (0 to 50 DAT; 0 to 900 GDD), further supporting the linear regression results.
Results and Discussion
Field Experiments
Given the different planting times and environmental conditions, the accumulated GDD at each sampling time (0, 10, 20, 30, 40, and 50 DAT) varied at each site-year (Tables 1 and 2). Nonetheless, 100, 500, and 900 GDD were selected as a representative range of GDD accumulated during the soil sampling interval in this study to assist interpretations of PRE herbicide impact on bioindicator biomass (Tables 4 and 5). The days after PRE herbicide application that represent 100, 500, and 900 GDD were 5, 27, and 48 for the Arlington 2018 experiment; 11, 38, and 59 for Arlington 2019; 5, 32, and 53 for Lancaster 2018; and 8, 36, and 55 for Lancaster 2019.
Table 4. Estimated parameter values for the linear regression model for plant biomass of each bioindicator species by PRE herbicide combination evaluated in the greenhouse bioassays.

a Abbreviations: DAT, days after treatment; GDD, growing degree days; SOA#, site of action group.
b The intercept indicates the injury expected at the highest herbicide concentration in soil (i.e., day of herbicide application [0 DAT = 0 GDD]) and the slope represents the biological response to herbicide dissipation over time for each species tested (0–900 GDD).
Table 5. Area under biomass production curve estimated for the percent biomass compared to the nontreated control of each bioindicator species by PRE herbicide combination over time.

a Abbreviations: SOA, site of action.
b Means within a column followed by the same letter are not significantly different at the 5% level according to Fisher’s LSD test.
Greenhouse Bioassays
The PRE herbicide × bioindicator species interaction was significant (P < 0.01), thus AUBPC was analyzed separately for each bioindicator species (Table 5), which further supported the decision to fit an individual linear regression model to each PRE herbicide by bioindicator species combination evaluated. Herein, the lower the intercept (% biomass at 0 GDD; Table 4), the lower the biomass estimated at 100, 500, and 900 GDD (Figure 1), and the lower the AUBPC values (Table 5), the more detrimental the impact of the PRE herbicide on the bioindicator species of interest.
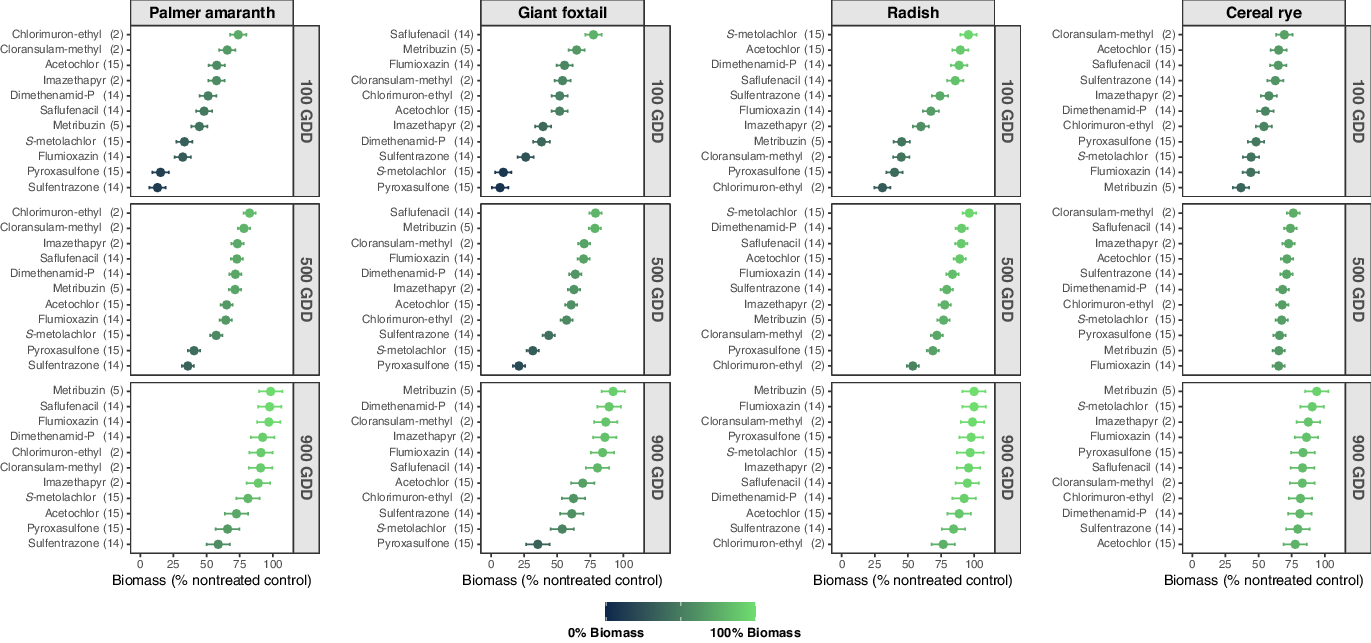
Figure 1. Estimated biomass (% biomass compared with the nontreated control) of each bioindicator species by PRE herbicide at 100, 500, and 900 growing degree days (GDD) across 4 site-years in southern Wisconsin. The days after PRE herbicide application that represent 100, 500, and 900 GDD were 5, 27, and 48 at Arlington 2018; 11, 38 and 59 at Arlington 2019; 5, 32, and 53 at Lancaster 2018; and 8, 36 and 55 at Lancaster 2019. Dots represent the means and dashes represent the 95% confidence intervals. PRE herbicides are ranked within each subfigure (bioindicator species by GDD combination) according to their impact on bioindicator biomass accumulation from least (100% biomass; light green) to highest (0% biomass; dark teal).
Palmer Amaranth
Sulfentrazone (intercept = 7.2%, slope = 0.057) and pyroxasulfone (intercept = 8.9%, slope = 0.063) were the most detrimental PRE herbicides to Palmer amaranth through the soil sampling period (Table 4). Palmer amaranth grown in soil treated with sulfentrazone produced 13%, 36%, and 59% biomass compared with the nontreated control at 100, 500, and 900 GDD, respectively (Figure 1). In soil treated with pyroxasulfone, Palmer amaranth presented 15%, 41%, and 66% biomass compared with the nontreated control at 100, 500, and 900 GDD, respectively. The AUBPC analysis corroborates these results with the lowest AUBPC values with sulfentrazone (AUBPC = 25,597) and pyroxasulfone (AUBPC = 28,722) compared with the nontreated control (AUBPC = 71,619; Table 5). Sulfentrazone and pyroxasulfone are selective herbicides that provide effective residual weed control of small-seeded broadleaf species including Palmer amaranth (Gregory et al. Reference Gregory, Porpiglia and Chandler2005; Grey et al. Reference Grey, Cuts, Newsome and Newel2014; Olson et al. Reference Olson, Zollinger, Thompsom, Peterson, Jenks, Moechnig and Stahlman2011; Sweat et al. Reference Sweat, Horak, Peterson, Lloyd and Boyer1998). Moreover, flumioxazin and S-metolachlor also resulted in significant Palmer amaranth biomass reduction (≤33% biomass compared with the nontreated control) at 100 GDD (Figure 1). These results are validated by the high biomass reduction shortly after application of these herbicides (intercept = 23.9%, 27.3% for flumioxazin and S-metolachlor, respectively; Table 4) and supported by the lower AUBPC for Palmer amaranth with flumioxazin and S-metolachlor (AUBPC = 49,062 and 43,851, respectively; Table 5). The Palmer amaranth population used in this study was confirmed to be resistant to an ALS-inhibitor herbicide (imazethapyr at 70 g ai ha−1; Oliveira et al. Reference Oliveira, Giacomini, Arsenijevic, Vieira, Tranel and Werle2020), explaining why ALS-inhibitor herbicides were not as effective. The additional PRE herbicides evaluated were less effective in controlling Palmer amaranth, showing ≥48%, ≥57%, and ≥73% biomass compared with the nontreated control at 100, 500, and 900 GDD, respectively (Figure 1). Thus, according to our results, flumioxazin, pyroxasulfone, S-metolachlor, and/or sulfentrazone, can be effective PRE herbicide options to control ALS-inhibitor-resistant Palmer amaranth populations in soybeans.
Giant Foxtail
Pyroxasulfone (intercept = 3.3%, slope = 0.035) and S-metolachlor (intercept = 3.7%, slope = 0.055) had the greatest impact on giant foxtail biomass through the soil sampling period (Table 4). Giant foxtail produced 7%, 21%, and 35% biomass on the nontreated control at 100, 500, and 900 GDD in soil treated with pyroxasulfone (Figure 1). In soil treated with S-metolachlor, giant foxtail produced 9%, 32%, and 54% biomass at 100, 500, and 900 GDD, respectively. The AUBPC results corroborate the linear regression analysis showing lower biomass production over time by giant foxtail in the soil treated with pyroxasulfone (AUBPC = 13,478) and S-metolachlor (AUBPC = 20,476) compared with that of the nontreated control (AUBPC = 65,974; Table 5). The effectiveness of pyroxasulfone and S-metolachlor reducing giant foxtail growth supports the efficacy and selectivity of VLFCA-inhibitor herbicides on small-seeded annual grass species (Parker et al. Reference Parker, Simmons and Wax2005; Yamaji et al. Reference Yamaji, Honda, Kobayashi, Hanai and Inoue2014). Sulfentrazone (intercept = 21.8%; Table 4) also reduced giant foxtail growth by 26%, 44% and 61% of biomass at 100, 500, and 900 GDD, respectively (Figure 1) and AUBPC = 29,526 (Table 5). The remaining PRE herbicides investigated were less effective on giant foxtail, with ≥38%, ≥44%, and ≥61% biomass compared with the nontreated control 100, 500, and 900 GDD, respectively.
Radish
Chlorimuron-ethyl was the most detrimental herbicide to radish (intercept = 25%, slope = 0.057; Table 4). Radish biomass was lowest in soil treated with chlorimuron-ethyl throughout the sampling period, producing 31%, 54%, and 77% biomass compared with the nontreated control at 100, 500, and 900 GDD, respectively (Figure 1). Validating these findings, radish growing in the soil treated with chlorimuron-ethyl presented the lowest AUBPC (AUBPC = 39,315) compared with the nontreated control (AUBPC = 73,374; Table 5). Chlorimuron-ethyl soil residual efficacy has been shown to influence the establishment of subsequent broadleaf cover crop species (Bedmar et al. Reference Bedmar, Perdigon and Monterubbianesi2006; Brown et al. Reference Brown, Robinson, Nurse, Swanton and Sikkema2009; Ren et al. Reference Ren, Wang and Zhou2011). Previous research has also indicated that chlorimuron-ethyl reduced biomass and stand of radish seeded after soybean harvest by 19% and 40%, respectively (Cornelius and Bradley Reference Cornelius and Bradley2017). The persistence of chlorimuron-ethyl in soil varies according to the pH (Sharma et al. Reference Sharma, Banerjee and Choudhury2012). For instance, chlorimuron-ethyl persistence increased from 30 d at pH 5.9 to 69 d at pH 6.8 (Bedmar et al. Reference Bedmar, Perdigon and Monterubbianesi2006). Thus, it is likely that chlorimuron-ethyl was the most detrimental herbicide on radish due to the moderate soil pH in this study (6.5–7.0; Table 1). Conversely, cloransulam-methyl, metribuzin, and pyroxasulfone affected radish biomass (≤45% biomass compared with the nontreated control) at 100 GDD but not at 900 GDD (≥98% biomass compared with the nontreated control; Figure 1). The AUBPC findings support the linear regression results, showing lower AUBPC by cloransulam-methyl (AUBPC = 53,591), metribuzin (AUBPC = 58,839), and pyroxasulfone (AUBPC = 51,768) than the nontreated control (AUBPC =73,374; Table 5). The lack of radish response at 900 GDD to cloransulam-methyl (slope = 0.067), metribuzin (slope = 0.079), and pyroxasulfone (slope = 0.072) likely occurred due to the higher dissipation of these herbicides over time (Figure 1; Table 4). On the other hand, radish showed constant biomass (79% biomass compared with the nontreated control) in soil treated with sulfentrazone at 500 and 900 GDD (Figure 1). The consistent reduction in radish biomass with sulfentrazone at 500 and 900 GDD is likely due to the extended half-life of this herbicide (121–302 d; Table 3; Shaner Reference Shaner2014). Despite sulfentrazone being less harmful to radish at 100 GDD (74% biomass compared with the nontreated control), this herbicide appeared to be as injurious as chlorimuron-ethyl to radish at 900 GDD (Figure 1). Cornelius and Bradley (Reference Cornelius and Bradley2017) observed that radish showed 19% and 13% stand and biomass reduction, respectively, by sulfentrazone at 28 d after emergence. The residual efficacy of the remaining PRE herbicides evaluated in this bioassay were less detrimental to radish, presenting ≥60%, ≥78%, and ≥89% biomass compared with the nontreated control at 100, 500, and 900 GDD, respectively (Figure 1).
Cereal Rye
Metribuzin caused the highest injury to cereal rye growth at 100 and 500 GDD, producing 37% and 65% biomass compared with the nontreated control, respectively (intercept = 29.6%; Figure 1; Table 4). However, this herbicide presented rapid dissipation over time (slope = 0.071) resulting in minimal to no impact on cereal rye growth at 900 GDD (94% biomass compared with the nontreated control). The AUBPC analysis also indicates that metribuzin was the most detrimental herbicide to cereal rye growth (AUBPC = 49,850; Table 5) when compared with the nontreated control (AUBPC = 60,638). Metribuzin half-life is influenced by soil texture, organic matter content, temperature, and pH, and it tends to decrease as soil temperature and pH increase (Hyzak et al. Reference Hyzak and Zimdahl1974; Ladlie et al. Reference Ladlie, Meggitt and Penner1976; Savage Reference Savage1977; Sharom et al. Reference Sharom and Stephenson1976; Webster and Reimer Reference Webster and Reimer1976). Metribuzin degradation occurs primarily by soil microorganisms (Maqueda et al. Reference Maqueda, Villaverde, Sopeña, Undabeytia and Morillo2009; Savage Reference Savage1977); higher soil temperature and pH (>7) support higher microbial activity and consequently higher herbicide degradation (Maqueda et al. Reference Maqueda, Villaverde, Sopeña, Undabeytia and Morillo2009; Singh et al. Reference Singh, Walker, Morgan and Wright2003). Thus, the increased soil temperature during the sampling period and the moderate soil pH in this study (6.5–7.0; Table 1) support the rapid degradation of metribuzin resulting in a low impact on cereal rye at 900 GDD (˜50 DAT). Cornelius and Bradley (Reference Cornelius and Bradley2017) observed that metribuzin reduced biomass (23%) and stand density (22%) of cereal rye seeded after soybean harvest. Cereal rye biomass was also reduced by pyroxasulfone, S-metolachlor, and flumioxazin at 100 and 500 GDD, with ≤48% and ≤67% biomass compared with the nontreated control, respectively (Figure 1). By 900 GDD, these herbicides had a minimal effect on cereal rye, with at least 84% biomass accumulation. According to the AUBPC analysis (Table 5), flumioxazin (AUBPC = 50,908), pyroxasulfone (AUBPC = 51,803), and S-metolachlor (AUBPC = 52,322) were ranked as detrimental herbicides to cereal rye. The other PRE herbicides assessed presented ≥54%, ≥68%, and ≥78% biomass compared with the nontreated control at 100, 500, and 900 GDD, respectively (Figure 1). Cornelius and Bradley (Reference Cornelius and Bradley2017) reported that only 4 out of 27 herbicides tested adversely affected cereal rye establishment in terms of stand and biomass reduction. Furthermore, Smith et al. (Reference Smith, Legleiter, Bosak, Johnson and Davis2015) observed that cereal rye was not affected by commonly used soybean herbicides across 2 yr in Wisconsin and Indiana. Therefore, cereal rye is a resilient winter-hardy species and could fit well as a cover crop in systems where PRE herbicides are used for weed control purposes and the species is planted after soybean harvest (>50 DAT).
Sulfentrazone, pyroxasulfone, flumioxazin, and S-metolachlor were the most efficacious herbicides in the bioassay in terms of Palmer amaranth biomass production whereas pyroxasulfone, S-metolachlor, and sulfentrazone presented the highest residual impact on giant foxtail biomass. Thus, growers and practitioners should be able to use our results to support their selection of PRE herbicide(s) based on their weed infestations and benefit from a range of effective herbicide SOAs to include during multiyear crop rotations. Overall, our results showed that radish was less affected by PRE herbicides than cereal rye at 900 GDD (Figure 1). Most PRE herbicides evaluated herein would likely not affect radish and cereal rye established in the fall after soybean harvest under environmental conditions across southern Wisconsin.
Results of our bioassays can be of value to growers and practitioners considering herbicide options for enhanced control of small-seeded weed species such as Palmer amaranth and giant foxtail and reduced impact on establishment of subsequent cover crops such as radish and cereal rye. With caution, these results can also guide growers and practitioners with proper selection of herbicides to be used as part of a layered residual approach (i.e., inclusion of soil-residual herbicide with the POST program) in systems where a cover crop may be seeded after such applications. Additionally, our findings showcase the value of bioassays as a strategy to evaluate the biological residual efficacy of herbicides in soil using plant species of interest (e.g., weed and/or crops from a control and/or carryover perspective, respectively). The use of greenhouse bioassays can also reduce the impact of confounding environmental factors under field settings that may lead to uneven seedling establishment. Herein we also describe novel ways that bioassay results can be analyzed and displayed. Further research is needed to investigate the residual efficacy of PRE herbicide premixes containing multiple SOAs under different soil types and environments on different weed and cover crop species.
Acknowledgments
We thank staff members at the University of Wisconsin-Madison Arlington and Lancaster Agricultural Research Stations and personnel in the Wisconsin Cropping Systems Weed Science Laboratory for their technical assistance with the field and greenhouse projects. Thanks to Dr. Mark VanGessel and anonymous reviewers for their thoughtful edits and suggestions to this manuscript. No conflict of interest has been declared.









