Vitamin D3, cholecalciferol (Vit D3) and 25-hydroxycholecalciferol (25(OH)D3) are the two major dietary forms to supply the organism with vitamin D (Vit D). In recent years, 25(OH)D3 is being studied as an alternative to Vit D3, as it is more bioavailable, efficiently absorbed, bypasses hepatic metabolism and is three to five times more potent than Vit D3(Reference Quesada Gómez and Bouillon1). Nevertheless, both dietary forms of Vit D are biologically inactive and require two (Vit D3) and one sequential hydroxylation reaction (25(OH)D3) for activation (Fig. 1). After ingestion or dermal synthesis, Vit D3 is transported via Vit D-binding protein (DBP) and hydroxylated via Vit D 25-hydroxylase (encoded by CYP2R1) in liver to form 25(OH)D3 and via 25-hydroxyvitamin D 1-alpha-hydroxylase (encoded by CYP27B1) in kidney to 1,25(OH)2D3 (the active form of Vit D). The excess amount of 1,25(OH)2D3 is subject to renal elimination following CYP24A1-mediated hydroxylation(Reference Quach2). Nevertheless, both synthesis and elimination also occur in non-renal tissues (i.e. intestine)(Reference Hasan, Oster and Reyer3). After activation, 1,25(OH)2D3 migrates via DBP to various target tissues such as intestine, bone, muscle, immune system, kidney, parathyroid glands and reproductive system to attribute its functions.

Fig. 1. Schematic illustration of the endogenous production of active (1,25(OH)2D3) and inactive (1,24,25(OH)2D3) forms of vitamin D from supplemented or dermally synthesised vitamin D3.
The mediation of the biological functions of Vit D depends on its successful binding with the intracellular Vit D receptor (VDR, ligand-dependent transcription regulatory molecule) forming the VDR-1,25(OH)2D3 complex. This complex interaction initiates the formation of two autonomous protein interaction surfaces on the VDR; one of them modulates interplay with the retinoid X receptor (RXR) for DNA binding and the other recruits co-regulators to control gene expression. After dimerisation of VDR-1,25(OH)2D3 with RXR, the heterodimer translocates to the nucleus, binds to the Vit D responsive element (VDRE), which in turn regulates the expression of numerous genes (> 900), and implements the specific function of Vit D in particular tissues(Reference Pike, Meyer and Bishop4,Reference Kongsbak, Levring and Geisler5) .
The secosteroid hormone Vit D is critical for the maintenance of serum Ca and P homeostasis for optimum bone development, as it modulates the active uptake of minerals through the intestine(Reference Holick6,Reference Sutton and MacDonald7) . The tissue-wide expression of VDR and other genes involving Vit D metabolism underscores the fact that the function of Vit D is not limited to osteogenesis or serum mineral balance(Reference Hasan, Oster and Reyer3,Reference Bouillon, Marcocci and Carmeliet8) . In fact, an array of tissues are capable to express relevant genes encoding hydroxylation enzymes for calcitriol synthesis and elimination in mammals(Reference Hasan, Oster and Reyer3,Reference Hewison, Burke and Evans9) . Thus, it turns out to be equally important for growth(Reference Zhang, Yang and Piao10–Reference Wilborn, Kerth and Owsley13), immunity(Reference Zhang, Liu and Piao14,Reference Konowalchuk, Rieger and Kiemele15) , oxidative status(Reference Yang, Tian and Chen16,Reference Rey, Segura and Castejón17) , reproductive capacity(Reference Upadhaya, Jung and Kim18,Reference Weber, Witschi and Wenk19) and progeny performance(Reference Upadhaya, Chung and Jung20). In pig feeding, legislation limits the Vit D supply to 1000–2000 μg/kg diet, which corresponds to 25–50 µg/kg feed(21,22) , while 25(OH)D3 in combination with Vit D3 is allowed up to doses of 50 µg/kg feed(23). However, the most potent form of Vit D in terms of nutritional benefits remains unclear, largely due to divergent functional demands of tissues and cell types(Reference Hasan, Oster and Reyer3,Reference Hewison, Burke and Evans9) . Given the background, this review summarises the available literature for molecular and phenotypic outcomes of Vit D3 in comparison with 25(OH)D3 supplements in terms of reproductive capacities, growth performance, immunity and bone development in pigs.
Search strategies and selection of articles
We conducted a systematic query in Web of Science and PubMed to retrieve all the articles dealing with Vit D3 and 25(OH)D3 in pigs published from 1 January 2000 to 23 May 2022. The search strategy included appropriate MeSH terms (supplementary material S1) without any language restriction. In total, 454 articles from the Web of Science and 241 articles from PubMed were identified using the appropriate search query. Of these, a total of thirty-five articles were selected for review, paying attention to relevance, overlap and experimental design (supplementary material S2).
Comparative performance of vitamin D3 and 25(OH)D3 on reproduction and growth
Recent investigations suggest that the dietary supply of both Vit D3 and 25(OH)D3 plays an important role in fertility and maturation. The objective of this section was to identify arguments for the more effective form of Vit D in the diet to improve sow reproductive capacities and growth performance of the piglets (Table 1). Parts of the reported findings could be attributed to adaptive responses resulting from maternal nutritional programming, i.e. long-term consequences for growth, function and structure of various tissues and cell types, and thus for health and welfare(Reference Fleming, Watkins and Velazquez24).
Table 1. Overview of the comparative studies between vitamin D3 (Vit D3) and 25(OH)D3 supplementation, indicating the study designs, dosages of supplements, underlying conditions and significant results of the experiments with regard to sow reproductive capacity and growth performance of their progeny. Only supplemented diets (control and treatment) and the conditions that led to noticeable outcomes are listed

aET, explained on text; bS, sows; cP, piglets; dMNDE, maternal-nursery diet effect on piglets; ePDE, piglet diet effect; fMDE, maternal diet effect on piglets; g MSTN, myostatin; hADWG, average daily weight gain; iFE, feed efficiency; jADFI, average daily feed intake; k MYF5, myogenic factor 5; l MYOD, myogenic differentiation.
* Asterisk indicates significant outcomes (P < 0·05).
† DTP, dietary treatment period.
‡ 1 µg = 40 μg Vit D3.
Thayer et al. (Reference Thayer, Nelssen and Langemeier25) studied the effects of feeding Vit D3 and 25(OH)D3 on sow reproductive capacity, muscle fibre morphometry and subsequent growth performance of piglets. For this purpose, sixty-nine sows were randomly allocated to one of three dietary groups comprising (i) Vit D3 as control (37·5 µg/kg), (ii) Vit D3 and 25(OH)D3 at low levels (DL, 12·5 µg/kg Vit D3 + 25 µg/kg 25(OH)D3) or (iii) Vit D3 and 25(OH)D3 at high levels (DH, 37·5 µg/kg Vit D3 + 50 µg/kg 25(OH)D3). On the other hand, the piglets (n 216) from these sixty-nine sows were treated similarly to their mothers from birth until day 59 of life. No significant effect was observed on the reproductive performance of sows regardless of the dietary Vit D regimens. At the time of birth, piglets from DH-fed sows showed a significant increase in the number of primary muscle fibres in contrast to piglets from control sows at birth. However, this effect was no longer present when weaning piglets were investigated. During the nursery period, there was no effect of dietary Vit D sources on the growth performance, except for feed efficiency from day 28 to 59 and day 0 to 59. During this period, piglets from DH-fed sows showed a significant increase in feed efficiency, i.e., the body weight gain to feed intake ratio, in contrast to piglets from sows assigned to DL. Overall, dietary intake of 25(OH)D3 resulted in a significant increase in the number of primary muscle fibres at birth, but the total number of muscle fibres did not improve at birth or weaning.
Upadhaya et al. (Reference Upadhaya, Jung and Kim18,Reference Upadhaya, Chung and Jung20) conducted two different studies to compare the effects of maternal Vit D3 and 25(OH)D3 supplementations on sow reproduction and offspring growth performance. In one study(Reference Upadhaya, Chung and Jung20), forty-eight multiparous sows were provided a basal diet containing Vit D3 (CON, 50 µg/kg Vit D3) or 25(OH)D3 (TRT, CON + 50 µg/kg 25(OH)D3) depending on their body weight and expected farrowing date. At weaning, eighty piglets each from CON and TRT sows were fed diets containing 62·5 µg/kg Vit D3 (weaning diet) and 43·75 µg/kg Vit D3 (growing-finishing diet) with or without 25(OH)D3 (50 µg/kg) for 140 d. Unlike Vit D3, the maternal intake of 25(OH)D3 significantly improved the average daily weight gain (ADWG) and feed efficiency of the piglets at the early stage of the nursery period. However, in the later phase of the nursery period, maternal ingestion of either source of Vit D demonstrated a similar effect on the growth performance of the piglets. In this period, the piglets alone supplemented with 25(OH)D3 showed significant improvement in ADWG, feed efficiency and average daily feed intake. Moreover, in contrast to Vit D3, 25(OH)D3 significantly increased the water-holding capacity and reduced pork drip loss. Feeding 25(OH)D3 to sows and their progenies had a significant impact on the expression of candidate genes associated with muscle formation. After dietary supplementation to sows and their offspring (post-weaning diet) with 25(OH)D3, the up-regulation of myogenic markers MYOD1 (myogenic differentiation 1), MYF5 (myogenic factor 5) and down-regulation of MSTN (myostatin) were observed, suggesting the importance of 25(OH)D3 for muscle development. MYOD1 and MYF5 are involved in the regulation of myoblast proliferation and differentiation and critically determine the survival of muscle progenitor cells as well(Reference Alway, Morissette, Siu, Masoro and Austad26,Reference Megeney and Rudnicki27) . On the contrary, MSTN is the negative regulator of muscle development(Reference Elkina, von Haehling and Anker28). In the second study(Reference Upadhaya, Jung and Kim18), a total of forty-eight multiparous sows received either a basal diet fortified with Vit D3 (control, 50 µg/kg) or a control diet containing 25(OH)D3 (TRT, 50 µg/kg) based on their body weight and expected farrowing date. The sows fed 25(OH)D3 demonstrated a significant increase in body weight gain and body condition score during the suckling period of the piglets. Unlike Vit D3, piglets from sows fed 25(OH)D3 also had significantly higher survival, ADWG and weaning weight than piglets from sows fed the control diet. These results indicate that dietary supplementation with 25(OH)D3 significantly enhanced growth performance in both sows and their offspring.
To compare the two dietary forms of Vit D in terms of reproductive performance, Weber et al. (Reference Weber, Witschi and Wenk19) performed their study into two parts. First, 227 primi- and multiparous sows received a basal diet containing Vit D3 (114 sows, 50 µg/kg) and 25(OH)D3 (113 sows, 50 µg/kg) from mating to day 110 of gestation. 25(OH)D3-supplemented sows exhibited a significant positive impact on total litter weight and total weaning weight of the offspring compared with sows that received Vit D3. The intrauterine development of the embryos was also positively correlated with the maternal serum levels of 25(OH)D3. For the second experiment, thirty-nine sows received basal diets fortified with Vit D3 (DL, 5 µg/kg; DN, 50 µg/kg) and 25(OH)D3 (DH, 50 µg/kg) from the day of the mating and the treatments were continued for four reproductive cycles. Following the intake, the two dietary sources of Vit D exhibited similar impacts on sow reproductive performance. However, a significant increase in weight gain between birth and weaning was observed in the offspring of the DN-fed sows, in contrast to the offspring of DL- and DH-fed sows. In summary, the authors concluded that sows fed 25(OH)D3 showed a significant improvement in the birth weight of their offspring. But sows provided with either form of Vit D for more than one reproductive cycle may not show any noticeable impact on the growth performance of their progeny.
Witschi et al. (Reference Witschi, Liesegang and Gebert29) conducted a comparative study in thirty-nine primi- and multiparous sows randomly assigned to Vit D3 (DL, 5 µg/kg or DN, 50 µg/kg) and 25(OH)D3 (HD, 50 µg/kg) from the day of mating to day 21 of lactation. Similar to other studies, the serum level of 25(OH)D3 increased significantly in pigs after the administration of 25(OH)D3 compared with Vit D3. DL led to a decrease in average daily feed intake and a tendency for a decrease in body weight and body weight gain compared with the other groups. Additional supplementation of HD had no significant effect. So, maternal diets supplemented with either form of Vit D (50 µg/kg) demonstrated a similar impact on the growth performance of their offspring.
Zhang et al. (Reference Zhang, Li and Shang30) compared Vit D3 (50 µg/kg) and 25(OH)D3 (50 µg/kg) to determine their relative effects on the reproductive capacity of twenty-four sows and the growth performance of their offspring from day 107 of gestation to day 21 of lactation. No significant effect of Vit D treatments on sow reproductive performance was observed. However, in contrast to Vit D3, offspring from 25(OH)D3-fed sows showed a significant increase in litter weight, total litter weight and piglet weight gain during the lactation period. Unlike Vit D3, ADWG and total weight gain also tended to improve in piglets from 25(OH)D3-supplemented sows. Overall, the dietary supplementation of 25(OH)D3 in pregnant sows significantly improved piglet growth performance (5·3 % increase in weight gain on average).
Hines et al. (Reference Hines, Coffey and Starkey12) studied the effects of maternal supplementation of Vit D3 or 25(OH)D3 on piglet growth and muscle development. They provided forty sows with a control diet containing Vit D3 (62·5 µg/kg) and an experimental diet fortified with 25(OH)D3 (DH, 12·5 µg/kg Vit D3 + 50 µg/kg 25(OH)D3) beginning 43 d before artificial insemination through day 90 of gestation. Both diets contained 12·5 µg/kg of Vit D3 to avoid possible Vit D deficiency. In comparison with Vit D3, foetuses from 25(OH)D3-fed dams showed a significant increase in the number of longissimus dorsi muscle fibres by 9·3 %. However, there were no significant effects of the maternal diet on the cross-sectional area of the muscles in piglets. Piglets from 25(OH)D3-fed sows had more Pax7+ myoblast (72 and 96 h of post-plating) in the longissimus dorsi muscle than piglets from sows fed Vit D3. Foetuses from 25(OH)D3-fed sows also had increased total myoblast proliferative capacity. Thus, a maternal diet containing 25(OH)D3 exhibited a significant positive impact on foetal muscle development.
In contrast to the aforementioned studies, there are very few studies that have investigated the growth performance after supplementing two dietary sources of Vit D directly to piglets rather than to pregnant sows. In the study of Zhang et al. (Reference Zhang, Yang and Piao10), 144 piglets were randomly assigned to three different dietary treatments with a follow-up period of 28 d, namely a normal Ca-P (PC), low Ca-P (NC) diet supplemented with 62·5 µg/kg Vit D3 and low Ca-P diet supplemented with 25(OH)D3 (NC + 25D, 50 µg/kg 25(OH)D3). The experiment was conducted for 28 d and consisted of phase 1 (days 0–14) and phase 2 (days 15–28). PC diet contained 0·81 % Ca, 0·60 % total P and 0·72 % Ca, 0·53 % total P, and NC diet contained 0·56 % Ca, 0·47 % total P, and 0·45 % Ca, 0·39 % total P in phase 1 and phase 2, respectively. Compared to NC, the body weight (day 28) and ADWG (days 15–28 and days 1–28) of the weaned piglets increased significantly after their dietary ingestion of NC + 25D. Overall, the piglets fed 25(OH)D3 showed noticeable growth performance even when no dietary source of Vit D was provided to their mothers.
Corresponding to the study of Zhang et al., Zhao et al. also conducted feeding trials only in piglets to evaluate growth efficiencies(Reference Zhao, Wen and Xiao31). They supplemented a total of 240 weaned piglets (21 d of age, initial body weight about 6 kg) with a positive control diet (PSC, 50 µg/kg Vit D3), negative control diet (NC, –0·15 % P and –0·25 % Ca of PSC), phytase (Phy diet, NC + 37·5 µg/kg phytase) and 25(OH)D3 (HyD, NC + 50 µg/kg 25(OH)D3; Phy + HyD, NC + 37·5 µg/kg Phy + 50 µg/kg HyD). The experiment was divided into two phases (6–10 kg and 10–20 kg). Regardless of the body weight, HyD supplements showed no significant effect in average daily feed intake or feed efficiency of the weaned piglets in contrast to NC. However, ADWG of piglets (10–20 kg) increased noticeably after dietary intake of HyD compared with NC. In essence, piglets from Vit D-deprived sows demonstrated noticeable improvement in their growth after dietary ingestion of 25(OH)D3 without altering their feed intake or feed efficiency. However, data on body composition traits have not been reported in this study.
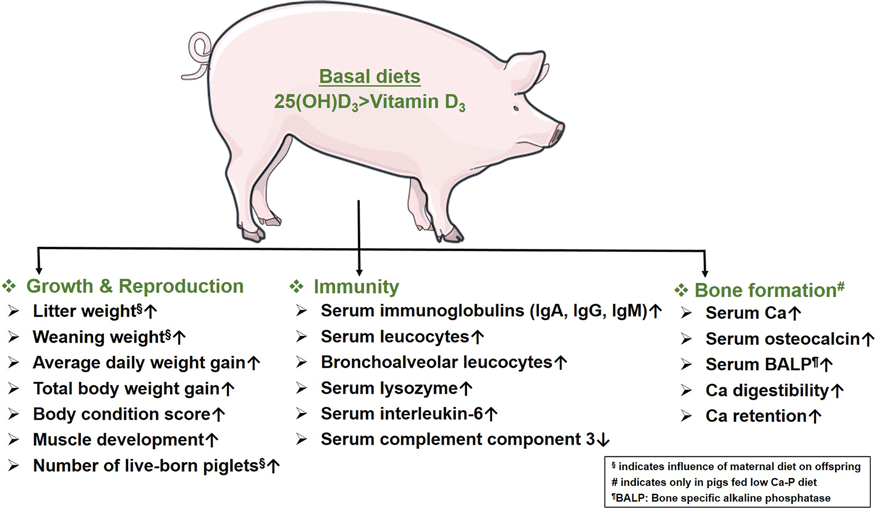
Thus, most studies show that sows fed either form of Vit D have a similar impact on their reproductive ability. However, the survivability of piglets from sows fed 25(OH)D3 is significantly better than piglets from sows fed Vit D3(Reference Zhou, Chen and Lv32,Reference Coffey, Hines and Starkey33) . Unlike Vit D3, feeding 25(OH)D3 to sows helps to noticeably increase offspring growth performance by improving weaning weight, ADWG, total body weight gain and body condition scores. In the absence of maternal supply of Vit D sources, supplementation of 25(OH)D3 to the offspring alone can also significantly boost their growth performance. 25(OH)D3 also outperforms Vit D3 in increasing the number of muscle fibres and improving the proliferation and differentiation ability of muscle cells.
Comparative performance of vitamin D3 and 25(OH)D3 on immunity
In recent years, a great deal of research has been conducted to investigate the immunoregulatory functions of Vit D. This section aimed to discuss the efficacy of Vit D3 and 25(OH)D3 to promote immunity in pigs (Table 2).
Table 2. Overview of the comparative studies between vitamin D3 (Vit D3) and 25(OH)D3, demonstrating the study designs, dietary doses, underlying conditions and significant outputs of the experiments corresponding to immunity. Only the dietary doses (control and treatment) and the conditions that led to noticeable outcomes are listed

aET, explained on text; bBA, bronchoalveolar; cPW, post-weaning; dC3, complement component 3; eIL-6, interleukin 6; fIgG, immunoglobulin G; gIgA, immunoglobulin A; hIgM, immunoglobulin M; iCAT, catalase; jSCFA, short-chain fatty acids; k ACCA, acetyl-CoA carboxylase α; l FAS, fatty-acid synthase; mTLC, total leucocyte count; nGLC, granulocytes; oLPC, lymphocytes; pTPLC, total phagocytic leucocytes; qPLC, phagocytic leucocytes; rTMNC, total monocytes; sTBLC, total bronchoalveolar leucocytes; tIL-1, Interleukin 1.
* Asterisk indicates significant outcomes (P < 0·05).
† DTP, dietary treatment period.
‡ 1 µg = 40 μg Vit D3.
In the study of Zhang et al. (Reference Zhang, Yang and Piao10), 25(OH)D3 was more effective than Vit D3 in fortifying the body’s immune system when receiving a low Ca-P diet. In contrast to NC, dietary supplementation of 25(OH)D3 (NC + 25D) reduced the incidence of streptococcal infections by significantly increasing the serum concentrations of immunoglobulin A (IgA) and G (IgG) in piglets at weaning. Since these two types of immunoglobulins (Igs) are involved in inhibiting streptococcal and other bacterial infections(Reference Janeway, Travers and Walport34,Reference Norrby-Teglund, Kaul and Low35) . Furthermore, in pigs receiving the 25(OH)D3 supplement, the serum concentration of IgM was enhanced at day 28, while one of the complement components (C3) was reduced at day 14. Compared to NC, diets containing 25(OH)D3 led to a significant rise in the serum concentration of catalase (CAT) at day 28, and this might be taken as a hint toward an improved antioxidant capacity. CAT is regarded as a first-line defence antioxidant enzyme that is fundamental and vital to the overall defensive mechanisms in biological systems(Reference Ighodaro and Akinloye36). However, NC and NC + 25D demonstrated similar effects on the serum status of other oxidative enzymes like super oxidase dismutase, total antioxidant capacity (T-AOC) and glutathione peroxidase (GSH-Px). In contrast to Vit D3, the intestinal concentration of SCFA significantly increased following the dietary intake of NC + 25D. For example, the abundance of Lachnospiraceae, which plays a crucial role in maintaining gut health, increased. The principal function of Lachnospiraceae is to break down the complex polysaccharide into SCFA, including acetate, butyrate and propionate(Reference Martin-Gallausiaux, Marinelli and Blottière37). SCFA are involved in boosting immunity and reducing inflammatory reactions in the gut and other organs by, i.e. acetyl-CoA synthesis, suppression of histone deacetylase, signalling via G protein-coupled receptors and metabolic integration(Reference Kim38). Thus, dietary inclusion of 25(OH)D3 resulted in a significant improvement in humoral as well as innate immunity and gut immunity of the weaned piglets.
In another study, Zhang et al. (Reference Zhang, Li and Shang30) compared the immune competence of sows supplemented with Vit D3 and 25(OH)D3. Sows fed either source of Vit D demonstrated a similar effect on the level of Igs (IgA, IgG and IgM) in colostrum. However, diet containing 25(OH)D3 significantly increased IgG levels in milk on day 21 of lactation. The expression of genes concerning fatty acid metabolisms, i.g., ACCa (Acetyl-CoA carboxylase α) and FAS (fatty-acid synthase) increased considerably in the mammary gland in response to 25(OH)D3 enriched diets. Lipid metabolism is directly involved in the regulation of the immune system through the activation of M1 and M2 macrophages(Reference Batista-Gonzalez, Vidal and Criollo39). The piglets from 25(OH)D3-fed sows demonstrated a significant elevation in the concentration of butyrate in the cecal digesta compared with piglets from sows fed Vit D3. The elevated concentration of butyrate following the breakdown of dietary fibres indicates improvement in bacterial metabolism(Reference Louis, Scott and Duncan40,Reference Lallès, Michel, Theodorou and Rosenfeld41) which plays a crucial role in adaptive immune response via two specific pathways: first, it acts directly on monocyte-derived dendritic cells(Reference Nastasi, Candela and Bonefeld42–Reference Nastasi, Fredholm and Willerslev-Olsen44) and second through its action on T lymphocytes. So, in contrast to Vit D3, the dietary inclusion of 25(OH)D3 during lactation period led to a significant increase in the concentration of milk IgG; and butyrate concentration in the cecal digesta of suckling piglets due to improved bacterial metabolism in the gut.
Konowalchuk et al. (Reference Konowalchuk, Rieger and Kiemele15) compared Vit D3 and 25(OH)D3 to evaluate the immune capacity by offering three different dietary treatments to 149 piglets weighing 5–7 kg for 14 or 21 d. The dietary supplements contained a baseline diet mixed with Vit D3 (NC, 37·5 µg/kg), which served as a control diet. The second regimen included the control diet mixed with an additional 50 µg/kg of Vit D3 (PC, NC + 50 µg/kg Vit D3). The third one consisted of the control diet mixed with 25(OH)D3 (HyD, NC + 50 µg/kg 25(OH)D3). Leucocytes are involved in both innate and humoral immune responses and play a critical role in fighting against infections and defence against foreign elements(Reference Tigner, Ibrahim and Murray45). The authors observed that supplementation of 25(OH)D3 resulted in a significant increase in total blood leucocyte (granulocytes, lymphocytes) counts in piglets, accompanied by a parallel increase in serum 25(OH)D3. Compared with NC, piglets fed HyD demonstrated a significant improvement in the viability of total blood leukocytes and monocytes. The HyD-fed group also showed a significant increase in the viability of bronchoalveolar leukocytes compared with PC. However, unlike Vit D3, 25(OH)D3 did not only increase the number and viability of leucocytes but also significantly improved their phagocytic capacity. Overall, dietary supplementation of 25(OH)D3 compared with Vit D3 showed a significant positive influence on systemic blood and peripheral bronchoalveolar mucosal compartments, resulting in an increase in leukocyte count as well as the survival and phagocytic ability of the discrete leucocyte populations.
In the study of Upadhaya et al. (Reference Upadhaya, Chung and Jung20), considerably lower serum levels of interleukin-6 (IL-6) at day 42 and higher serum levels of interleukin-1 (IL-1) at day 140 were observed in growing pigs supplemented with 25(OH)D3 compared with Vit D3. IL-6 is an anti-inflammatory cytokine that contributes to host defence via stimulation of the acute phase response, haematopoiesis and immune responses(Reference Tanaka, Narazaki and Kishimoto46). IL-1 is a strong inflammatory cytokine involved in a wide range of immunological responses. It is predominantly produced by macrophages during defensive reactions to protect the body from infection and disease through inflammation and innate and adaptive immune responses(Reference Fields, Günther and Sundberg47). The authors were unable to explain the underlying reason for this up- or down-regulation of the pro- and anti-inflammatory cytokines. According to Tanaka et al., the fluctuation of inflammatory cytokines is due to the maintenance of immune homoeostasis(Reference Alhassan Mohammed, Mirshafiey and Vahedi48). In conclusion, the supplementation of 25(OH)D3 exhibited a more positive impact than Vit D3 on pig health.
Meuter et al. (Reference Meuter, Thoby and Lichou49) compared two different dietary sources of Vit D to observe their effects on humoral immunity by measuring the serum concentration of IgG and lysozyme. For this purpose, 160 post-weaned piglets (weaned at 21 days of age) were divided into two groups matched for maternal origin and body weight. Each group included sixteen pens with five animals each and received the same diet, differing only in the source and doses of Vit D used. The piglets received either Vit D3 (50 µg/kg) or 25(OH)D3 (50 µg/kg) for 48 d. The serum concentration of 25(OH)D3 increased considerably after dietary supplementation of 25(OH)D3 compared to Vit D3. The increased concentration of 25(OH)D3 was associated with the positive modulation of parameters related to the humoral immune systems. In contrast to Vit D3, dietary supplementation of 25(OH)D3 led to a significant increase in the serum concentration of IgG and lysozyme. So, unlike Vit D3, the dietary supplementation of 25(OH)D3 significantly improved humoral immunity and strengthened the immune response of the piglets without compromising their health.
Thus, unlike Vit D3, the dietary intake of 25(OH)D3 significantly improves immune status of the body. In particular, the improvement of humoral immunity is reflected in the increased concentration of serum immunoglobulins and phagocytic capacity of the macrophages in piglets, which allows the animals to respond more effectively to potential health challenges. 25(OH)D3 also positively affects the serum concentration of inflammatory cytokines to maintain immune homoeostasis. Moreover, 25(OH)D3 is superior over Vit D3 in modulating the systemic and mucosal antimicrobial responses, suggested by increased numbers of leucocytes and their survival and phagocytic ability in blood and bronchoalveolar compartments. Compared to Vit D3, the significant expression of genes related to fatty acid metabolism due to dietary intake of 25(OH)D3 is also noteworthy. Fatty acids positively affect immune cell functions through a variety of complex mechanisms by improving phagocytosis, T-cell signalling and antigen presentation capability(Reference Calder50). In contrast to Vit D3, the role of 25(OH)D3 in improving gut immunity is also noteworthy. Dietary supplementation of 25(OH)D3 alters the gut microbiota and thus might promote specific metabolic processes, which plays a crucial role in maintaining intestinal health(Reference Belkaid and Hand51).
Comparative performance of vitamin D3 and 25(OH)D3 on bone development
Vit D regulates bone and mineral metabolism. This section reviews the available pig studies highlighting the efficacy of Vit D3 and 25(OH)D3 on mineral status and bone development (Table 3).
Table 3. Overview of the comparative studies between vitamin D3 (Vit D3) and 25(OH)D3, demonstrating the study designs, dietary doses, underlying conditions and significant outputs of the experiments corresponding to skeletal development. Only the dietary doses (control and treatment) and the conditions that led to noticeable outcomes are listed

aET, explained on text; bIN, insemination; cPI, post-insemination; dCa, calcium; eBALP, bone-specific alkaline phosphatase; fOC, osteocalcin.
* Asterisk indicates significant outcomes (P < 0·05).
† DTP, dietary treatment period.
‡ 1 µg = 40 μg Vit D3.
Zhang et al. (Reference Zhang, Yang and Piao10) compared the efficacy of Vit D3 and 25(OH)D3 in improving serum mineral status. In contrast to Vit D3 (NC), the dietary inclusion of 25(OH)D3 (NC + 25D) significantly normalised the serum levels of Ca, bone-specific alkaline phosphatase and osteocalcin (OC) in low Ca-P fed pigs depending on the duration of the dietary supplementation. The increased serum concentration of Ca indicates the compensatory efforts of 25(OH)D3 to maintain Ca-P homoeostasis in pigs fed with low Ca-P diets. Serum level of bone-specific alkaline phosphatase (synthesised by osteoblasts) positively correlates with bone formation(Reference Konukoglu52). OC is also considered as a serum marker of osteoblastic bone formation which acts in the bone matrix to regulate bone mineralisation via resorption(Reference Zoch, Clemens and Riddle53). Unlike OC, the supplementation of piglets with both forms of Vit D exhibited a similar impact on the serum status of bone resorption markers like tartrate-resistant acid phosphatase (TRAP) and pyridinoline (PYD). TRAPs perform bone resorption by catalysing the hydrolysis of various phosphates and anhydrides in an acidic environment,(Reference Oddie, Schenk and Angel54) and PYD is synthesised by the reaction at the side chain of the collagen residues during trimerisation in skeletal development(Reference Kline, Orton, Sadrzadeh, Sadrzadeh and Kline55). Overall, 25(OH)D3-enriched diets proved to be more effective in improving bone integrity in low Ca-P-fed pigs compared with Vit D3, as indicated by increased serum bone formation biomarkers to balance bone mineralisation and its homoeostasis.
Similar to the study of Zhang et al. (Reference Zhang, Yang and Piao10), Zhao et al. (Reference Zhao, Wen and Xiao31) also demonstrated the superiority of dietary 25(OH)D3 over Vit D3 in restoring bone mineral status in pigs supplemented with low Ca-P. Unlike Vit D3 (NC), the serum level of Ca increased significantly in piglets (10–20 kg) after dietary ingestion of 25(OH)D3 (HyD). Serum Ca needs to be regulated in narrow physiological ranges and serves as an important marker of bone turnover and osteoblast functions(Reference Kuo and Chen56,Reference Åkesson, Lau and Johnston57) . Its elevation in piglets fed low Ca-P indicates the body’s compensatory effort to balance the level of Ca and P to achieve optimal mineralisation. However, no major dietary effect on the serum status of Ca was observed in 6–10 kg piglets. Dietary supplementation of Vit D had also no discernible effect on the serum levels of P. This could be due to the body’s ability to maintain serum P levels in pigs fed low P(Reference Hasan, Oster and Reyer3,Reference Gerlinger, Oster and Reyer58) . Thus, the supplementation of dietary 25(OH)D3 led to a significant impact on increasing the serum Ca to improve the mineral homeostasis in pigs receiving low Ca-P diet.
Doherty et al. (Reference O’Doherty, Gahan and O’Shea59) performed a similar experiment, but with a different dosage of 25(OH)D3 compared with the aforementioned studies. For the mineral balance study, twenty-four finishing boars (13 weeks of age) with an initial live weight of 42 kg were used and fed the following diets containing low P (T1, 50 µg/kg Vit D3), T1 + Phytase (T2, 50 µg/kg Vit D3 + 750 μg/kg phytase), T1 + 25(OH)D3 (T3, 25 µg/kg Vit D3 + 25 µg/kg 25(OH)D3) or T1 + Phytase + 25(OH)D3 (T4, 25 µg/kg Vit D3 + 750 μg/kg Phytase + 25 µg/kg 25(OH)D3) for 66 d. Accordingly, in contrast to Vit D3 (T1), dietary inclusion of 25(OH)D3 (T3) significantly improved Ca digestibility, and retention in low Ca-P fed pigs, suggesting the same remedial endeavour as reported in the above studies to balance mineral homeostasis. Ca digestibility and P retention are closely associated with bone mineralisation and growth performance(Reference Lautrou, Narcy and Dourmad60). However, the dietary intervention did not affect P metabolism, serum mineral (Ca, P) status, bone ash and bone strength.
Weber et al. (Reference Weber, Witschi and Wenk19) compared the effectiveness of Vit D3 and 25(OH)D3 to determine the plasma mineral status following the supplementation of sows with basal diets containing Vit D3 and 25(OH)D3. Dietary intake of Vit D3 and 25(OH)D3 did not consistently affect the plasma status of Ca, P and OC associated with skeletal development and resorption throughout the reproduction cycle. However, the concentration of CrossLaps (β-CTx) increased significantly before parturition in both treatments and was the highest at the end of lactation. This observation suggests the mobilisation of Ca from the sow’s skeleton to the developing foetuses and piglets for their optimum bone development(Reference Bonde, Qvist and Fledelius61). Thus, in healthy pigs, Vit D3 and 25(OH)D3 resulted in similar impacts on the concentrations of bone mineralisation markers.
In the study of Witschi et al. (Reference Witschi, Liesegang and Gebert29), thirty-nine sows (thirteen in each treatment) were assigned to Vit D3 and 25(OH)D3 supplementation from the day of mating until day 21 of lactation. Rosenberg et al. (Reference von Rosenberg, Weber and Erhardt62) used six-week-old piglets (n 40) supplemented with Vit D3 (control, 50 µg/kg) and 25(OH)D3 (50 or 250 or 500 µg/kg) for 42 d. Witschi et al. and Rosenberg et al. reported no significant difference in ash%, weight, length, mineral content and mineral density of bone in response to the dietary intakes of Vit D3 and 25(OH)D3. However, low dietary intake of Vit D3 (200 μg) was associated with reduced bone-breaking strength, cortical bone mineral content and density at the tibial midshaft of the piglets(Reference Witschi, Liesegang and Gebert29). Thus, both forms of Vit D implied a similar effect on bone formation in healthy pigs.
In summary, 25(OH)D3 outperforms Vit D3 to improve the serum mineral status associated with bone mineralisation in pigs fed low Ca-P diet. Unlike Vit D3, the serum concentrations of Ca, bone-specific alkaline phosphatase and OC increase significantly in pigs receiving basal diets containing 25(OH)D3 and low Ca-P. Therefore, the upregulation of serum markers associated with bone turnover indicates a noticeable positive influence of 25(OH)D3 to balance mineral homeostasis in pigs experiencing Ca-P deficiency. The comparative efficacy of Vit D3 and 25(OH)D3 on mineralisation in healthy pigs appears to be similar.
Implications for further research
Vitamin D metabolism is attributed to endogenous adaptive mechanisms for maintaining mineral homeostasis, which is critical for animal health. In particular, potential long-term consequences due to nutritional strategies in the early life stages need to be addressed as summarised in the concept of nutritional programming, thereby inducing resource-efficient phenotypes. However, evaluation must also consider a range of effects in peripheral tissues to account for unintended metabolic effects (Fig. 1). With regard to alternative housing conditions of pigs, such as exposure to natural sunlight, dietary Vit D requirements and the physiological synergy with endogenous syntheses need to be investigated. Identifying the complex genetic architecture of the Vit D system is key to improving mineral efficiency and animal health aspects as a basis for developing new breeding criteria.
Conclusions
In sows, dietary supplementation of Vit D3 and 25(OH)D3 results in similar reproductive outcomes. The growth performance of piglets and sows fed 25(OH)D3 is significantly better than those fed Vit D3. The superiority of 25(OH)D3 over Vit D3 is evident in enhancing innate and humoural immunity. Improved bone mineralisation in pigs supplemented with 25(OH)D3 on diets low in Ca and phosphorus provides an option to balance animal welfare and resource efficiency. Thus, 25(OH)D3 is a promising and potential alternative to Vit D3 for promoting growth, immunity and bone development in the pigs.
Acknowledgement
None
This research received funding from the Leibniz ScienceCampus Phosphorus Research Rostock, Germany. This study was further funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under project number 503753038. The funders had no role in the design, analysis or writing of this article. The project received additional funding from the FBN, in particular from the FBN Open Access Fund.
Conceptualisation: M. H., M. O., H. R., K. W. and D-C. F.; systematic search and articles selection: M. H.; first draft: M. H.; revision: M. H., M. O., H. R., K. W. and D-C. F.
The authors declare no conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523000442