Maintaining good nutritional status among HIV-infected children is clearly of paramount importance( Reference Rose, Hall and Martinez-Alier 1 ). WHO guidelines for incorporating nutritional support into care programmes for HIV-infected children are available for local adaptation( 2 ) and nutritional support services appear to be generally available at HIV care and treatment sites in sub-Saharan Africa( Reference Anema, Zhang and Wu 3 ). However, the evidence base for the effectiveness of specific nutritional interventions in HIV-infected children is weak( Reference Irlam, Siegfried and Visser 4 ) and nutritional interventions impose additional costs on HIV care programmes( Reference Cobb and Bland 5 ).
These costs could be reduced by developing locally produced food-based supplements, which are also likely to be more palatable and culturally acceptable. Leaf concentrate is a food-based nutritional supplement( Reference Davys, Richardier and Kennedy 6 ) that has been shown to be effective at preventing anaemia in adolescent girls( Reference Vyas, Collin and Bertin 7 ) and pregnant women( Reference Magon, Collin and Joshi 8 ) in India. Although anaemia may actually be less prevalent among HIV-infected than among uninfected children( Reference Esan, Jonker and Hensbroek 9 ), it remains an important co-morbidity and, together with other nutritional deficiencies and diarrhoeal disease, is a strong predictor of poor outcomes among children after initiation of antiretroviral therapy (ART)( Reference Auld, Tuho and Ekra 10 – Reference Zanoni, Phungula and Zanoni 12 ).
Leaf concentrate was discovered in France in the 18th century and was developed as a foodstuff in England between 1940 and 1970( Reference Pirie 13 ). It has since been promoted by several non-governmental organizations, including Leaf for Life in the USA( Reference Kennedy 14 ) and the Association pour la Promotion des Extraits Foliaires en Nutrition (APEF) in France( 15 ), as a sustainable form of protein and micronutrient supplementation for low-income communities( Reference Davys, Richardier and Kennedy 6 ). In the present study, we investigated the potential benefits of dried lucerne (alfalfa) leaf concentrate as a nutritional supplement for HIV-infected ART-naïve children living in two cities in Burundi. The aim of the study was to determine the acceptability and palatability of leaf concentrate powder (LCP) and to measure its effectiveness (compared with skimmed milk powder (SMP)) in relation to HIV-specific (viral load, CD4+ count) and general (haematological, anthropometric) parameters and overall health.
Methods
Study population and recruitment
Our target population was antiretroviral-naïve HIV-positive children aged 5–14 years. Children were recruited from two clinical centres (in Bujumbura and Kirundo) run by the Association Nationale de Soutien aux Séropositifs et malades du Sida (ANSS; National Association of Support for People Living with HIV and AIDS). Founded in 1993, ANSS’ mission is to promote the prevention of HIV transmission and to improve the well-being of people living with and affected by HIV. The ANSS was the first Burundian civil society organization to provide HIV services to people living with HIV, including the distribution of ART. At the end of 2013, the ANSS was providing treatment to more than 6000 people in Burundi. From 2007 to 2013, the Association provided HIV counselling and testing to more than 56000 people as part of its HIV prevention efforts. Recruitment was conducted by medical staff at the two centres, who identified eligible children at routine clinic appointments to which HIV-positive children are invited for medical and psychosocial monitoring purposes. The same staff explained the purpose of the study to children and their parent(s)/guardian(s), who were provided with a study information sheet and consent form in the local language (Kirundi).
Ethical approval, trial registration and consent
Ethical approval for the study was granted on 16 June 2012 by the ‘Comité National d’Ethique pour la protection des êtres humains participants à la recherche biomédicale et comportementale’ of Burundi. Written informed consent was obtained from the parent(s)/guardian(s) of eligible children, with original consent forms being held by ANSS.
Inclusion criteria
Children were eligible if they were ≥5 and ≤14 years old, had tested positive for HIV-1 (HIV 1/2 STAT-PAK® DIPSTICK Assay; Chembio Diagnostic Systems, Inc., Medford, NY, USA) and had a CD4+ cell count of ≥350 cells/mm3 ( 16 ). Children with a CD4+ cell count of <500 cells/mm3 were required to receive prophylactic sulfamethoxazole/trimethoprim( 2 ). All children were dewormed before the study began using an appropriate dose of albendazole.
Exclusion criteria
Children were ineligible if they had a major concurrent illness (e.g. cancer, tuberculosis or diabetes), a BMI of <12 kg/m2, were liable to move out of the study area or were unlikely to be able to receive the weekly supplements, or had participated in a clinical trial in the preceding three months.
Primary and secondary outcomes
Primary outcomes were percentage of CD4+ T lymphocytes, HIV viral load and Hb measured after 6 months of follow-up. Secondary outcomes (also measured at 6 months) included: anthropometric indices (height-for-age, weight-for-age, handgrip strength); episodes of illness (diarrhoea, respiratory); overall health (anaemia, inflammation (C-reactive protein (CRP)), fatigue, appetite); and palatability of the supplements (appearance, texture, taste).
Sample size
We aimed to recruit forty-two children into each arm of the trial. This sample size was calculated to yield 90 % power at a 5 % level of significance to detect: (i) equivalence between LCP and SMP in percentage of CD4+ T lymphocytes at 6 months, by excluding a difference in means of 5 % (assuming that the sd in the outcome=7 %); (ii) equivalence between LCP and SMP in log10 HIV viral load, by excluding a difference in means of 0·5 (assuming sd=0·7 and 15 % of participants lost to follow-up); and (iii) superiority of LCP over SMP in increasing mean Hb from 115 (sd 14) g/l to 125 (sd 14) g/l, compared with no change in the SMP arm (because LCP provides 5·4 mg Fe compared with 0·1 mg Fe in SMP).
Randomization, allocation and adherence
Eligible children with consent to participate were allocated to LCP or SMP using a computer-generated random number sequence and consecutively numbered sealed envelopes. Envelopes were held and opened by a member of the ANSS administrative staff not otherwise involved in the study, i.e. allocation was concealed from the ANSS medical staff who were running the trial. Delivery of supplements to all participants was made by ANSS health workers at an interval of 2 weeks and was recorded for each child. Observance of supplement consumption was monitored and discussed collectively (with all children and their parents) at each monthly monitoring visit in order to highlight difficulties and elaborate solutions. For the few parents who were not present at a supplement delivery time or reported difficulties in supplement consumption by their child, ANSS health workers visited them at home to see how supplements were consumed.
Leaf concentrate powder
The LCP group received 10 g of lucerne (Medicago sativa) leaf concentrate powder (France-Luzerne Agricultural Co-operative, Aulnay-aux-Planches, France). The LCP was contained in sachets and could be taken orally by mixing with water or tea. The composition of the LCP is shown in Table 1.
Table 1 Composition of leaf concentrate powder (LCP) and skimmed milk powder (SMP)
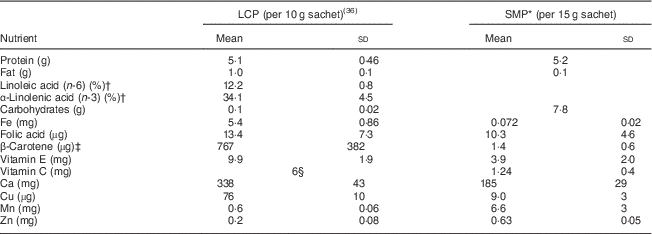
* From French Agency for Food, Environmental and Occupational Health and Safety (https://pro.anses.fr/TableCIQUAL/, accessed July 2015).
† Percentage of fat content.
‡ Retinol activity equivalents (RAE).
§ Corresponding to ascorbic acid added during manufacturing to prevent oxidation.
Skimmed milk powder
The SMP group received 15 g of skimmed milk powder (Société Industrielle Laitière du Léon, Plouvien, France). The SMP was given in a sachet which could be taken orally by mixing with water or tea. The composition of the SMP is shown in Table 1.
HIV-specific parameters and non-specific blood parameters
HIV-1 viral load, absolute count and percentage of CD4+ T lymphocytes, and general blood parameters (Hb, white blood cell count, lymphocytes, platelets and CRP) were measured at baseline, 3 and 6 months in blood samples which were collected at ANSS clinics and transferred to the National Institute of Public Health (INSP), Bujumbura and to a laboratory at the New Hope Centre, Bujumbura. HIV viral load was measured at INSP using real-time PCR (Abbott RealTime HIV-1 assay; Abbott Laboratories, Abbott Park, IL, USA). CD4+ absolute count and percentage were measured at the New Hope Centre using a BD FACSCount™ system (BD Biosciences, San Jose, CA, USA). Non-specific blood parameters were measured at INSP. Anaemia was defined as Hb <115 g/l, CRP positivity as CRP ≥6 mg/l.
Anthropometric parameters
Weight and height were measured using electronic scales and standing measurement boards during clinic visits at baseline, 3 and 6 months. Height-for-age and weight-for-age Z-scores were estimated against British 1990 (UK90) growth charts( Reference Cole, Freeman and Preece 17 , Reference Cole, Williams and Wright 18 ). The UK90 BMI reference provides centile curves for children from birth to 23 years, based on a sample of 32222 measurements from twelve distinct surveys in British children collected between 1978 and 1994.
Handgrip strength was measured, using a Jamar® hydraulic hand dynamometer (Patterson Medical, Nottinghamshire, UK), by two successive trials on the dominant side with the elbow at 90°, the maximal score of the two trials being recorded.
Diarrhoea, respiratory infection, appetite, fatigue and overall health levels
Diarrhoea, respiratory infection and other illnesses were recorded by ANSS clinicians monthly and during clinic visits at baseline, 3 and 6 months. Disease occurrences and corresponding treatments were recorded for each child in order to detect the medical events during each three-month period (0–3 months, 3–6 months). Appetite, fatigue and overall health were assessed by questioning parents/carers at baseline, 3 and 6 months, with the following possible responses: fatigue (none, moderate, severe); appetite (weak, satisfactory, good, very good); overall health (very poor, poor, satisfactory, good, excellent).
Palatability
Three aspects of palatability (appearance, texture and taste) were recorded by ANSS health workers during clinic visits at 3 and 6 months, with the following possible responses: appearance (unappealing, acceptable, appealing, very appealing); texture (unappealing, acceptable, appealing, very appealing); taste (very bad, bad, acceptable, good, very good).
Statistical analyses
Data were entered into a computer using Microsoft® Excel and were analysed using the statistical software package Stata release 13. Between-group differences in continuous outcome measures were estimated using linear regression models. Between-group differences in binary outcome measures were tested using Fisher’s exact tests. We fitted linear regression models using generalized estimating equations for each continuous outcome measure, including an interaction term between study arm and time point (0, 3 or 6 months) to estimate within- and between-group changes at each time point. We used an exchangeable within-child correlation structure (which assumes that the observations within a subject are equally correlated) and we verified by inspection that other correlation structures (ar1 and ar2) did not produce different results.
Results
Characteristics of trial participants
The median age of the participants was 10 years (range 5–14 years) and 39·8 % (33/83) were male (Table 2). Forty-two children were randomized to receive SMP and forty-one to LCP (Fig. 1).

Fig. 1 CONSORT (Consolidated Standards of Reporting Trials) flow diagram (ART, antiretroviral therapy; SMP, skimmed milk powder; LCP, leaf concentrate powder)
Table 2 Characteristics of participants in each arm at the beginning of the study (baseline); HIV-positive antiretroviral-naïve children aged 5–14 years, Bujumbura and Kirundo, Burundi

SMP, skimmed milk powder; LCP, leaf concentrate powder; IQR, interquartile range; CRP, C-reactive protein.
Loss to follow-up and adherence
One child in the SMP arm and two children in the LCP arm did not receive their allocated intervention and dropped out at the beginning of the trial (Fig. 1). One child in the LCP arm dropped out just after the 3-month follow-up. Three children in each arm had a CD4+ count of <350 cells/mm3 measured at the 3-month follow-up and proceeded to receive ART. At 6 months, primary and secondary outcome measures were available for 90·5 % (38/42) and 85·4 % (35/41) of participants in the SMP and LCP groups, respectively. The final analysis was performed on the thirty-eight SMP and thirty-five LCP children who had data on one or more outcome measures at the 6-month follow-up.
Blood parameters
The 95 % CI of the between-group differences in percentage of CD4+ T lymphocytes and HIV-1 viral load at 6 months suggested non-equivalence between SMP and LCP (Table 3). For CD4+ percentage, equivalence had been defined as a difference not greater than 5 %, but the 95 % CI for the between-group difference in this parameter at 6 months was −1·17 %, 6·07 %; i.e. the upper bound exceeded 5 % (in favour of LCP). Similarly, equivalence for HIV viral load had been defined as a difference not greater than 0·5 logs, but the 95 % CI of the observed difference was −0·19, 0·67; i.e. the upper bound exceeded 0·5 (in favour of SMP). However, the 95 % CI for the baseline values of these two HIV-related measures were also consistent with non-equivalence (suggesting the role of chance). Adjusted for baseline, the between-group differences at 6 months for CD4+ percentage and HIV viral load were 0·59 (95 % CI −1·42, 2·61) % and 0·18 (95 % CI −0·08, 0·44), respectively. These differences and their 95 % CI are consistent with equivalence between SMP and LCP.
Table 3 Percentage of CD4+ T lymphocytes, HIV-1 viral load and Hb at baseline, 3 and 6 months among HIV-positive antiretroviral-naïve children aged 5–14 years, Bujumbura and Kirundo, Burundi

SMP, skimmed milk powder; LCP, leaf concentrate powder; CRP, C-reactive protein.
* The between-group difference corresponding to the ‘0–6 month change’ shows the estimated additional effect of LCP compared with SMP, i.e. the interaction term between the intervention and 6-month time point.
† Adjusted for age, sex, all blood parameters, CRP positivity and height-for-age Z-score.
The estimated additional effects of LCP (compared with SMP) on the changes in CD4+ percentage and HIV viral load over 6 months indicate no additional effect of LCP: for CD4+ percentage, 0·97 (95 % CI −0·86 2·80) %, P=0·30; for log10 HIV viral load, 0·20 (95 % CI −0·03, 0·43), P=0·09. LCP was not superior to SMP in improving Hb, with similar increases over the 6 months in both arms, and no additional effect of LCP compared with SMP (−1·71 (95 % CI −6·49, 3·07) g/l, P=0·48). LCP did not have any additional effect over SMP on 0–6 month changes in any of the other blood parameters (see online supplementary material, Supplemental Table 1).
We found only slight changes in the interaction (adjusted 0–6 month change) estimates and their 95 % CI when we used ar1 and ar2 autoregression correlation structures, instead of an exchangeable (exch) structure: percentage CD4+ T lymphocytes, exch 0·97 (95 % CI −0·86, 2·80) %, ar1 0·98 (95 % CI −0·93, 2·88) %, ar2 0·90 (95 % CI −1·03, 2·83) %; log10 HIV-1 viral load, exch 0·20 (95 % CI −0·03, 0·43), ar1 0·19 (95 % CI −0·04, 0·43), ar2 0·23 (95 % CI −0·01, 0·46); Hb exch −1·71 (95 % CI −6·49, 3·07) g/l, ar1 −2·14 (95 % CI −7·20, 2·91) g/l, ar2 −1·51 (95 % CI −6·47, 3·45) g/l.
Anthropometric parameters
Height-for-age and weight-for-age Z-scores increased to the same degree in both groups of children over the 6 months and there was no additional effect of LCP over SMP (Table 4). There were no between-group differences in median handgrip strength scores at 3 or 6 months, but children in the LCP group increased their (geometric mean) handgrip strength score 2·4-fold over the 6 months, compared with no change in the SMP group (Table 4).
Table 4 Anthropometric parameters at baseline, 3 and 6 months among HIV-positive antiretroviral-naïve children aged 5–14 years, Bujumbura and Kirundo, Burundi

SMP, skimmed milk powder; LCP, leaf concentrate powder; CRP, C-reactive protein.
* The between-group difference corresponding to the ‘0–6 month change’ shows the estimated additional effect of LCP compared with SMP, i.e. the interaction term between the intervention and 6-month time point.
† Adjusted for age, sex and all blood parameters.
‡ Values shown for baseline, 3- and 6-month levels of handgrip strength are geometric mean (95 % CI). Between-group difference and 0–6 month changes are ratios of geometric means from linear regression of log-transformed handgrip strength values, e.g. coefficient=1·10 indicates that strength is 10 % higher in LCP group compared with SMP group, coefficient=0·90 indicates that strength is 10 % lower in LCP group compared with SMP group, coefficient=1·00 indicates no difference/change.
Diarrhoea, respiratory infection and other illnesses
There were few reported cases of diarrhoea during the study in either group: in the LCP group, four cases at baseline, then one case during 0–3 months and one case during 3–6 months of follow-up; in the SMP group, three cases at baseline, no cases during 0–3 or 3–6 months of follow-up. Pneumonia was reported to be present only once in each group; in both cases, during 3–6 months of follow-up. Ear/nose/throat infections (tonsillitis, ear infection) occurred in two LCP group children and in five SMP group children. Malaria was diagnosed during an acute clinic access by two children of the LCP group and three children of the SMP group.
Anaemia, inflammation, fatigue, appetite and overall health
Overall prevalence of anaemia (Hb <115 g/l) fell from 39·7 % (29/73; 95 % CI 28·5, 51·9 %) at baseline to 24·7 % (18/73; 95 % CI 15·3, 36·1 %) at 3 months and 20·6 % (15/73; 95 % CI 12·0, 31·6 %) at 6 months, with similar prevalence in both groups at each time point. CRP positivity fell from 23·3 % (17/73; 95 % CI 14·2, 34·6 %) at baseline to 15·1 % (11/73; 95 % CI 7·8, 25·4 %) and 9·6 % (7/73; 95 % CI 3·9, 18·8 %) at 3 and 6 months, respectively, again with no detectable between-group differences (see online supplementary material, Supplemental Table 2). Appetite and overall health appeared to be better at 6 months in the LCP group, although there were no differences if the responses ‘very good’ and ‘good’ to the appetite question were merged (92·1 % (35/38; 95 % CI 78·6, 98·3 %) in the SMP group compared with 97·1 % (34/35; 95 % CI 85·1, 99·9 %) in the LCP group, P=1·00) and if the ‘excellent’ and ‘good’ overall health responses were merged (73·7 % (28/38; 95 % CI 56·9, 86·6 %) in the SMP group compared with 85·7 % (30/35; 95 % CI 69·7, 95·2 %) in the LCP group, P=0·31).
Palatability
The appearance, texture and taste of SMP were rated more favourably than for LCP. All (38/38) participants in the SMP arm rated appearance and texture as appealing/very appealing at 3 and 6 months, and 97·4 % (37/38; 95 % CI 86·2, 99·9 %) and 97·3 % (36/37; 95 % CI 85·5, 99·9 %) rated taste as good/very good at 3 and 6 months, respectively (see online supplementary material, Supplemental Table 3). In contrast, 80·0 % (28/35; 95 % CI 63·1, 91·6 %) and 82·9 % (29/35; 95 % CI 66·4, 93·4 %) of participants in the LCP arm rated appearance and texture as appealing/very appealing, and 77·1 % (27/35; 95 % CI 59·9, 89·6 %) rated taste as good/very good at 3 months. At 6 months, 85·7 % (30/35; 95 % CI 69·7, 95·2 %) and 80·0 % (28/35; 95 % CI 63·1, 91·6 %) rated the appearance and texture of LCP as appealing/very appealing, and 68·6 % (24/35; 95 % CI 50·7, 83·1 %) the taste as good/very good. There were fewer negative ratings of the palatability of LCP at 6 months compared with 3 months, but with insufficient numbers to provide statistical evidence of change: unappealing appearance 11·4 % (4/35; 95 % CI 3·2, 26·7 %) to 0·0 % (0/35); unappealing texture 5·7 % (2/35; 95 % CI 0·7, 19·2 %) to 2·9 % (1/35; 95 % CI 0·1, 14·9 %); and bad taste 17·1 % (6/35; 95 % CI 6·6, 33·6 %) to 11·4 % (4/35; 95 % CI 3·2, 26·7 %).
Discussion
During 6 months of follow-up in this population of HIV-positive ART-naïve children, LCP was equivalent to SMP, after adjustment for baseline values, in relation to HIV-specific blood parameters (which showed little or no change in either group) and did not demonstrate superiority over SMP in relation to Hb levels (which increased in both groups). Higher levels of appetite and overall health were reported for children in the LCP group, and they also had higher handgrip strength scores. There were no differences in any of the other outcome measures. In terms of palatability, LCP was less acceptable than SMP to the children in this population, but with few negative ratings of appearance, texture and taste.
Our study was powered to detect equivalence in relation to HIV-specific blood parameters (CD4+ percentage and HIV viral load) and superiority in relation to Hb levels (because of the higher Fe content of LCP). Differences between the two groups at baseline (which could only have arisen by chance) exceeded the thresholds for equivalence, suggesting that a wider a priori equivalence interval was required. However, the fully adjusted analysis using longitudinal linear regression models gave estimates which were consistent with equivalence in the primary outcome measures. No differences were detected in any of the other outcome measures, although assessment of ‘appetite’, ‘overall health’ and handgrip strength at 6 months appeared to favour LCP. Several participants were lost to follow-up from each arm of the study, which would have reduced the power of the study, and the study was not sized to detect pre-specified differences in the secondary outcome measures. Allocation to the supplementation arms was concealed, but children and their parents/carers and field workers were not blinded to allocation.
In the fifteen or so years since paediatric HIV came to be regarded as a chronic disease( Reference Miller 19 ), very little research has been conducted into the nutritional aspects of HIV infection in children aged ≥5 years. Even for children <5 years old, the evidence base for nutritional interventions remains weak. The most recent Cochrane review reported some evidence for beneficial effects of vitamin A, Zn and micronutrient supplements, based on seven trials in sub-Saharan African countries( Reference Irlam, Siegfried and Visser 4 ). This dearth of evidence may have arisen because children and adolescents fall into a gap between research into HIV infection during pregnancy and infancy (because of the focus on vertical transmission) and during adulthood (wherein lies the main ‘burden’ of HIV infection, in terms of numbers of people infected), or it may have arisen because nutritional interventions have proven to be less effective than expected, given observational evidence for the importance of nutrition among people infected with HIV( Reference Salomon, De and Melchior 20 ).
In policy terms, it has been acknowledged that children, together with adolescents (girls in particular), are being ‘left behind’ in global efforts to end the AIDS epidemic( 21 ). This may be attributable in part to success in reducing the incidence of new infections in children, which have fallen by 58 % from a peak of 580000 in 2002. However, in 2013 there were an estimated 240000 new infections, and 3·2 million children under the age of 15 years were living with HIV, 91 % of whom were in sub-Saharan Africa. Given that these children are more likely to live in food-insecure households( Reference Rose, Hall and Martinez-Alier 1 ), that HIV infection is associated with faltering growth to at least 10 years of age( Reference Newell, Borja and Peckham 22 ) and that micronutrient sufficiency might be an important adjunct to ART( Reference Drain, Kupka and Mugusi 23 ), effective and sustainable approaches to maintaining adequate nutrition for HIV-infected children in resource-poor settings should be a priority area for research.
In the absence of comparable studies of nutritional interventions among ART-naïve children aged 5–14 years and because our study did not have an unsupplemented control group, we can only conclude that children receiving leaf concentrate and dairy supplement had equivalent outcomes in terms of CD4+ percentage and HIV viral load. We cannot conclude that either supplement had any effect on these two outcomes, i.e. equivalence may indicate lack of effect. The higher Fe content of the leaf concentrate supplement did not have any additional effect on Hb levels, for which there was evidence of improvement in both groups. This result may be attributable to the relatively normal baseline Hb levels and/or to the consumption of LCP within tea (an inhibitor of Fe absorption). Similar improvements in inflammation and fatigue were detected in both arms. Although both supplements contained Zn, the amounts are unlikely to have had an effect on diarrhoea, for which some evidence of a protective effect of Zn supplementation has been reported( Reference Irlam, Siegfried and Visser 4 ). The leaf concentrate supplement contained much higher levels of β-carotene than did the skimmed milk powder, but our study was not designed to detect differential effects of leaf concentrate on outcomes such as diarrhoea and cough, for which evidence of beneficial effects of vitamin A supplementation is reasonably consistent( Reference Irlam, Siegfried and Visser 4 ).
That growth indices improved in both groups is reassuring, given the accuracy with which these outcomes can be measured and the 6-month duration of our study. Classical anthropometric indices improved to the same degree in both groups, whereas handgrip strength score increased at a higher level in the LCP group. These data, combined with impact on appetite and overall health, suggest that LCP may increase the nutritional status more efficiently than SMP, because a relationship has been highlighted between muscle mass and function in children( Reference Marrodan Serrano, Romero Collazos and Moreno Romero 24 , Reference Schweizer, Martin and Schonau 25 ). These differences need to be confirmed by a larger study, ideally comparing LCP with no supplement (or to a supplement with known effects), and perhaps providing larger amounts of supplement or in conjunction with other nutrients.
HIV-infected adults have increased energy requirements( Reference Kosmiski 26 ) and additional kilojoules are recommended by WHO for HIV-positive children( 27 ), particularly in children experiencing weight loss. In our study, even greater gains in height and weight might have been achieved had the supplements been used to fortify an energy-providing snack, something which should be considered for future trials in this population. Incorporating the supplements into isoenergetic biscuits could also address the issue of blinding (if the non-LCP biscuits were dyed green) and might improve adherence and acceptability( Reference Ernst, Ettyang and Neumann 28 ). The Ca content of both supplements might be expected to ameliorate low bone mass, an effect of HIV infection that has been reported for children and adolescents( Reference Lima, Silva and Giuliano Ide 29 ), although longer follow-up would probably be required to detect any such effects. Trials that aim to test novel dietary interventions would benefit from information obtained from qualitative research into local tastes and preferences( Reference Hong, Hendricks and Wanke 30 ). Such research would, ideally, be conducted before the trial, but could also be conducted during the trial.
Leaf concentrate might be considered a candidate ingredient for the food multimix concept( Reference Zotor and Amuna 31 ). The aim of food multimix is to combine locally available and commonly consumed food ingredients into a culturally acceptable end product which makes the best use of each ingredient’s nutritional ‘strengths’. Although our study compared LCP against SMP, parallel production of the two supplements is rendered eminently feasible by the unique capacity of the leaf fractionation concept to provide direct access to the edible fraction of the leaves and to provide feed for livestock. For example, lucerne grown on 1ha can generate the production of 2000 kg of SMP if consumed directly by cattle; the same lucerne, fractionated into LCP for human consumption, fibre cake and whey for cattle can still produce 1800 kg of SMP, with the added production of 1200 kg of LCP( Reference Davys, Richardier and Kennedy 6 ). In this context, our demonstration that the nutritional qualities of SMP and LCP are comparable is an important finding.
The acceptability of leaf concentrate, and any economic advantage that it may have over industrially sourced skimmed milk powder, remain to be determined. Much as local and sustainable production of leaf concentrate is espoused as a key selling point( Reference Davys, Richardier and Kennedy 6 ), this remains somewhat theoretical and the development of the leaf fractionation concept is an ongoing process. In the case of Burundi, as in many tropical and subtropical countries, vegetables constitute a far greater component of the traditional diet than dairy products( 32 ) and vegetable crops (including those with leaves that are suitable for leaf concentrate production, e.g. beans) are one of the main agricultural products( 33 ).
These uncertainties should not deter further pursuit of alternative food-based solutions, including leaf fractionation, to nutritional problems among impoverished and vulnerable communities, particularly where local resilience might be needed in the face of endemic food insecurity and episodic civil instability( Reference Rossi, Verna and Villeneuve 34 , Reference Verwimp 35 ). In the meantime, our small study in a particularly vulnerable group has demonstrated that a leaf-derived food supplement could meet this need as effectively as a conventional milk-based supplement.
Acknowledgements
Acknowledgements: The authors thank Dr Pelagie Nimbona and Dr Anita Munyana, from the ANSS clinical centre, Bujumbura, and Dr Franck Kavabushi from the ANSS clinical centre, Kirundo. They thank the chair of ANSS, Jeanne Gapiya, and the ANSS nursing and care staff and social psychologists. They also thank Dr Donavine Hakizimana and Francine Kabatesi from INSP in Bujumbura, Dr Emery Barutwanayo from Nouvelle Espérance in Bujumbura, and Joyce Nsengiyumva (Université des Sciences et Technologies de Lille). Financial support: This study was funded by the Tolkien Trust (UK) and the Fondation Mérieux (France). Neither funder played any role in the design, analysis or writing of this article. Conflict of interest: B.L. and F.-C.R. undertake voluntary work on behalf of APEF. APEF is a non-profit organization whose remit is to explore the potential role of leaf concentrate in human nutrition. Authorship: B.L., F.-C.R., L.A. and E.B. formulated the research question, obtained funding and designed the study. The study was conducted by N.T. and E.B. S.M.C. performed the statistical analyses and wrote the first draft of the paper. All authors contributed to interpretation of the results and revisions of the manuscript, and approved of the final version. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the ‘Comité National d’Ethique pour la protection des êtres humains participants à la recherche biomédicale et comportementale’ of Burundi. Written informed consent was obtained from all subjects/patients.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980015003456








