In the UK, high blood pressure (BP) is a significant cause of premature morbidity and mortality, increasing the risk of developing stroke and heart disease( 1 ). At least 25 % of adults and more than 50 % of people over 60 years old in the UK have elevated BP( 1 ). Estimates from the results of the Framingham Study in the USA suggest that achieving normal BP in the community could significantly reduce the lifetime risk of developing CVD such as stroke( Reference Seshadri, Beiser and Kelly-Hayes 2 ).
Epidemiological evidence suggests that green leafy vegetables significantly protect against both CHD and stroke( Reference Joshipura, Hu and Manson 3 , Reference Hung, Joshipura and Jiang 4 ). For example, an increase of just one serving of green leafy vegetables per day is associated with an 11 % lower risk of CVD( Reference Hung, Joshipura and Jiang 4 ). Green leafy vegetables, as well as beetroot, are naturally high in dietary nitrate( 5 ). A recent systematic review concluded that nitrate, either as inorganic nitrate supplements or beetroot juice, significantly reduced systolic BP (SBP) by 4·4 mmHg in predominantly healthy, normal-weight men( Reference Siervo, Lara and Ogbonmwan 6 ). It was noted that further research is required to assess the interaction of dietary nitrate with gender( Reference Siervo, Lara and Ogbonmwan 6 , Reference Kapil, Milsom and Okorie 7 ). These authors speculated that gender differences in the processing of dietary nitrate may account for lower BP observed in premenopausal women( Reference Kapil, Milsom and Okorie 7 ). Changes in BP normally occur during the menstrual cycle, so that BP is lower at days 17–26 of the menstrual cycle( Reference Dunne, Barry and Ferriss 8 ). Unless adequately controlled, this could confound results of studies into the effects of dietary nitrate in women.
After consumption, inorganic nitrate is absorbed into the circulation, increasing plasma nitrate (NO3 −) concentration( Reference Larsen, Ekblom and Sahlin 9 ). Plasma nitrate is actively absorbed by the salivary glands and secreted into the oral cavity( Reference Lundberg, Carlstrom and Larsen 10 ). Oral bacteria reduce salivary nitrate to nitrite (NO2 −), which is swallowed and either absorbed, increasing plasma nitrite concentration, or reduced to nitric oxide (NO) in the acidic conditions of the stomach( Reference Lundberg, Carlstrom and Larsen 10 ). In hypoxic conditions, circulating plasma nitrite can be reduced to nitric oxide, a powerful vasodilator, subsequently increasing blood flow( Reference Cosby, Partovi and Crawford 11 ). It has been suggested that the nitrate–nitrite–nitric oxide pathway could have an important role in protection against CVD( Reference Lundberg, Carlstrom and Larsen 10 ).
Current public health advice includes recommendations to eat at least five portions of a wide variety of fruit and vegetables each day (‘5 A Day’)( 12 ). Fruit and vegetables contain many different nutrients such as folate, fibre and K, all of which have been proposed to have hypotensive properties( Reference He, Nowson and MacGregor 13 ). In addition, biologically active polyphenols, such as quercetin, have been shown to reduce BP( Reference Barona, Aristizabal and Blesso 14 , Reference Perez-Vizcaino and Duarte 15 ), potentially via increasing the biological activity of nitric oxide and protecting it from oxidation( Reference Hord, Tang and Bryan 16 ). However, specific nutrients or polyphenols in fruit and vegetables responsible for their health-promoting effects have not been identified.
Inorganic nitrate has historically been considered a contaminant due to concerns regarding increased risk of gastric cancer and infant methaemoglobinaemia (‘blue baby’ syndrome)( Reference McKnight, Duncan and Leifert 17 ). The WHO recommends an Acceptable Daily Intake (ADI) of 3·7 mg nitrate/kg body mass( 18 ). Regulatory bodies control maximum levels of nitrate in spinach, lettuce, baby foods and in water supplies so that the ADI for nitrate is not exceeded( 5 ). However, more recently, the WHO has stated that ‘there is inadequate evidence in humans for the carcinogenicity of nitrate in food’( 19 ). In addition, the European Food Safety Authority concluded that the benefits gained from eating vegetables outweigh the potential risks( 5 ). Due to emerging evidence for the health benefits of inorganic nitrate, there have been calls from the research community for a review of the ADI( Reference Lundberg, Carlstrom and Larsen 10 , Reference Hord, Tang and Bryan 16 , Reference Sobko, Marcus and Govoni 20 ).
There have been no published studies investigating the physiological effects of increasing dietary nitrate intake in women using fresh, green leafy vegetables readily available in the UK. We supplemented the diets of premenopausal, healthy, women volunteers with high-nitrate (HN) vegetables for 1 week. We compared this with the effects of a ‘Control’ diet, in which HN vegetables were avoided for 1 week. The study was designed to accommodate for potential changes in BP over the menstrual cycle. We carried out nutritional analyses to estimate nitrate, total polyphenol and quercetin contents of the fruit and vegetable component of two dietary interventions. We hypothesized that consuming HN vegetables would increase intake of dietary nitrate, resulting in increased plasma nitrate and nitrite concentrations and reduction of BP. We also hypothesized that the Control diet would not affect these variables significantly.
Experimental methods
Participants
Thirty healthy, adult women were enrolled and assessed for eligibility. Participants were non-smoking, premenopausal women with self-reported, regular (28 d) menstrual cycles, who were not taking any medications. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the local institutional Ethics Committee. All participants were provided with a written information sheet about the study and all provided written informed consent prior to being enrolled in the study. Ten participants were excluded or withdrew after enrolment due to personal reasons, time constraints or illness unrelated to the study, or difficulties in obtaining vascular access. One participant was subsequently excluded from the final analysis on the basis that her baseline plasma nitrite concentration lay >3 sd outside the group mean (Fig. 1). Data analysis was performed on the remaining nineteen participants (mean age 20 (sd 2) years, mean body mass 62·7 (sd 10·3) kg and mean BMI 22·5 (sd 3·8) kg/m2 at baseline). The study took place between September and December 2012.
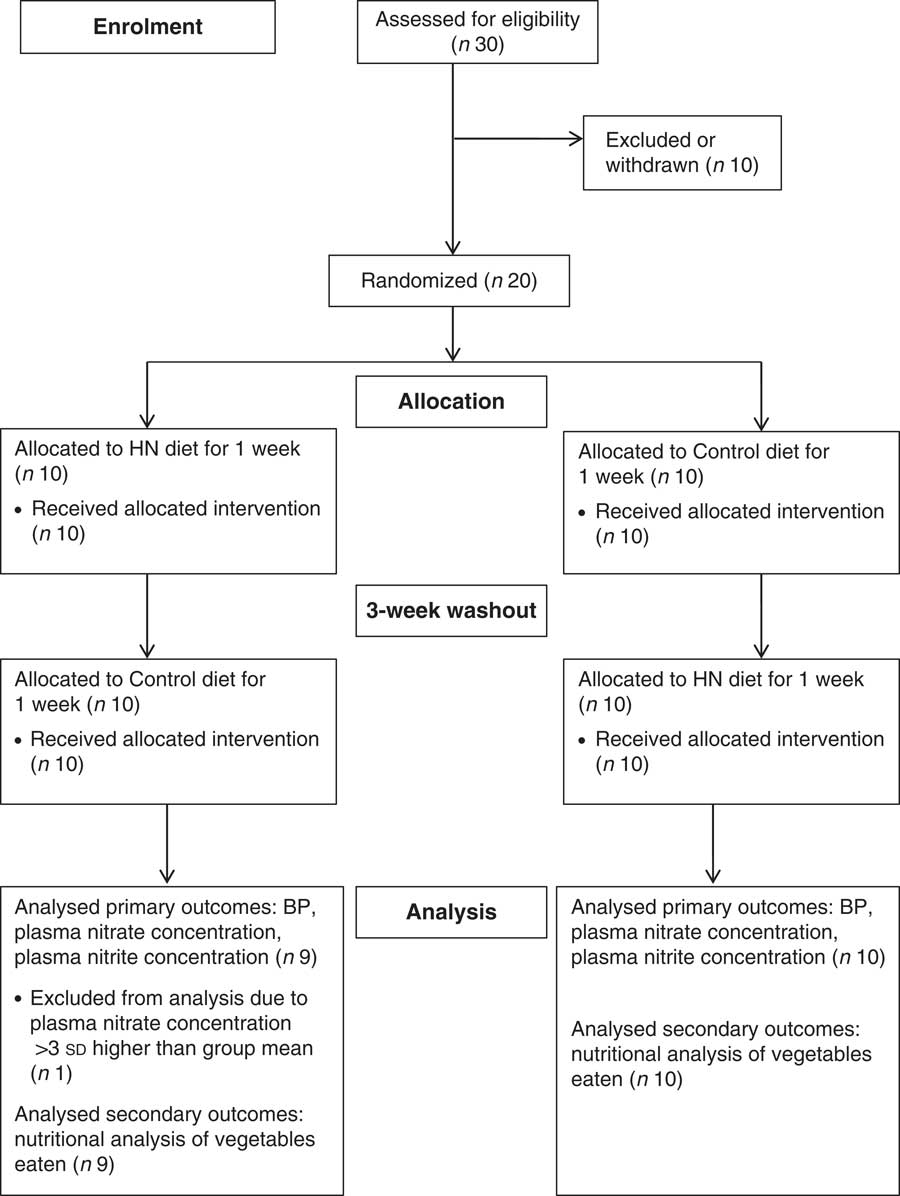
Fig. 1 Flowchart illustrating the overall study design (HN, high-nitrate; BP, blood pressure)
Study design
The study had a randomized, crossover design (Fig. 1). Participants were weighed on digital scales (XWM-105 K; Hampel Electronic Co. Ltd, Taipei, Taiwan), their height was measured (Harpenden Portable Stadiometer; Holtain Ltd, Crymych, UK) and their BMI calculated as weight/height2 (kg/m2). Participants were randomized using a sealed envelope technique to receive a box of HN vegetables (HN diet) for 1 week or were given written instructions to avoid HN vegetables for 1 week (Control diet), so that each participant consumed both HN and Control diets over a 5-week period with a ‘wash-out’ of 3 weeks between conditions. A 3-week wash-out was selected in order to synchronize dietary interventions with participants’ menstrual cycles. Before and after each HN and Control diet, participants were weighed, their BP measured and subsequently a venous blood sample was obtained for analysis of plasma nitrate and nitrite concentrations. Participants were not informed of the study hypothesis until the end of the study. The laboratory technician who carried out the analysis of plasma nitrate and nitrite concentrations was blinded to the test conditions.
Participants were advised to maintain their normal body mass throughout the study to avoid the effects of weight loss on BP( Reference Blumenthal, Babyak and Hinderliter 21 ). They were requested to record their exercise pattern using an exercise diary and to maintain their normal exercise pattern throughout the study in order to avoid exercise-induced reduction in BP( Reference Blumenthal, Babyak and Hinderliter 21 , Reference Whelton, Chin and Xin 22 ). Participants were requested to avoid using mouthwash throughout the study as antibacterial mouthwash has been shown to attenuate the rise in plasma nitrite( Reference Govoni, Jansson and Weitzberg 23 ). At each visit, participants were asked to confirm that they had taken no medications, including the contraceptive pill.
Dietary protocol
Participants randomized to receive the HN diet were requested to avoid HN vegetables for 3 d before their initial laboratory visit. The HN diet consisted of a box containing up to five types of HN vegetables including salad, such as lettuce, rocket, celery, leeks, fennel and mixed salad leaves (Table 1)( 5 , Reference Santamaria, Elia and Serio 24 , 25 ). Nitrate content of vegetables is influenced by environmental factors (humidity, temperature, water content and exposure to sunlight), as well as agricultural factors( Reference Lidder and Webb 26 ). In order to control for this as much as possible, vegetables were obtained from one supplier (Riverford Organic Farms Ltd, Buckfastleigh, UK) and the trial was completed in a 4-month period. Vegetable box contents were weighed using digital kitchen scales (Hanson Scale Company, Chicago, IL, USA) and then given directly to each participant. Quantities were calculated so that participants would be able to consume up to five portions (one portion=80 g)( 27 ) of vegetables and/or salad daily. Participants were requested to eat some HN vegetables every day for 7 d, including the final day (2–3 h before testing), but not alter their normal diet in any other way. Previous studies have suggested that the peak action time for dietary nitrate to reduce blood pressure is ~2·5–3 h after ingestion( Reference Lidder and Webb 26 ). Participants were not given a daily specific target of HN vegetables to be consumed. In order to preserve nutritional content, participants were requested to eat vegetables raw or stir-fried. At the end of each HN diet, participants were asked to quantify the amount of HN vegetables not eaten, if any, and the weight of wastage was estimated. Participants were requested to avoid HN vegetables on the Control diet, but were permitted to consume low-nitrate vegetables (Table 2) as well as fruit, and requested not to alter their normal diet in any other way.
Table 1 Estimated mean nitrate and total polyphenol contents of vegetables consumed by participants during the high-nitrate diet( 5 , Reference Santamaria, Elia and Serio 24 , 25 , Reference Neveu, Perez-Jiménez and Vos 41 ); nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012
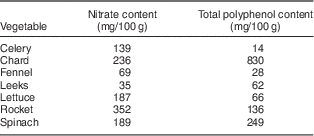
Table 2 Estimated mean nitrate and total polyphenol contents of vegetables consumed by participants during the Control diet( 5 , Reference Santamaria, Elia and Serio 24 , 25 , Reference Neveu, Perez-Jiménez and Vos 41 ); nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012

Participants were requested to provide an estimated record of their intake during both HN and Control diets using a 7 d food diary and to describe portions as household measures. They were not asked to weigh their food intake, as experience from a previous study suggested this would be too inconvenient for participants. ‘Medium’ portions were used for analysis, as quantified by Microdiet, when household measures were unavailable. As the HN vegetables had been weighed beforehand, the quantity of HN vegetables consumed was calculated as:
The ‘edible portion’ refers to the ‘edible material remaining after the inedible waste has been trimmed away’( 28 ) (e.g. in celery and leeks). A quantitative assessment of the vegetables and fruit consumed during both HN and Control diets was carried out by a registered dietitian using a nutritional analysis program (Microdiet version 2·8·8; Downlee Systems Limited, Chapel-en-le-Frith, UK). Direct measurement of nitrate content of the vegetables consumed was beyond the scope of the study.
Blood pressure
Participants were seated, undisturbed, for a minimum of 5 min prior to BP measurements. Each participant was tested at approximately the same time of day to avoid diurnal variation. Four readings were obtained using an automated sphygmomanometer (Dinamap PRO 100V2; GE Medical Systems Information Technology, Tampa, FL, USA) and mean SBP, mean diastolic BP (DBP) and mean arterial pressure (MAP) were calculated using the final three readings. The study was designed so that participants would be at the same stage of their 28 d menstrual cycle for both the HN and Control diets in order to control for possible BP fluctuation (SBP ~−0·65 mmHg during days 17–26)( Reference Dunne, Barry and Ferriss 8 ).
Analysis of plasma nitrate and nitrite concentrations
In order assess the effects of HN vegetables on plasma nitrate and nitrite concentrations, venous blood samples were collected into lithium-heparin tubes (BD Vacutainer Plasma Tube, 6 ml; Becton Dickinson, Plymouth, UK) after the BP measurement. Samples were placed within 3 min into a refrigerated (4°C) centrifuge (Sorvall ST16R; Thermo Scientific, Hemel Hempstead, UK) and centrifuged at 4000 rpm for 10 min. Plasma was frozen at −80°C for later analysis of plasma nitrate and nitrite concentrations using a modified chemiluminescence technique described previously( Reference Bailey, Winyard and Vanhatalo 29 ).
Statistical analysis
Analysis was carried out using the statistical software package SPSS Statistics version 19 and statistical significance was accepted when P<0·05. Paired-sample t tests were used to assess differences in body mass, hours of exercise and nutritional analysis of fruit and vegetables content of vegetables consumed during HN and Control diets. Two-way repeated-measures ANOVA were used to assess the effect of HN and Control diets on plasma nitrate and nitrite concentrations, SBP, DBP and MAP. Simple contrasts (least significant difference) were used to follow up any significant main and interaction effects. Results are presented as means and standard deviations. Relationships between changes in the variables were assessed using Pearson’s product-moment correlation coefficients. The primary end points were blood pressure (SBP, DBP and MAP) and plasma nitrate and nitrite concentrations. Sample size calculation was estimated from a previous study which found a reduction in SBP in healthy young men after sipping beetroot juice (containing 346 mg nitrate) or placebo drink throughout the day over 6 d( Reference Bailey, Winyard and Vanhatalo 29 ). In order to detect a significant reduction in SBP with α level of 5 % and statistical power of 80 %, it was estimated that a sample size of twenty participants was necessary. No participant withdrew after allocation, such that the analysis was carried out on an intention-to-treat basis (Fig. 1).
Results
High-nitrate and control diets
Participants on the HN diet consumed a mean raw weight of 180 (sd 75) g of HN vegetables and salad daily, equivalent to approximately two 80 g portions. Nutritional analysis of the fruit and vegetables eaten suggested that participants consumed significantly more nitrate, total polyphenols, protein, fat, K, Ca and Mg during the HN diet than the Control diet (Table 3), whereas total energy and carbohydrate intakes were similar on both diets. Food diaries suggested good compliance. Six participants consumed small portions of HN vegetables during the Control diet and five participants did not consume HN vegetables 2–3 h prior to the laboratory visit.
Table 3 Estimated quantitative nutritional analysis of fruit and vegetables eaten during high-nitrate (HN) and Control diets in terms of mean daily intakes of nitrate, total polyphenols, macronutrients (energy, protein, fat and carbohydrate) and minerals (potassium, calcium and magnesium); nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012

There was no significant difference in body mass of participants from baseline (mean 62·7 (sd 10·3) kg) to the end of the study period (mean 62·4 (sd 10·4) kg). Similarly, there was no significant difference between Control and HN diets in duration of exercise undertaken (Control diet: mean 4·0 (sd 2·6) h/week; HN diet: mean 4·2 (sd 2·6) h/week).
Plasma nitrate and nitrite concentrations
Having completed the study, one participant had a vasovagal response before the final venous sample collection, resulting in one missing data value. Plasma nitrate concentration was higher after 7 d on the HN diet (mean 61·0 (sd 44·1) µmol/l) than after the Control diet (mean 26·0 (sd 9·8) µmol/l). There were significant main effects by diet (F (1,17)=13·3, P=0·002) and by time (F (1,17)=11·8, P=0·003), as well as an interaction effect (F (1,17)=15·8, P=0·001) on mean plasma nitrate concentration. Follow-up tests revealed that the HN diet significantly increased plasma nitrate (P=0·002), whereas the Control diet had no significant effect (P=0·52; Fig. 2).
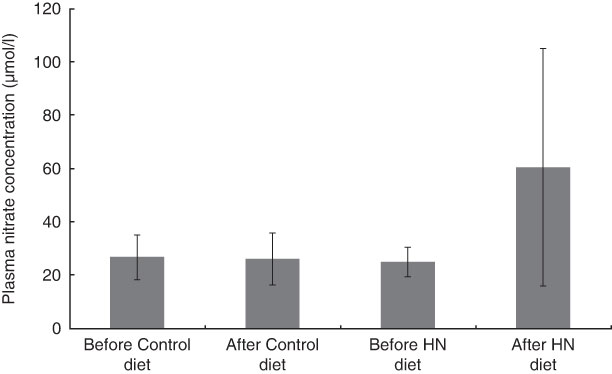
Fig. 2 Effects of high-nitrate (HN) vegetables on plasma nitrate concentration among nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012. Values are means with standard deviations represented by vertical bars. There was no significant difference after consuming the Control diet for 1 week (P=0·52). After the HN diet, plasma nitrate concentration was increased significantly (P=0·002)
There was a significant main effect by time (F (1,17)=7·3, P=0·015), a trend for a main effect by diet (F (1,17)=4·0, P=0·06) and no interaction effect on plasma nitrite concentration (F (1,17)=2·7, P=0·12). Follow-up tests revealed that plasma nitrite concentration increased after the HN diet (P=0·027), whereas the Control diet had no significant effect (P=0·23; Fig. 3). Plasma nitrite concentration was higher after 7 d on the HN diet (mean 185 (sd 146) nmol/l) than after the Control diet (mean 101 (sd 76) nmol/l; P=0·048).
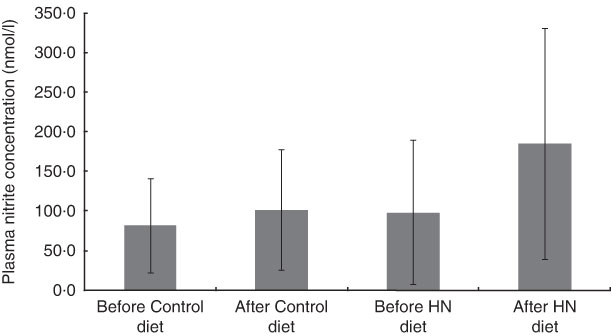
Fig. 3 Effects of high-nitrate (HN) vegetables on plasma nitrite concentration among nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012. Values are means with standard deviations represented by vertical bars. There was no significant difference after consuming the Control diet for 1 week (P=0·23). After the HN diet, plasma nitrite concentration was increased significantly (P=0·027)
Blood pressure
Group mean values and standard deviations of SBP, DBP and MAP before and after the Control and HN diets are given in Table 4. There was a significant main effect by time on SBP (F (1,18)=5·07, P=0·037), but no significant main effect by diet and a trend for an interaction effect (F (1,18)=3·39, P=0·082). Follow-up tests revealed that SBP was significantly reduced after the HN diet (P=0·008; Fig. 4). There was no significant difference between SBP before the HN diet and SBP before the Control diet (P=0·68). There was no significant change in SBP after the Control diet (P=0·94; Fig. 5) and there were no significant main or interaction effects on DBP or MAP (Table 4).
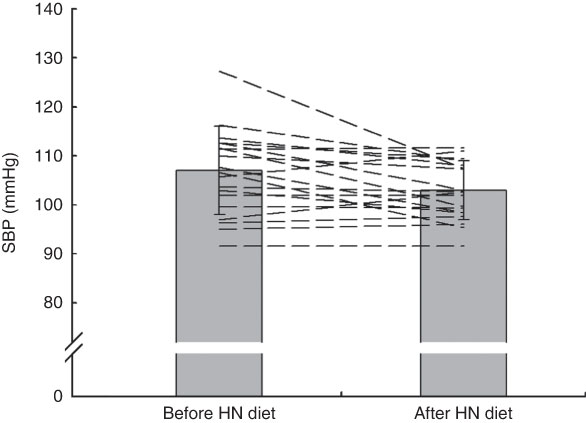
Fig. 4 Effects of 7 d high-nitrate (HN) diet on systolic blood pressure (SBP) among nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012. Values are means with standard deviations represented by vertical bars; the dashed lines illustrate individual responses. After the HN diet, SBP was reduced significantly (P=0·008)
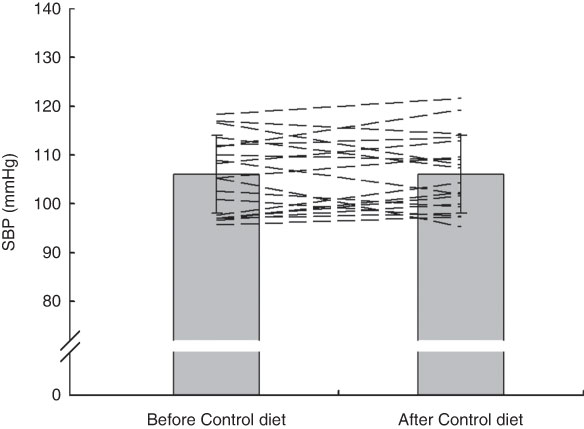
Fig. 5 Effects of 7 d Control diet on systolic blood pressure (SBP) among nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012. Values are means with standard deviations represented by vertical bars; the dashed lines illustrate individual responses. There was no significant difference after consuming the Control diet (P=0·94)
Table 4 Group mean values of systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) before and after the Control and high-nitrate (HN) diets; nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012
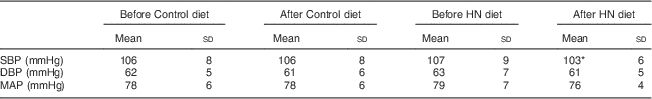
Statistical significance was determined by repeated-measures ANOVA and follow-up tests (least significant difference).
* SBP was significantly reduced after the HN diet (P=0·008), but not after the Control diet.
There was a significant correlation between baseline SBP and change in SBP following the HN diet (r=−0·74, P<0·001; Fig. 6). There was no correlation between estimated nitrate intake and change in SBP (r=0·002, P=0·99).
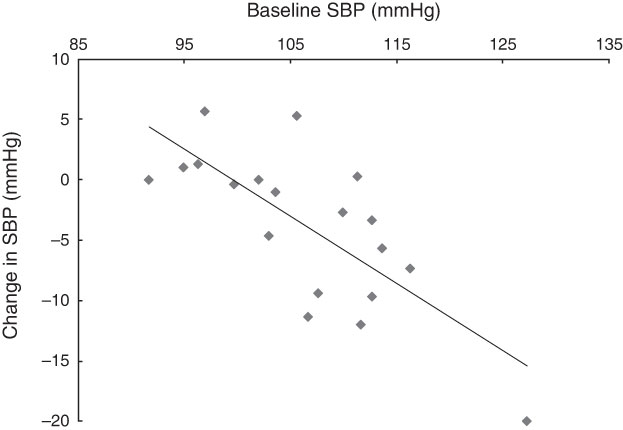
Fig. 6 The relationship between baseline systolic blood pressure (SBP) and the change in SBP following the high-nitrate diet (r=−0·74, P<0·001) among nineteen healthy women (mean age 20 years, mean BMI 22·5 kg/m2), Exeter, UK, September–December 2012
Discussion
The main finding of the present study was that consumption of approximately two portions of a variety of HN vegetables daily over 7 d reduced SBP (by ~4 mmHg) in healthy, normotensive young women. This supports our hypothesis that consumption of HN vegetables significantly reduces SBP. DBP and MAP were not significantly affected by the HN diet and no significant changes in SBP, DBP or MAP were observed when participants avoided HN vegetables during the Control diet. These findings are important as they suggest that consumption of HN vegetables, as part of the ‘5 A Day’ message, may promote cardiovascular health.
Dietary nitrate intake during high-nitrate and control diets
In the present study, participants consumed approximately two portions daily of a variety of HN vegetables and salad as part of a normal diet. The recommendation of a daily intake of at least five portions of fruit and vegetables, using a portion size of 80 g, equates to a target of at least 400 g/d( 27 ). Current advice to eat ‘5 A Day’ does not, however, specify which types of fruit and vegetables should be consumed( 12 ).
Mean nitrate intake on the HN diet was approximately 340 mg/d (5·5 mg nitrate/kg body mass per d), calculated using available data in the diet diaries (Table 1). This is in agreement with previous findings which indicate that it is comparatively easy to consume amounts of nitrate in excess of the ADI of 3·7 mg nitrate/kg body mass by eating a normal diet. For example, Hord et al. ( Reference Hord, Tang and Bryan 16 ) calculated that the DASH (Dietary Approaches to Stop Hypertension) diet could provide more than 1200 mg nitrate/d (equivalent to ~17 mg nitrate/kg body mass per d), from eight to ten portions of fruit and vegetables, including two portions of green leafy vegetables. Sobko et al. ( Reference Sobko, Marcus and Govoni 20 ) estimated that a traditional Japanese diet, including vegetables, mushrooms and seaweed, could provide five times the ADI, as it could theoretically contain 18·8 mg nitrate/kg body mass per d (1109 mg nitrate/d). Our findings support the suggestion that dietary inorganic nitrate has an important role in BP regulation and that a review of the ADI is warranted( Reference Lundberg, Carlstrom and Larsen 10 , Reference Hord, Tang and Bryan 16 , Reference Sobko, Marcus and Govoni 20 ).
Participants continued to consume fruit and low-nitrate vegetables (with avoidance of HN vegetables) during the Control diet, which resulted in no significant changes in BP. A study by Berry et al. ( Reference Berry, Mulla and Chowienczyk 30 ) showed no change in BP following a dietary intervention of increased fruit and vegetable intake. However, in that study, participants tended to consume fruit and vegetables that were convenient to carry around, such as apples, tomatoes and dried fruit( Reference Sanders 31 ). All fruits (apart from rhubarb, which is technically a vegetable) are low in nitrate, as are tomatoes( 5 ). Therefore, our finding of no significant change of BP after the Control diet is consistent with the findings of the study by Berry et al. ( Reference Berry, Mulla and Chowienczyk 30 ), which demonstrated that fruit and low-nitrate vegetables do not induce BP-lowering effects.
Effects of whole high-nitrate vegetables on plasma nitrate and nitrite concentrations
Plasma nitrate concentration increased by ~250 % and plasma nitrite concentration increased by ~190 % after 1 week of supplementation with HN vegetables, while no significant changes were seen after the Control diet. The effects of the HN diet in the present study are comparable to those in a previous study where supplementation with Japanese HN vegetables for 10 d resulted in a 360 % increase in plasma nitrate and a 150 % increase in plasma nitrite( Reference Sobko, Marcus and Govoni 20 ). Both our study and that by Sobko et al. ( Reference Sobko, Marcus and Govoni 20 ) suggest that supplementation of diets with whole HN vegetables significantly affects both plasma nitrate and nitrite concentrations. These findings are supported by a study which used beetroot-enriched bread to obtain significant reductions in both SBP and DBP( Reference Hobbs, Kaffa and George 32 ).
Effects of high-nitrate vegetables on blood pressure
Our 7 d supplementation study is novel in that we used fresh, whole HN vegetables readily available in the UK and found a reduction of SBP of ~4 mmHg. Baseline SBP and DBP were in line with current mean UK population levels in 16–24-year-old women of 110 mmHg and 66 mmHg, respectively( 33 ). Sobko et al. ( Reference Sobko, Marcus and Govoni 20 ) reported a significant reduction in DBP of ~5 mmHg after 10 d but no change in SBP, whereas our study resulted in a significant reduction in SBP but not DBP. The study by Sobko et al. ( Reference Sobko, Marcus and Govoni 20 ) used a different methodology, with an older population (mean age 36 (sd 10) years), Japanese HN foods (such as seaweed), and included both men and women. However, both our 7 d study and the 10 d study by Sobko et al. ( Reference Sobko, Marcus and Govoni 20 ) suggest that diets containing HN vegetables may be an effective, acceptable way to reduce BP. The results of our 7 d study, the 10 d study described above( Reference Sobko, Marcus and Govoni 20 ) and a 15 d study by Vanhatalo et al. ( Reference Vanhatalo, Bailey and Blackwell 34 ) suggest that HN vegetables do not induce tolerance, which is a potential advantage over the therapeutic use of organic nitrates( Reference Lidder and Webb 26 ).
A recent systematic review found the pooled effect for inorganic nitrate and beetroot juice supplementation to be a significant reduction of SBP of ~4–5 mmHg( Reference Siervo, Lara and Ogbonmwan 6 ). However, most of the study populations have consisted of healthy, young men and may not be representative of the whole population( Reference Siervo, Lara and Ogbonmwan 6 ). Kapil et al. ( Reference Kapil, Milsom and Okorie 7 ) observed a change in SBP (~4 mmHg) in women 3 h after consuming potassium nitrate. Our results suggest that the consumption of whole HN vegetables may be as effective in reducing SBP in women.
The present results suggest that dietary nitrate from HN vegetables may facilitate BP reduction. The significant correlation between baseline SBP and change in SBP following the HN diet suggests that people with higher SBP may benefit more from eating HN vegetables. This is consistent with previous findings from the DASH( Reference Appel, Moore and Obarzaneck 35 ) diet, which found that participants with higher BP experienced greater reductions following dietary intervention. However, both fruit and vegetables are a key part of the DASH diet( Reference Appel, Moore and Obarzaneck 35 ). The DASH diet has been endorsed as an effective treatment for high BP in the USA( Reference Blumenthal, Babyak and Hinderliter 21 ), but it is not widely advised in the UK( Reference Harnden, Frayn and Hodson 36 ). The DASH diet recommends a high intake of fruit and vegetables (8–10 portions/d), together with whole grains, low-fat dairy products and reduced intakes of Na, fat and saturated fat. The specific constituent(s) of the DASH diet which contribute to its success is/are unknown. The DASH diet provides more K, Mg, Ca, fibre and less total and saturated fat than a control diet( Reference Appel, Moore and Obarzaneck 35 ). Our results also suggest that K, Ca and Mg intakes from fruit and HN vegetables were higher than when on the control diet. However, Cochrane reviews of the effectiveness of K, Mg and Ca supplements concluded that there was insufficient evidence to support their use as hypotensive agents( Reference Dickinson, Nicolson and Campbell 37 – Reference Dickinson, Nicolson and Cook 39 ). In addition, there is a lack of evidence to promote the use of antioxidant supplements, which have been associated with increased mortality( Reference Bjelakovic, Nikolova and Gluud 40 ). It has been suggested that the success of the DASH diet could be partly due to the increased nitrate intake from HN green leafy vegetables and salads( Reference Hord, Tang and Bryan 16 ) and our study would appear to support this hypothesis. However, there are many other nutrients or biologically active compounds, such as polyphenols, found in both fruit and vegetables, which may have physiological effects( Reference Neveu, Perez-Jiménez and Vos 41 ). The estimated total polyphenol intake of participants was significantly higher on the HN diet than on the Control diet, suggesting that green leafy vegetables contain more polyphenols. It has been proposed that polyphenols may increase generation of nitric oxide in the gastrointestinal tract and protect nitric oxide from oxidation( Reference Hord, Tang and Bryan 16 ). It is possible that both nitrate and polyphenols contained in HN vegetables reduce BP. Therefore, although our study suggests that consumption of HN vegetables is important in BP regulation, it remains to be determined to what extent polyphenols contributed to the lower BP we observed.
Study limitations
Food diaries were used to analyse consumption of vegetables and fruit on both diets, but they were not analysed for total intakes of macro- and micronutrients from all foods eaten. The effect of eating two daily portions of HN vegetables, which can add bulk to the diet, on normal dietary intake was not assessed. There was no significant change in body mass throughout the study, suggesting that overall energy intake was not significantly affected. A potential limitation in dietary control was that nitrate intake was estimated based on self-reported vegetable consumption and indirect estimation of nitrate content of vegetables, which may vary according to factors such as crop variety, season and light conditions. Future research should consider direct measurement of nitrate concentrations in the HN diet, as well as assessment of the effects of HN vegetables on macro- and micronutrient intakes.
The study was designed to assess the effects of 7 d consumption of HN vegetables in free-living participants. It could be argued, however, that the acute effect was measured as participants were requested to eat vegetables on the final day 2–3 h before testing. However, the five participants who did not eat HN vegetables before the final test also experienced reductions in BP.
The possibility of a ‘placebo effect’ should be considered because participants were given HN vegetables to consume in the experimental arm but asked to maintain their normal diet during the control arm. However, participants were blind to the purpose of the study, i.e. they were not informed that the vegetables they were given were high in inorganic nitrate or that this might influence BP.
BP is known to vary during the menstrual cycle( Reference Dunne, Barry and Ferriss 8 ). The current study design relied on recruitment of participants having a 28 d menstrual cycle, with testing timetabled such that the participants would be at the same stage of their cycle for both the HN and Control diets. As the length of the menstrual cycle is subject to variability (21–35 d), a sliding wash-out period might have allowed for shorter or longer cycle length and could be included in the design of further studies.
Finally, one of the participants allocated to the HN group was excluded following analyses of primary outcome variables based on the criterion that a measurement lay >3 sd beyond the group mean. The principal results of the study would not be altered if this participant had not been excluded. However, this meant that the statistical power of the present data (64 %) was somewhat lower than that predicted (80 %) in the sample size calculation based on a previous study (n 20)( Reference Bailey, Winyard and Vanhatalo 29 ). It should be noted, however, that despite moderate statistical power, the P value for the primary outcome variable (SBP) was clearly significant (P=0·008) and our findings are consistent with previous studies showing significant elevations in plasma nitrite concentration and reductions in BP following nitrate ingestion( Reference Kapil, Milsom and Okorie 7 , Reference Bailey, Winyard and Vanhatalo 29 ). The present findings suggest that nitrate-rich vegetables represent a promising intervention to improve cardiovascular health in women and warrant further investigation.
Conclusions
Our results suggest that consumption of HN vegetables over 7 d reduces BP in normotensive women. People with hypertension may obtain even greater reductions in BP through dietary intervention( Reference Appel, Moore and Obarzaneck 35 ). Therefore, consumption of HN vegetables as part of the ‘5 A Day’ may be an effective strategy to reduce population-wide BP and hypertension. He and MacGregor( Reference He and MacGregor 42 ) estimated that reducing BP by 5 mmHg could result in a reduction in stroke death in the UK by 23 %, which could equate to 13 700 deaths prevented per year. Not only would this BP reduction save lives and prevent significant disabilities, it would also represent a major cost saving to health services. Stroke costs the National Health Service £3 billion per year, with a wider economic cost of £8 billion per year( 43 ).
Further research into gender differences in the metabolism of nitrate is required to identify whether this is the reason why premenopausal women have significantly lower BP than men of comparable age( 33 ). Longer-term supplementation studies are required to address this. In addition, the availability and acceptability of HN vegetables to various population groups should be explored.
Supplementing the diets of healthy, normotensive, premenopausal women with HN vegetables for 7 d resulted in a significant reduction in SBP. This simple intervention could be incorporated into current health advice to eat at least ‘5 A Day’, which could result in a population-wide reduction of BP, reduce morbidity and mortality from stroke, and reduce costs for health-care services.
Acknowledgements
Acknowledgements: The authors thank the study participants for taking part and Riverford Organic Farms Ltd for providing the vegetable boxes. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Vegetable boxes were provided free of charge by Riverford Organic Farms Ltd, who had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: A.A. was responsible for the study design, recruitment, data collection, statistical analysis, data interpretation, drafting of the manuscript and was involved in plasma analysis. K.M. was involved in the study design, recruitment and data collection. J.R.B. was responsible for plasma analysis. A.V. and A.M.J. were responsible for study design, recruitment, data interpretation, study supervision and approval of the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Ethics Committee of Sport and Health Sciences, College of Life and Environmental Sciences, University of Exeter. All participants were provided with a written information sheet about the study and all provided written informed consent prior to being enrolled in the study.













