Clinical Implications
∙ The hippocampus is involved in the regulation of emotional and cognitive processing, both of which are compromised in patients with major depressive disorder (MDD).
∙ Serotonin (5-HT) plays an important role in hippocampal function.
∙ Multiple 5-HT receptors are often co-expressed on the same cell types in the hippocampus with functions that can either be complementary or opposing. Overall, 5-HT appears to inhibit pyramidal cells in the hippocampal circuit in rodents.
∙ While selective serotonin reuptake inhibitors (SSRIs) have the potential to normalize hippocampal output under stress conditions and to treat mood symptoms in MDD, their effects on cognitive function are less clear.
∙ The multimodal antidepressant vortioxetine has shown clinical efficacy on mood as well as cognitive symptoms in patients with MDD. 5-HT3 receptor inhibition of GABAergic interneurons is thought to play an important role in mediating these effects.
Introduction
There is extensive evidence that depression and other stress-related conditions are associated with hippocampal dysfunction.Reference Femenia, Gomez-Galan, Lindskog and Magara1, Reference Small, Schobel, Buxton, Witter and Barnes2 In several magnetic resonance studies, patients with major depressive disorder (MDD) have reduced hippocampal volumes compared with matched control subjects.Reference Bell-McGinty, Butters, Meltzer, Greer, Reynolds and Becker3–Reference Frodl, Koutsouleris and Bottlender5 Furthermore, Sheline et al Reference Sheline, Gado and Kraemer6 have shown that there is an inverse correlation between hippocampal volume and the duration of untreated depression. A meta-analysis of 12 clinical studies indicated that the number of depressive episodes may be correlated with a reduction of hippocampal volume in the right hemisphere.Reference Videbech and Ravnkilde7 Reduced hippocampal volume in MDD patients has also been associated with impaired memory (eg, MacQueen et al Reference MacQueen, Campbell and McEwen8). Memory function related to hippocampal integrity decreases with increasing numbers of depressive episodes.Reference Gorwood, Corruble, Falissard and Goodwin9 In addition, functional magnetic resonance imaging (fMRI) studies of depressed patients have consistently shown overactivity in the frontolimbic circuitry, including the dorsolateral prefrontal cortex and hippocampus during working memory performance.Reference Harvey, Fossati and Pochon10, Reference Walsh, Williams and Brammer11
In addition to disturbances in mood and emotional processing, MDD is associated with deficits in several cognitive domains, including executive function, processing speed, and attention, as well as learning and memory.Reference Hammar and Ardal12–Reference Porter, Bourke and Gallagher14 There is evidence that cognitive impairment varies independently of mood state and does not necessarily resolve when the patient is considered to be in clinical remission.Reference Hasselbalch, Knorr and Kessing15 This may imply that cognitive control and the regulation of emotion have distinct neuronal bases in depression.Reference Campbell and MacQueen16 While the literature suggests that antidepressants may potentially treat cognitive dysfunction in some patients with MDD, these studies were not designed to distinguish between the direct effects on cognitive domains versus indirect effects on cognition via improvements in mood. Overall, small sample sizes, methodological constraints, and the absence of replication make it difficult to draw firm conclusions from the majority of these studies.Reference Biringer, Rongve and Lund17, Reference McIntyre, Cha and Soczynska18
Since the selective serotonin (5-HT) reuptake inhibitors (SSRIs) and serotonin norepinephrine (NE) reuptake inhibitors (SNRIs) are the predominant pharmacotherapies used for the treatment of MDD, modulation of serotonergic neurotransmission is assumed to play a pivotal role in achieving their antidepressant efficacy. Many 5-HT receptor subtypes are extensively expressed in the hippocampus. However, even though a large number of preclinical studies in rodents are strongly supportive of antidepressant treatments restoring hippocampal function, their mechanisms of action have not been fully elucidated. Furthermore, it is not well understood how the clinical efficacy of currently used antidepressants might be related to changes in hippocampal function in patients with MDD.Reference Vythilingam, Vermetten and Anderson19 Therefore, a thorough understanding of how 5-HT receptor modulation affects hippocampal functions is essential to the understanding of how antidepressants might work. Here we review the current knowledge of how 5-HT, through its various receptor subtypes, might modulate hippocampal activity in rodents. In addition, we discuss its relevance for cognitive processing and the possible clinical implications for patients with MDD. Finally, we review available data on how SSRIs and vortioxetine, an antidepressant that, in addition to inhibition of 5-HT reuptake, also modulates a number of 5-HT receptor subtypes, affect hippocampus function from a preclinical and clinical perspective.
Anatomy of the Hippocampus
To understand how 5-HT modulates hippocampal function at a molecular level, it is necessary to gain insights into how 5-HT modulates the different cell types and subregions that comprise the hippocampal microcircuits. Along the longitudinal axis, the hippocampus is segregated into dorsal, intermediate, and ventral regions in rodents (reviewed in Fanselow and DongReference Fanselow and Dong20 and Moser and MoserReference Moser and Moser21), and analogous posterior and anterior regions in primates and humansReference Poppenk, Evensmoen, Moscovitch and Nadel22 that project to distinct brain areas.Reference O’Leary and Cryan23 Lesion and electrophysiology studies in rodents have shown that the dorsal hippocampus is primarily involved in the cognitive functions, including spatial learning and memory,Reference Moser, Moser and Andersen24, Reference Pothuizen, Zhang, Jongen-Relo, Feldon and Yee25 whereas the ventral hippocampus is primarily involved in regulating stress, emotion, and anxiety.Reference Bannerman, Grubb, Deacon, Yee, Feldon and Rawlins26–Reference Kjelstrup, Tuvnes, Steffenach, Murison, Moser and Moser28 However, this division of functions is somewhat ambiguous, since parts of the ventral hippocampus have been also shown to be involved in memory tasks.Reference Rudy and Matus-Amat29
The hippocampus is subdivided into several distinct zones: the dentate gyrus (DG), CA3, CA2, CA1, and the subiculum regions that were first described by Ramon y Cajal in 1911Reference Ramon y30 and Lorente de Nó in 1934 Reference Lorente de Nó31 (Figure 1). The CA3, CA2, and CA1 regions are sometimes called the hippocampal gyrus or Ammon’s horn. Granule cells in the DG receive projections from the surrounding entorhinal cortex and send their axons, called mossy fibers, to the CA3 area. Pyramidal cells in the CA3 area project axons, known as Schaffer collaterals, to the CA2 and CA1 areas. Pyramidal cells in the CA1 area send their axons to the surrounding deep cortical layers of the entorhinal cortex and to the subiculum which is the final processing stage of the hippocampal microcircuitry (Figure 1). In addition to this main “trisynaptic circuit,” there are direct connections from the superficial layers of the entorhinal cortex to the CA3 and CA1 areas, and synaptic connections from inhibitory gamma-butyric acid (GABA)ergic interneurons to excitatory glutamatergic pyramidal and granule cells within the hippocampus.Reference Lavenex32

Figure 1 Schematic illustration of the rat hippocampal circuit with 5-HT receptor localization. The main areas of the hippocampus, including the dentate gyrus (DG), CA3, CA2, CA1, and the subiculum regions, and synaptic connections between them are indicated. Principal (granule and pyramidal) cells are shown in blue, and interneurons are shown in green. Expression of 5-HT receptor subtypes on hippocampal CA1 and CA3 pyramidal cells, granule cells, and interneurons are shown. References for 5-HT receptor localization are listed Table 1. At least 16 subtypes of interneurons have been identified in the hippocampus; one representative interneuron is shown for illustrative purposes. Note that the 5-HT1A heteroreceptor is expressed at high levels throughout the hippocampus. The 5-HT1B receptor is found at highest levels in the subiculum. Based on histology data, the 5-HT3 receptor is only expressed on the interneurons, and the 5-HT4 receptor is only expressed on pyramidal cells. Other 5-HT receptors subtypes are found on both principal cells and interneurons.
There are 2 types of principal cells in the hippocampal circuit: glutamatergic pyramidal cells in the Ammon’s horn and subiculum regions, and glutamatergic granule cells in the DG (Figure 1). They generally have excitatory effects on the neurons to which they send axon terminals including other glutamatergic and GABAergic, as well monoaminergic [5-HT, norepinephrine (NE), dopamine (DA)], cholinergic, and histaminergic (HA) cells. There are also 3 major populations of GABAergic inhibitory interneurons that can be identified by the expression of the calcium-binding proteins parvalbumin, calbindin, and calretinin. These interneurons can be further subdivided based on their morphology and presence of receptors for neuropeptides and other neurotransmitters. In total at least 16 different subtypes of interneurons have been identified in the hippocampus with different firing properties and functions.Reference Freund and Buzsáki33, Reference Parra, Gulyas and Miles34 Each of these interneuron subpopulations has a distinct placement within the network, as well as a distinct role in modulating the behavior of pyramidal neurons.
Parvalbumin-immunoreactive interneurons are present within the pyramidal cell body layer (divided into stratum oriens and pyramidale), and synapse onto the soma and/or axons of pyramidal neurons.Reference Ribak, Nitsch and Seress35 In contrast, calbindin immunoreactive interneurons are present in the dendritic layers of the hippocampus (divided into stratum radiatum and moleculare)Reference Sloviter36 and are thought to synapse onto pyramidal neuron dendrites.Reference Aznar, Qian, Shah, Rahbek and Knudsen37 Finally, calretinin immunoreactive interneurons frequently form local synaptic connections onto other interneurons.Reference Gulyas, Hajos and Freund38 Thus, hippocampal interneurons can modulate the activity of both pyramidal cells and other interneurons.Reference Gulyas, Hajos and Freund38
The processing of information within the hippocampus is complex and is influenced by multiple neurotransmitters and neuromodulators, including glutamate, GABA, DA, NE, HA, acetylcholine (ACh), and 5-HT. Serotonergic receptors are found on both excitatory cells and inhibitory interneurons. As will be discussed in the following sections, 5-HT neurotransmission can have a direct effect on pyramidal neuron firing by modulating its membrane potential and indirect effects via modulating GABA neurotransmission.
5-HT Receptors in the Rodent Hippocampus
Nearly all of the identified 5-HT receptor subtypes are expressed in the hippocampal circuit in rodents.Reference Berumen, Rodriguez, Miledi and Garcia-Alcocer39 Interestingly, 5-HT fibers often lack direct synaptic contacts, and in many cases 5-HT receptors have been detected on neurons that do not receive serotonergic innervation.Reference Oleskevich, Descarries, Watkins, Seguela and Daszuta40–Reference Vizi and Kiss42 This suggests that in the hippocampus, as in other brain areas, 5-HT is released diffusely by volume transmission and acts more as a neuromodulator whose function might be to maintain homeostasis in the brain.
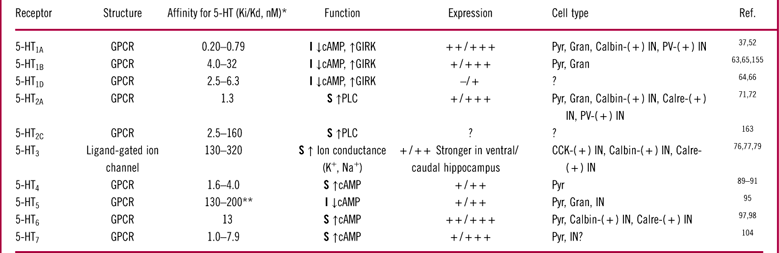
The specificity and diversity of 5-HT signaling arises from at least 14 different receptor subtypes grouped in 7 receptor families with distinct characteristics and expression patterns (Table 1, Figures 1 and 2). The 5-HT1 receptor family is inhibitory; it is coupled to G-protein-coupled inwardly rectifying potassium (GIRK) channels and Gi/o proteins. Activation of GIRK channels exerts hyperpolarizing effects on cell function. Activation of Gi/o proteins inhibits adenylyl cyclase and decreases cyclic adenosine monophosphate (cAMP) concentration. The 5-HT2 receptor family is stimulatory and signals via activation of Gq proteins that are coupled to phospholipase C. Phospholipase C hydrolyses membrane phosphoinositides into inositol triphosphate (IP3) and diacylglycerol (DAG), which elevate intracellular calcium. One of the downstream targets of 5-HT2 receptors are potassium leak channels. Activation of 5-HT2 receptors closes these channels, resulting in cell depolarization. The 5-HT3 receptor family is stimulatory and the only non-G-protein coupled receptor. Activation of 5-HT3 receptors opens a non-selective Na+/K+ ion channel that depolarizes neurons and increases neurotransmitter release. The 5-HT4, 5-HT6, and 5-HT7 receptors are stimulatory and increase neuronal excitability. They are coupled to Gs protein and signal via activation of adenylyl cyclase and elevation in cAMP levels. Finally, 5-HT5 receptors are inhibitory, and, as for the 5-HT1 receptor family members, are coupled to Gi/o proteins that suppress adenylyl cyclase and decrease cAMP levels.Reference Aghajanian and Sanders-Bush43, Reference Glennon, Dukat and Westkaemper44 In the hippocampus, cells often co-express several types of 5-HT receptors that can have either complementary or opposing effects on cell function (Figure 1 and Table 1). Moreover, 5-HT receptors can form homodimers or heterodimers with other G-protein coupled receptors, which adds further complexity to 5-HT signaling.Reference Herrick-Davis45 For example, heterodimers of 5-HT1A-5HT7 receptors and 5-HT2A-mGlu2 (metabotropic glutamate 2) receptors have been shown to have characteristics that differ from their individual counterparts.Reference Herrick-Davis45–Reference Naumenko, Popova, Lacivita, Leopoldo and Ponimaskin47 Below we describe the expression patterns of 5-HT receptors and discuss their effects on hippocampal circuitry and hippocampus-mediated behavioral responses based on published results and our data in rodents. We chose to only include behavioral models of memory and learning, since these models have the best link to hippocampal function.Reference Jarrard48–Reference Knierim and Hamilton50

Figure 2 Expression of several classes of 5-HT receptors and the 5-HT reuptake transporter (SERT) by ex vivo autoradiography in the rat hippocampus. Autoradiographic images representing total (left panels) and non-specific binding (right panels) for each of 5 separate serotonergic targets in coronal brain sections (20 µm in thickness). 5-HT1A receptors were mapped using 3 nM [3H]8-OH-DPAT (A) alone or (B) in combination with 1 µM of the 5-HT1A receptor selective antagonist WAY100635 to determine the level of nonspecific binding. 5-HT1B/1D receptors were mapped using 1 nM [3H] GR125743 (C) alone or (D) in combination with 1 µM of the 5-HT1B receptor preferring SB216641 to determine the level of nonspecific binding. 5-HT3 receptors were mapped using 3 nM [3H] LY278584 (E) alone or (F) in combination with 1 µM ondansetron to determine the level of nonspecific binding. 5-HT7 receptors were mapped using 4.5 nM [3H]SB269970 (G) alone or (H) in combination with 1 µM of unlabeled SB269970 to determine the level of nonspecific binding. Finally, SERT was mapped using 4.5 nM [3H] escitalopram (I) alone or (J) in combination with 1 µM paroxetine to determine the level of nonspecific binding. Scale bars represent 5 mm.
Table 1 5-HT receptor subtypes in the rodent hippocampus

* Affinities for 5-HT were calculated from pKi/pKd data obtained from the IUPHAR data base. For further details and references see http://www.iuphar-db.org.
** 5-HT5a. Expression strength is indicated by –: absent, +: low, ++: moderate, +++: strong, ?: unknown.
Abbreviations used: GPCR: G-protein-coupled receptor; I: inhibitory; S: stimulatory; ↑: increase; ↓: decrease; Pyr: pyramidal; Gran: granule; IN: interneuron; PV: parvalbumin; CCK: cholecystokinin; Calbin: calbindin; Calre: calretinin.
5-HT1A receptors
Among all 5-HT receptor subtypes, 5-HT1A receptors have the highest affinity for 5-HT (Table 1). In the hippocampus, they are found on non-serotonergic cells as heteroreceptors and inhibit cellular activity via activating GIRK channels.Reference Luscher, Jan, Stoffel, Malenka and Nicoll51 They are defined as heteroreceptors because they control release of neurotransmitters other than 5-HT. 5-HT1A receptors are moderately to highly expressed throughout the hippocampusReference Pompeiano, Palacios and Mengod52 (Figure 2A). They have been detected on both glutamatergic principal cells and at least two subtypes of GABAergic interneurons (Table 1).
Activation of 5-HT1A receptors primarily leads to inhibition of hippocampal pyramidal cells.Reference Andrade and Nicoll53–Reference Tada, Kasamo, Suzuki, Matsuzaki and Kojima59 Interestingly, in the prefrontal cortex, 5-HT1A receptor agonists produce both excitatory and inhibitory effects on cortical pyramidal cells.Reference Llado-Pelfort, Santana, Ghisi, Artigas and Celada60 This might be ascribed to a difference in the distribution of 5-HT1A receptors on interneurons versus pyramidal cells in these 2 brain regions.
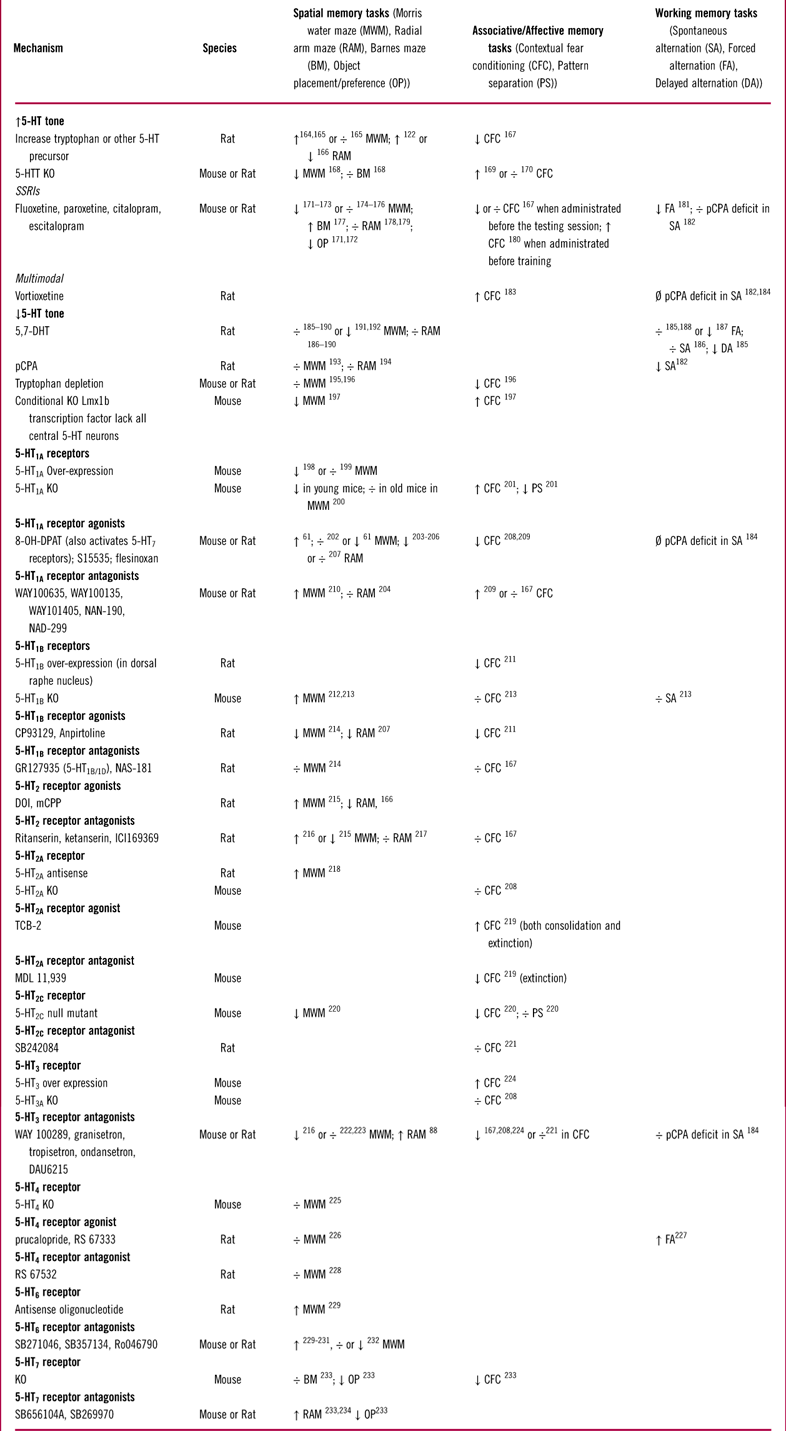
The function of 5-HT1A receptors has been extensively studied in multiple behavioral studies, 16 of which are listed in Table 2. Modulation of 5-HT1A receptor activity in animal models of memory and learning has produced inconsistent results that range from impairment to improvement (Table 2). Some of these inconsistencies might be due to differences in experimental design across studies. Multiple factors, such as drug dose, length of treatment (acute vs chronic), whether the drug was administered before or after the training period, and also the age and stain of animals, could all influence the behavioral outcome. However, in some studies (eg, Haider et al Reference Haider, Khaliq, Tabassum and Haleem61), opposing results were obtained with different doses of the same compound under the same experimental conditions. This suggests that the variability in results might be partially due to the complex effects of 5-HT1A receptors expressed on different cell types. For instance, 5-HT1A receptors can inhibit both principal (glutamatergic) neurons and GABAergic interneurons. Inhibition of GABAergic interneurons would disinhibit principal cells and thus counteract the direct effects of 5-HT1A receptors expressed on principal neurons. Therefore, selectively targeting 5-HT1A receptors may not be an optimal strategy for modulating hippocampal function, unless these receptors could be targeted in a regional or cell-specific manner.Reference Newman-Tancredi, Martel and Assie62
Table 2 Effects of serotonergic manipulations on hippocampal dependent memory tests in rodents

÷: no effect; ↑: increase/improve; ↓: decrease/impair; Ø: prevented/reduced deficits.
Abbreviations used: MWM: Morris Water Maze; RAM: Radial Arm Maze; BM: Barnes Maze; OP: Object Placement (Preference); CFC: Contextual Fear Conditioning; PS: Pattern Separation; SA: Spontaneous Alternation; FA: Forced Alternation; DA: Delayed Alternation.
5-HT1B and 5-HT1D receptors
5-HT1B heteroreceptors are found throughout the hippocampus at levels ranging from low to very high.Reference Boschert, Amara, Segu and Hen63–Reference Sari, Lefevre and Bancila65 They are expressed on axonal terminals and dendrites of principal cells, which include pyramidal cells in Ammon’s horn and granule cells in the DG (Table 1). The highest expression is found in the dorsal subiculum, which might originate from axonal terminals of CA1 pyramidal cells that project to that region (Figure 2C).Reference Sari, Lefevre and Bancila65 Interestingly, in our experiments, the subiculum had the strongest signal for 5-HT1B receptor expression in the rodent forebrain.
Much less is known about the 5-HT1D receptor. 5-HT1D receptors are generally thought to be expressed at much lower levels than 5-HT1B receptors in the rodent brain.Reference Bruinvels, Landwehrmeyer and Gustafson64, Reference Bruinvels, Palacios and Hoyer66 5-HT1D and 5-HT1B receptors are often expressed in the same brain regions.Reference Bruinvels, Landwehrmeyer and Gustafson64 However, no 5-HT1D receptor-specific binding has been detected in the dorsal subiculum, where 5-HT1B receptor-specific binding is very strong.Reference Bruinvels, Palacios and Hoyer66 Interestingly, Xie et al Reference Xie, Lee, O’Dowd and George67 suggest that when 5-HT1B and 5-HT1D receptors are co-expressed, they might exist in a heterodimerized state. Thus, it can be questioned if the effects of 5-HT1B and 5-HT1D receptors should be considered separately.
Activation of 5-HT1B receptors attenuates glutamate transmission in the subiculum and CA1 regions of the hippocampus.Reference Boeijinga and Boddeke68, Reference Mlinar, Falsini and Corradetti69 The effect of 5-HT1B receptor modulation in behavioral models of memory and learning has been far less studied than that for 5-HT1A receptors (Table 2). In general, several studies suggest that 5-HT1B receptor stimulation may negatively affect performance in hippocampal-dependent memory tests. Antagonism of 5-HT1B receptors, in spite of its associated increase in extracellular ACh levels in the dorsal hippocampus,Reference Hu, Wang, Stenfors, Ogren and Kehr70 does not seem be effective in these models (Table 2).
5-HT2A and 5-HT2C receptors
5-HT2A receptors are broadly present within the hippocampus, but little is known about the expression of 5-HT2C receptors.Reference Bombardi71, Reference Cornea-Hebert, Riad, Wu, Singh and Descarries72 5-HT2A receptors are expressed on both principal glutamatergic cells (on their somatic and dendritic regions) and on all known subtypes of hippocampal interneurons (Table 1). There is also some evidence suggesting that 5-HT2A receptors are expressed on mossy fibers in the CA3 region.Reference Bombardi71, Reference Luttgen, Ove Ogren and Meister73 Thus, since 5-HT2A receptors are stimulatory and are expressed on both principal cells and GABAergic interneurons, it would be expected that they would have mixed effects on the firing of principal cells. However, data from 2 electrophysiological studies in brain slices suggest that the effects that 5-HT2 receptors have on interneurons might overwhelm their direct excitatory effects on principal cells.Reference Shen and Andrade74, Reference Wang and Arvanov75 Further research is needed to confirm these observations and clarify which of the 2 effects of 5-HT2 receptors (indirect inhibition or direct excitation of principal neurons) prevails in physiological conditions.
Studies of selective 5-HT2 receptor ligands in behavioral models show variable effects (Table 2), possibly reflecting differences in experimental design across studies and the fact that 5-HT2 receptors are expressed on multiple cell types in the hippocampus (Table 1). Little is known about the effects of 5-HT2C receptor modulation due to a lack of selective compounds. Thus, as with the 5-HT1A receptor, selective targeting of 5-HT2 receptors may not be an optimal strategy for modulating hippocampal function due to their varying functions in this brain region.
5-HT3 receptors
5-HT3 receptors are also found throughout the hippocampus.Reference Morales, Battenberg, de Lecea and Bloom76, Reference Morales, Battenberg, de Lecea, Sanna and Bloom77 Autoradiographic data from our laboratory suggest that 5-HT3 receptors have a distinct expression gradient within the hippocampus, with the highest expression observed in the caudal and ventral portions (Figure 2E). Interestingly, histological evidence in the rodent forebrain suggests that 5-HT3 receptors are almost exclusively expressed on GABAergic interneurons.Reference Bloom and Morales78, Reference Morales and Bloom79 5-HT3 receptor-expressing interneurons are generally immunopositive for cholecystokinin, and for the calcium binding proteins calretinin and calbindin.Reference Morales and Bloom79
Based on this histological evidence, it can be hypothesized that 5-HT3 receptors provide a fast excitatory drive onto hippocampal GABAergic interneurons and inhibit hippocampal principal cells. Consistent with this hypothesis, pharmacological activation of 5-HT3 receptors depolarizes hippocampal interneuronsReference Kawa80, Reference McMahon and Kauer81 and increases inhibitory drive onto CA1 pyramidal cells.Reference Dale, Zhang and Leiser82–Reference Turner, Mokler and Luebke85 Conversely, 5-HT3 receptor antagonists inhibit hippocampal interneurons, increase the firing rate of pyramidal cells, and enhance long-term potentiation (LTP) in in vivo electrophysiology recordings in rats.Reference Reznic and Staubli86–Reference Staubli and Xu88 Taken together, these mechanistic findings might point to a pro-cognitive effect of 5-HT3 receptor antagonism. However, behavioral studies of selective 5-HT3 receptor antagonists in models of memory and learning have again shown inconsistent results (Table 2).
5-HT4 receptors
Autoradiographic studies have demonstrated the presence of 5-HT4 receptors throughout the hippocampus.Reference Vilaro, Cortes and Mengod89, Reference Waeber, Sebben, Nieoullon, Bockaert and Dumuis90 In general, protein expression is low-to-moderate, with the highest levels found in the stratum oriens and pyramidale of Ammon’s horn, subiculum, and the molecular layer of the DG (Table 1). 5-HT4 receptor mRNA has been detected in hippocampal pyramidal cells.Reference Peñas-Cazorla and Vilaró91 Interestingly, 5-HT4 receptor mRNA was not found in cells expressing glutamic acid decarboxylase 65 (GAD65), which is thought to be a selective marker of GABAergic neurons.Reference Peñas-Cazorla and Vilaró91 Thus, it appears that 5-HT4 receptors preferentially act to stimulate pyramidal neurons, without directly modulating GABA neurotransmission. In support of this hypothesis, 2 electrophysiology studies have shown that stimulation of 5-HT4 receptors increases the excitability of CA1 pyramidal cells.Reference Chapin, Haj-Dahmane, Torres and Andrade92, Reference Mlinar, Mascalchi, Mannaioni, Morini and Corradetti93
5-HT4 receptors have been shown to modulate the cholinergic system. In microdialysis recordings, application of the 5-HT4 receptor agonist SC53116 causes a release of ACh, and this effect is blocked by the 5-HT4 receptor antagonist GR113808.Reference Matsumoto, Togashi and Mori94 Thus in theory, 5-HT4 receptor agonists should be pro-cognitive. This hypothesis has been investigated in preclinical models, but the results to date have been disappointing (Table 2).
5-HT5 receptors
Immunohistochemical expression studies have shown that 5-HT5 receptors are present in some portions of the hippocampus. For example, Oliver et al Reference Oliver, Kinsey, Wainwright and Sirinathsinghji95 observed moderate immunoreactivity levels in CA1, CA2, and CA3 regions and weak immunoreactivity levels in the DG. This study also reported that 5-HT5 receptors were present on pyramidal and granule principal cells and on interneurons in the DG.Reference Oliver, Kinsey, Wainwright and Sirinathsinghji95 Due to the lack of selective compounds, no mechanistic and behavioral studies targeting 5-HT5 receptors have been performed in rodents.
5-HT6 receptors
Histochemical studies suggest that 5-HT6 receptors are expressed at moderate-to-high levels in all subfields of the rodent hippocampus.Reference Kinsey, Wainwright, Heavens, Sirinathsinghji and Oliver96, Reference Gerard, Martres and Lefevre97 The expression is particularly strong in the molecular layer of the DG and in the stratum oriens and stratum radiatum of CA1, where 5-HT6 receptors are thought to be expressed on dendritic processes of pyramidal cells.Reference Gerard, Martres and Lefevre97 Moderate levels of 5-HT6 receptor immunoreactivity have been observed in CA2 and CA3 regions. There has been also one report showing the expression of 5-HT6 receptors on a subset of calretinin- and calbindin-positive hippocampal interneurons.Reference de Jong and Helboe98
Pharmacological stimulation of 5-HT6 receptors by the 5-HT6 receptor agonist WAY-181187 increases GABA transmission and attenuates LTP in the CA1 area of the hippocampus.Reference West, Marcy, Marino and Schaffhauser99 Both of these effects were blocked by the selective 5-HT6 receptor antagonists SB-399885.Reference West, Marcy, Marino and Schaffhauser99 Furthermore, systemic administration of WAY-181187 increases GABA levels in several brain regions, including the dorsal hippocampus.Reference Schechter, Lin and Smith100 Consistent with its enhancing effect on GABA transmission, antagonism of 5-HT6 receptors increases extracellular glutamate levels in the frontal cortex and dorsal hippocampus.Reference Dawson, Nguyen and Li101 However, although the potentiating effects of 5-HT6 receptors on GABA transmission have been well documented, it is not clear whether these responses are due to direct effects of 5-HT6 receptors on GABAergic interneurons.Reference Pehrson and Sanchez102
Several 5-HT6 receptor antagonists are currently in clinical development for the treatment of Alzheimer’s disease.Reference Wilkinson, Windfeld and Colding-Jorgensen103 However, in rodent hippocampus-dependent behavioral models, the effects of 5-HT6 receptor antagonists have been only investigated in a small number of studies with variable results (Table 2).
5-HT7 receptors
Immunohistochemical data suggest that 5-HT7 receptors are expressed throughout Ammon’s horn, especially on the soma and dendrites of pyramidal neurons.Reference Neumaier, Sexton, Yracheta, Diaz and Brownfield104 There is also weak 5-HT7 receptor expression in the DG.Reference Neumaier, Sexton, Yracheta, Diaz and Brownfield104 It is important to note that in the rodent brain expression of 5-HT7 receptors changes during development. It is the highest during the first 2 post-natal weeks and progressively decreases with age.Reference Garcia-Alcocer, Segura, Garcia Pena, Martinez-Torres and Miledi105–Reference Muneoka and Takigawa107 Our autoradiographic data using the selective 5-HT7 receptor antagonist [3H]SB269970 did not show strong binding in the hippocampal sections taken from adult rats (Figure 2G). This result raises questions regarding the level of expression of 5-HT7 receptors in the adult rodent hippocampus.
5-HT7 receptor stimulation increases the firing of pyramidal neurons and glutamatergic transmission in hippocampal brain slices.Reference Ciranna108–Reference Tokarski, Zahorodna, Bobula and Hess111 Stimulation of 5-HT7 receptors also enhances inhibitory transmission in the hippocampus.Reference Tokarski, Kusek and Hess112 This suggests that 5-HT7 receptors might be expressed on GABAergic interneurons; however, there are no histological data available to support this notion. In behavioral studies, both 5-HT7 agonists and antagonists have shown both memory-enhancing and memory-impairing properties depending on the animal model and test conditions (such as 5-HT tone) (Table 2).Reference Gasbarri and Pompili113, Reference Meneses114 Thus, additional research is needed to determine the role of 5-HT7 receptors on cognition and memory function.
Effect of 5-HT and SSRIs on CA1 Pyramidal Cells and Hippocampal Function
Given that most 5-HT receptors are expressed on both excitatory cells and inhibitory interneurons and can function in either a stimulatory or inhibitory manner depending on the receptor subtype, it would be expected that the net effect of 5-HT on hippocampal function depends on local 5-HT concentration, the ratio of different 5-HT receptor subtypes expressed, and the density of 5-HT receptors in a particular population of cells. In general, 5-HT inhibits CA1 pyramidal cells and thereby decreases hippocampal output in rodents (reviewed by CirannaReference Ciranna108). Stimulation of the serotonergic projection from the dorsal raphe nucleus to the hippocampus decreases the firing rate of CA1 pyramidal cells in anesthetized animals.Reference Chaput, Araneda and Andrade55, Reference Chaput, Lesieur and de Montigny115–Reference Segal118 In a similar manner, application of 5-HT to hippocampal brain slices inhibits the function of pyramidal neurons by hyperpolarizing their membrane potential and increasing local GABA transmission.Reference Andrade and Nicoll53, Reference Shen and Andrade74, Reference Passani, Pugliese, Azzurrini and Corradetti83–Reference Turner, Mokler and Luebke85 There have also been reports of excitatory effects of 5-HT on pyramidal cell function, but the magnitude of excitation was much smaller than the 5-HT-induced inhibition.Reference Andrade and Nicoll53, Reference Andrade and Chaput119 However, one has to be careful in interpreting the ex vivo results obtained in brain slices; in most of these studies, 5-HT was exogenously applied at moderately high concentrations (15–50 micromolar), which might be higher than physiologically relevant concentrations of 5-HT in the brain. Therefore, these studies might exaggerate a contribution of certain subtypes of 5-HT receptors to its overall response. In summary, it seems that the overall effect of 5-HT on the hippocampal circuit in rodents is to inhibit pyramidal cell output. However, a majority of the studies that have led to this conclusion were either done in anesthetized animals or in brain slice preparations, and conclusions from such studies should be therefore interpreted with caution.
The inhibitory effect of 5-HT in the hippocampus is mediated via its actions on 5-HT1A, 5-HT1B, 5-HT2A/5-HT2C, 5-HT3, 5-HT6, and possibly 5-HT7 receptors.Reference Andrade and Nicoll53–Reference Chaput, Araneda and Andrade55, Reference Shen and Andrade74, Reference McMahon and Kauer81–Reference Ropert and Guy84, Reference West, Marcy, Marino and Schaffhauser99, Reference Tokarski, Kusek and Hess112, Reference Piguet and Galvan120 Activation of 5-HT1A receptors has a direct inhibitory effect on pyramidal cell firing by hyperpolarizing their membrane potential via activating a potassium conductance.Reference Andrade and Nicoll53–Reference Chaput, Araneda and Andrade55 Other 5-HT receptors subtypes decrease the activity of pyramidal cells indirectly by mainly activating interneurons and enhancing GABA transmission onto pyramidal cells.Reference Shen and Andrade74, Reference McMahon and Kauer81–Reference Ropert and Guy84, Reference West, Marcy, Marino and Schaffhauser99, Reference Tokarski, Kusek and Hess112, Reference Piguet and Galvan120 Multiple synaptic connections from hippocampal interneurons onto pyramidal cells might further amplify the inhibitory effect of 5-HT in the hippocampus.Reference Pehrson and Sanchez102
SSRIs, which act through the inhibition of the 5-HT transporter, are among the most studied serotonergic agents. Microdialysis studies in rodents have shown that systemic administration of SSRIs rapidly enhances extracellular 5-HT concentrations in multiple brain regions, including the ventral hippocampus.Reference Cremers, Rea and Bosker121–Reference Hervas and Artigas123 Although SSRIs are selective for the serotonergic system, they do not show selectivity for 5-HT receptor subtypes and could, in theory, simultaneously activate all 5-HT receptors. However, since 5-HT receptors have different affinities for 5-HT, with 5-HT1A receptors being the most sensitive type (Table 1), local 5-HT concentrations in the brain would determine which 5-HT receptor subtypes become engaged upon SSRI treatment. In addition, chronic treatment with SSRIs, which is often required to achieve clinical efficacy, can desensitize and change expression patterns of 5-HT receptor subtypes.Reference Le Poul, Boni and Hanoun124 For instance, chronic treatment with paroxetine desensitizes presynaptic 5-HT1A autoreceptors in the raphe nuclei, which leads to increased serotonergic transmission.Reference Blier, de Montigny and Chaput125, Reference Chaput, de Montigny and Blier126
In electrophysiology studies, the effect of SSRIs in the hippocampus has been mostly studied in relationship to hippocampal LTP, which is thought to be important for learning and memory.Reference Malenka and Nicoll127 In a majority of studies in normal animals, both the application of 5-HT and acute and chronic treatments with SSRIs inhibit hippocampal LTP.Reference Staubli and Otaky87, Reference Corradetti, Ballerini, Pugliese and Pepeu128–Reference Stewart and Reid132 Interestingly, exposure to stress also impairs LTP in the CA1 and DG regions of the hippocampus (reviewed by Pittenger et al Reference Pittenger and Duman133 and Popoli et al Reference Popoli, Gennarelli and Racagni134). Chronic treatments with SSRIs can reverse these stress-induced deficits in LTP,Reference Holderbach, Clark, Moreau, Bischofberger and Normann135, Reference Matsumoto, Tachibana and Togashi136 which suggests that SSRIs can restore hippocampal function in disease-like conditions. The positive effects of SSRIs are thought to be mediated, at least in part, by increasing the expression of brain-derived neurotrophic factor (BDNF) and neurogenesis in the hippocampal and cortical circuits.Reference O’Leary and Cryan23, Reference Pittenger and Duman133
Consistent with the hypothesis that SSRIs might stimulate multiple hippocampal 5-HT receptors expressed on different cell types, their net effects in behavioral cognition models have been limited and variable. The clinical experience with SSRIs is aligned with the preclinical data. Thus, while fMRI studies reveal that the neural systems important for emotional processing are adequately normalized by SSRIs in the treatment of depression,Reference Godlewska, Norbury, Selvaraj, Cowen and Harmer137 SSRIs are unable to correct the over-activation of the frontolimbic circuitry important for the non-emotional cognition.Reference Walsh, Williams and Brammer11 Although there are studies showing that SSRIs can remediate “hippocampal-related” cognitive deficits in patients with depression,Reference Vythilingam, Vermetten and Anderson19 a recent study by Herzallah et al Reference Herzallah, Moustafa and Natsheh138 indicates that SSRIs can also impair hippocampus-dependent generalization of past learning to novel contexts.
In conclusion, the regulation of hippocampal function by 5-HT is complex, involving multiple receptor subtypes and diverse expression patterns of 5-HT receptors on principal glutamatergic cells and GABAergic interneurons. Thus, administration of an SSRI may not be a rational approach to achieve enhanced hippocampal output and subsequent improvement of cognitive function in patients with MDD due to the potential of activation of multiple receptor subtypes, which may have opposing effects on cell function. On the other hand, targeting a single 5-HT receptor subtype may not be a viable strategy either due to redundancies in the serotonergic system. The consequences of modulating one 5-HT receptor may be attenuated by effects through other 5-HT receptor subtypes. Drugs designed to target single 5-HT receptors have so far not yielded new pharmacological treatments. For example, the selective 5-HT1A receptor agonist flesinoxan was under development for the treatment of generalized anxiety disorder for many years, but its clinical program was stopped in the late 1990s after it failed to show efficacy in 2 large phase-3 clinical trials.Reference Olivier139 The 5-HT1B/1D receptor antagonist elzasonan (CP-448187) was recently under development for the treatment of MDD and was tested in several phase-2 clinical trials, but its development program was also discontinued.Reference Kirchhoff, Nguyen, Soczynska, Woldeyohannes and McIntyre140 An alternative approach to targeting a single receptor subtype could be to target a combination of 5-HT receptor subtypes that would work in a concerted manner. The multimodal antidepressant vortioxetine is an example of such an approach.
Effects of the Multimodal Antidepressant Vortioxetine on Hippocampus Function
Vortioxetine is a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist, a 5-HT1B receptor partial agonist, a 5-HT1A receptor agonist, and a SERT inhibitor in cellular assays.Reference Westrich, Pehrson and Zhong141, Reference Bang-Andersen, Ruhland and Jorgensen142 Vortioxetine has been approved for the treatment of MDD in the US, the EU, Australia and several other countries. Furthermore, clinical studies with cognitive outcome measures have shown that vortioxetine significantly improves cognitive function in MDD patients compared with placebo treatment. The efficacy of vortioxetine on cognitive function has been demonstrated in 3 randomized, double-blinded, placebo-controlled studies in MDD patients.Reference Katona, Hansen and Olsen143–Reference Mahableshwarkar, Zajecka, Jacobson, Chen and Keefe145 One clinical trial was conducted in elderly MDD patients with cognition as a secondary pre-defined outcome and included 128 patients in the placebo group, 136 patients in the vortioxetine-treated group, and 128 patients in the duloxetine-treated group.Reference Katona, Hansen and Olsen143 The other 2 clinical trials were designed to compare the efficacy of vortioxetine to that of placebo on cognitive function as the primary efficacy outcome and on depressive symptoms as the secondary efficacy outcome.Reference McIntyre, Lophaven and Olsen144, Reference Mahableshwarkar, Zajecka, Jacobson, Chen and Keefe145 The study by McIntyre et al Reference McIntyre, Lophaven and Olsen144 included 196 patients in the placebo group, 195 patients in the 10 mg vortioxetine group, and 207 patients in the 20 mg vortioxetine group. The study by Mahableshwarkar et al Reference Mahableshwarkar, Zajecka, Jacobson, Chen and Keefe145 had 194 patients in the placebo group, 198 patients in the vortioxetine-treated group, and 210 patients in the duloxetine-treated group. These clinical studies demonstrated that vortioxetine improves objective measures of processing speed, executive function, attention and learning, and memory, including hippocampus-dependent memory measures.Reference Katona, Hansen and Olsen143, Reference McIntyre, Lophaven and Olsen144, Reference Mahableshwarkar, Zajecka, Jacobson, Chen and Keefe145 Path analyses suggested that the effect on cognitive function was largely independent of its effect on improvements in mood symptoms, supporting the hypothesis that these domains do not necessarily track together. Furthermore, an fMRI study showed that vortioxetine reduced neural activity in the left hippocampus during a working memory task in patients remitted from depression.Reference Browning, Smith and Conen146 This indicates that vortioxetine, unlike SSRIs, might restore compensatory over-activation in the hippocampus by increasing neural efficiency.Reference Walsh, Williams and Brammer11
Consistent with clinical findings, vortioxetine has shown antidepressant as well as pro-cognitive activities in a number of preclinical animal models.Reference Sanchez, Asin and Artigas147 Furthermore, in several behavioral and mechanistic studies that engaged hippocampal and cortical circuitry, vortioxetine’s effects differentiated from those of SSRIs and SNRIs.Reference Sanchez, Asin and Artigas147 In the following paragraphs, we review these preclinical results and discuss how vortioxetine might modulate hippocampal function and affect hippocampus-dependent cognitive behaviors in rodents.
Acute and chronic treatments with vortioxetine increase extracellular 5-HT levels in the rat ventral hippocampus to a much greater extent than those observed with SSRIs.Reference Mork, Pehrson and Brennum148, Reference Pehrson, Cremers and Betry149 Interestingly, combining an SSRI with the 5-HT3 receptor antagonist ondansetron resulted in a similar potentiating effect on 5-HT levels.Reference Mork, Pehrson and Brennum148 This suggests that the effect of vortioxetine was at least partially due to its 5-HT3 receptor antagonism. Since 5-HT3 receptors are expressed on GABAergic neurons,Reference Morales, Battenberg, de Lecea and Bloom76, Reference Puig, Santana, Celada, Mengod and Artigas150 it was hypothesized that vortioxetine, through its blockade of 5-HT3 receptors, reduces GABA release and thereby attenuates the inhibitory effect that GABA exerts on 5-HT release in the hippocampus.Reference Riga, Celada, Sanchez and Artigas151 Recent data by Riga et al Reference Riga, Celada, Sanchez and Artigas151 support this hypothesis. In their study, local application of ondansetron to the ventral hippocampus augmented the effect of the SSRI escitalopram on increasing extracellular 5‐HT levels. This effect was reversed by the local application of the GABAB receptor agonist baclofen, which restored GABAB receptor tone in the hippocampus.Reference Riga, Celada, Sanchez and Artigas151 Locally applied baclofen also attenuated the potentiating effect of vortioxetine on extracellular 5‐HT. Taken together, these results indicate that 5-HT3 receptor antagonism plays a prominent role in the vortioxetine’s effect on 5-HT levels in the hippocampus.
Although 5-HT3 receptor antagonism is important in the pharmacology of vortioxetine, contributions from its other receptor activities cannot be ruled out. For instance, an in vivo electrophysiology study of pyramidal neurons in the CA3 area of the hippocampus by El Mansari et al Reference El Mansari, Lecours and Blier152 showed that vortioxetine acts as a partial agonist of 5-HT1B receptors and can function as either an agonist or an antagonist depending on the endogenous 5-HT tone. Vortioxetine enhanced the inhibitory effect of the stimulation of the 5-HT bundle at a high, but not at a low frequency, and reversed the inhibitory effect of the 5-HT1B receptor agonist CP 94253.Reference El Mansari, Lecours and Blier152 Thus, vortioxetine also modulates the intra-hippocampal circuitry through its effects at 5-HT1B receptors. 5-HT1B receptors are densely expressed in the subiculum, the main output area of the hippocampus (Figure 2C), and are believed to play an important role in memory function.Reference O’Mara, Sanchez-Vives, Brotons-Mas and O’Hare153, Reference Sari154 Thus, vortioxetine, through its partial agonism at 5-HT1B receptors, might have a positive outcome on memory processing. Additional studies are needed to confirm this hypothesis, as well as to test the potential role of vortioxetine’s other receptor activities on hippocampal function.
Acute and sub-chronic treatments with vortioxetine also increase extracellular levels of NE and HA in the ventral hippocampus.Reference Pehrson, Cremers and Betry149, Reference Smagin, Song, Budac, Pehrson, Li and Sanchez155 Increases in DA and ACh levels have also been observed, but only after the acute treatment.Reference Mork, Pehrson and Brennum148, Reference Pehrson, Cremers and Betry149 Furthermore, a study in rat hippocampal slices showed that vortioxetine disinhibited CA1 pyramidal neurons in response to 5-HT, again most likely through its 5-HT3 receptor antagonism, whereas escitalopram had no effect on this measure.Reference Dale, Zhang and Leiser82 In line with these findings, several results indicate that vortioxetine promotes glutamate-dependent neuronal plasticity in the hippocampus to a greater degree than SSRIs. For example, vortioxetine, unlike escitalopram, produced a significant increase in LTP in rat hippocampal slices.Reference Dale, Zhang and Leiser82 Furthermore, in mice aged 12 months, chronic treatment with vortioxetine activated neuronal plasticity–related genes and improved hippocampus-dependent, visual-spatial memory deficits, whereas fluoxetine had no effect on these readouts.Reference Li, Abdourahman and Tamm156 In another study performed in rats, vortioxetine increased cell proliferation in the hippocampal DG faster than fluoxetine (3 days for vortioxetine compared to 10 days for fluoxetine).Reference Bétry, Etiévant, Pehrson, Sanchez and Haddjeri157 Vortioxetine also produced a larger degree of hippocampal dendritic branching than fluoxetine after 2 weeks of dosing in mice.Reference Guilloux, Mendez-David and Pehrson158 In line with these mechanistic data, vortioxetine showed pro-cognitive effects in hippocampus-dependent cognition models in rodents, such as footshock-induced fear conditioning and spontaneous alternation (Table 2). However, these findings are relatively recent, and vortioxetine has been studied less than other serotonergic drugs and receptors. For instance, there have been only 3 behavioral studies on the effects of vortioxetine on hippocampal-dependent memory versus 16 studies on 5-HT1A receptors and 10 studies on 5-HT2 receptors with different pharmacological and genetic approaches (Table 2). Thus, confirmation and expansion of these results are important.
Taken together, vortioxetine’s effects in the hippocampus support the notion that its antidepressant activities and pro-cognitive effects are mediated, at least to some extent, through increased glutamate neurotransmission and increased neuroplasticity. It is important to note that although increased glutamate neurotransmission is thought to favor neuronal plasticity, it is also clear that excessive glutamate release (for instance, in relation to stress) can be neurotoxic (reviewed in Sanacora and BanasrReference Sanacora and Banasr159 and Sanacora et al Reference Sanacora, Treccani and Popoli160). Vortioxetine’s effects on glutamate are limited to enhanced neuronal function. This has been shown in microdialysis studies, where the treatment with vortioxetine did not result in measureable changes in extracellular glutamate in the ventral hippocampus and prefrontal cortex.Reference Pehrson and Sanchez161
In conclusion, vortioxetine’s combined inhibition of 5-HT reuptake and 5-HT receptor modulation results in a differentiated effect on hippocampus function and hippocampal-dependent behavior compared to SSRIs. The full implication of vortioxetine’s modulation of multiple neurotransmitter systems on its antidepressant and pro-cognitive potential is complex and remains to be elucidated in future studies.
Overall Conclusion and Future Directions
There is considerable evidence to support the notion that the hippocampus plays an important role in emotional and cognitive processing, and that both of these functions are affected in patients with MDD. SSRIs and SNRIs are the predominant pharmacotherapies for treating MDD, and their enhancing effects on 5-HT levels are believed to be important for their therapeutic efficacy. However, the biological processes that lead to the recovery from the depressive state remain poorly understood. Furthermore, despite several decades of extensive research, the role of 5-HT in regulating hippocampal function in normal or disease states is not well understood, probably due to the high degree of complexity of the serotonergic system.
Multiple classes of 5-HT receptors are often co-expressed on the same cell types with functions that can either be complementary or opposing, and little is known about the interactions between different 5-HT receptors subtypes. Furthermore, the majority of 5-HT receptors in the hippocampus are found on both principal glutamatergic cells and GABAergic interneurons. The 2 known exceptions are the 5-HT3 receptor subclass, which has only been found on interneurons, and the 5-HT4 receptor subclass, which has only been found on pyramidal cells. 5-HT3 receptors are also the only non–G-protein-coupled receptors that function as a ligand-gated ion channel. The impact of the unique expression pattern and effector system of the 5-HT3 receptor remains to be elucidated. However, given the key role that 5-HT3 receptor antagonism appears to have in mediating the pharmacological effects of vortioxetine, at least in preclinical behavioral, electrophysiology, and microdialysis studies,Reference Mork, Pehrson and Brennum148, Reference Bétry, Etiévant, Pehrson, Sanchez and Haddjeri157, Reference Bétry, Pehrson, Etiévant, Ebert, Sánchez and Haddjeri162 this receptor subtype might have an important role in the hippocampus. 5-HT4 receptors have been less studied, and understanding their function in the hippocampus remains to be elucidated in further detail.
The effect of SSRIs on hippocampal function remains poorly defined. While SSRIs have the potential to normalize hippocampal plasticity under stress conditions and to treat mood symptoms in MDD, their effects on cognitive function are less clear. Since SSRIs do not possess selectivity for 5-HT receptor subtypes, their efficacy might be weakened due to opposing activities of different 5-HT receptor subtypes. In this context, multitarget drugs or combination therapies might be a better strategy to modulate both emotional and cognitive processes in the hippocampus. Future insights into interactions between different serotonergic subtypes might therefore lead to novel treatment options for the treatment of MDD.
Disclosures
All authors are full-time employees of H. Lundbeck A/S.