Dementia is a major, growing public health problem and, with disappointing results from treatment-orientated studies, identifying risk factors for primary prevention is key.Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames1 Whereas genetic risk and lifestyle factors are important in the development of dementia, there is evidence that environmental factors may also play a role.Reference Russ, Gatz, Pedersen, Hannah, Wyper and Batty2–Reference Cacciottolo, Wang, Driscoll, Woodward, Saffari and Reyes5 One potentially important environmental risk factor is drinking water quality. Aluminium can occur naturally in water but is also widely used in water treatment, including in Scotland.6, 7 Aluminium has a wide variety of neurotoxic effects and there is some evidence supporting aluminium influencing β-amyloid oligomerization;Reference Kawahara and Kato-Negishi8 it has not been linked with other health outcomes.7 In terms of epidemiological evidence, only one longitudinal study has investigated aluminium in drinking water and dementia,Reference Rondeau, Jacqmin-Gadda, Commenges, Helmer and Dartigues9 although a number of cross-sectional studies – many small in size – make up a complex literature:Reference Killin, Starr, Shiue and Russ4 seven studies found a positive association between aluminium levels in drinking water and dementia and five found no association or conflicting findings. Furthermore, fluorine can increase aluminium absorption from drinking water because aluminium fluoride has greater bioavailability.Reference Lubkowska, Zyluk and Chlubeka10 Fluoride occurs naturally in water and is not added in Scotland.6 High levels of fluoride affects skeletal tissues and possible links with cancer have been identified, but low levels of intake protect against dental caries.7 Accordingly, the present study aims to explore the association between aluminium and fluoride levels in drinking water with dementia in a large longitudinal study in Scotland where such levels are below levels considered acceptable according to guidelines (the mean aluminium level in Scotland is 37.4 µg/L compared to regulatory limits in Scotland, which are <200 µg/L; the mean fluoride [F−] level in Scotland is 53.4 µg/L compared to the World Health Organization fluoride [F] guideline value, which is <1500 µg/L). This is the largest longitudinal study on the topic to date, and the first to explore aluminium and fluoride together.
Method
Study sample
This study used data from the Scottish Mental Survey 1932 (SMS1932); in this almost all people born in 1921 and at school in Scotland in June 1932 took part in a comprehensive national intelligence test at a mean age of 11 years, as previously described in detailReference Deary, Whalley and Starr11; 5.6% of participants did not take part in the test because they were absent from school but were still recorded in the test ledgers without a test score. The intelligence test was the Moray House Test no. 12, from which an IQ score was derived, corrected for age in days at the time of testing. Participants have been passively followed up into later life by anonymised probabilistic record linkage to hospital admissions and death certificate data, as described in detail in a previous report.Reference Russ, Gatz, Pedersen, Hannah, Wyper and Batty2 Approximately 43% of the 86 250 test participants (apart from those living in the counties of Angus, Fife and Wigtown; SMS1932 ledgers for these locations have been lost) were identified in later life. Dementia cases were identified by any mention of ICD-9 codes 290.0–290.4, 290.8, 290.9, 291.1, 291.2, 294.1, 294.2, 294.8, 294.9, and 331.0–331.912 and ICD-10 codes F00-F05.1, F09, G30, and G3113 recorded on electronic medical records or death certificates after 2004. A subsample was also identified from primary care records, specifically the Greater Glasgow & Clyde Nursing Homes Medical Practice, which exclusively treated residents of nursing homes. An individual's residential location (postcode sector) was recorded from the record that first mentioned dementia for those who developed this condition. For those who did not develop dementia, their residential location was recorded from the first record after the age of 60 years (the earliest possible because of the electronic medical records), which may have been at death. We excluded individuals who died before the exposure period, which began in 2005. This study received ethical approval from the Scotland A Research Ethics Committee (10/S1103/6). Collecting individual informed consent from participants was not feasible as this was a retrospective study design.
Environmental data
Water quality data were obtained from the Drinking Water Quality Regulator for Scotland (DWQR) for the years 2005–2014. The DWQR is responsible for regulating public water supplied by Scottish Water. Prior to the establishment of Scottish Water in 2002, monitoring water quality was the responsibility of separate local authorities. Aluminium and fluoride levels in drinking water (micrograms per litre) were extracted from the database.
Sampling sites were identified by longitude and latitude and were widely distributed across Scotland, particularly where the population is more concentrated (Supplementary Fig. 1 available at https://doi.org/10.1192/bjp.2018.287). Of 50 378 aluminium and 15 808 fluoride sampling sites, the precise location of the site was missing for 1128 and 556 locations, respectively. These sites were assigned the location from the closest row above in the database. Supplementary Table 1 shows the number of sampling sites for aluminium and fluoride in each year (2005–2012) and summarises the levels of these substances in drinking water in Scotland.
We used the idw() function from the gstat package for R for Windows, version 3.4.3, to interpolate values for aluminium and fluoride, using inverse distance weighting across a spatial grid with spacing of 0.1 degrees of longitude or latitude for each year separately. This allowed us to estimate values for areas where no measurements had been made. The mean values for each grid area within every postcode sector in Scotland were then calculated (again for each year separately), which were assigned to each individual based on their residential location. We used each individual's age to create dummy variables indicating whether they were alive for any of each of the years from 2005 to 2012. These dummy variables were then used to calculate a ‘personal’ mean value for aluminium and fluoride, using only data from the years in which the individual was alive to be exposed. For example, an individual who died at 88 years of age would have been alive in the years 2005–2009 inclusive; data from 2010–2012 would be ignored for this person. These ‘personal’ mean values were standardised and centred on zero such that a unit increase indicated 1 s.d. increase in the original scale (10.0 µg/L for aluminium and 16.0 mg/L for fluoride). We also calculated quartiles of aluminium and fluoride values to allow us to examine the shape of any association identified.
Socioeconomic data
To account for socioeconomic position, we obtained Scottish Index of Multiple Deprivation (SIMD), which provides a relative measure of deprivation by small area (datazone, of which there are 6505 in Scotland in the 2012 data we used) and incorporates the following domains: employment; income; health; education, skills and training; geographic access to services; crime; and housing.14 Each individual was assigned a rank based on the data-zone in which they lived, either at the time dementia was first mentioned or the first record after the age of 60 years.
Statistical modelling
After confirming that the proportional hazards assumption was valid using the cox.zph() function from the survival package in R (all P > 0.1), we constructed Cox proportional hazards models for the association between aluminium and fluoride levels in drinking water with dementia in men and women separately. Age in years over the age of 84 years was the timescale and all models were additionally adjusted for IQ at age 11 years because this has also been linked with dementia risk in this cohort.Reference Doubal, Ali, Batty, Charidimou, Eriksdotter and Hofmann-Apitius15 We made the decision to analyse separately by gender, despite preliminary analyses suggesting there was no statistical interaction by gender (P > 0.5), because of evidence that the pattern of geographical variation in dementia risk varies between men and women (Supplementary Fig. 2).Reference Russ, Gatz, Pedersen, Hannah, Wyper and Batty2 We conducted a sensitivity analysis, additionally adjusting the above models for SIMD rank. We additionally constructed a joint model investigating for a statistical interaction between aluminium and fluoride. Maps were produced in R, using the ggplot2 package.
Results
The sample comprised 19 272 men and 18 325 women, but 4408 men and 3446 women were missing residential location in later life. Men were overrepresented in those missing residential location (χ1 = 93.8, P < 0.001) but, although statistically significant because of to the large sample size, individuals with missing residential location scored only 0.9 IQ points lower than those who had location data (P < 0.001). Given the known effect size of IQ in relation to dementia, a difference of 0.06 s.d. is unlikely to be important.Reference Doubal, Ali, Batty, Charidimou, Eriksdotter and Hofmann-Apitius15 A further 9536 men and 7984 women died before the monitoring period began in 2005, and 2600 men and 2633 women were missing childhood IQ. This resulted in an analytic sample of 2728 men and 4262 women alive in 2005, of whom 622 men and 1350 women were identified as having subsequently developed dementia. All participants were approximately 84 years old at the start of the exposure period and were followed up for a mean of 2.7 (s.d. 2.1, range 0–7) years.
Levels of aluminium and fluoride in drinking water derived from DWQR data are shown in Supplementary Table 1 and Fig. 1. Mean aluminium levels in drinking water in participants were 37.4 µg/L (s.d. 10.0, range 10.5–92.8) and mean fluoride levels were 53.4 µg/L (s.d. 16.0, range 23.8–181.1).
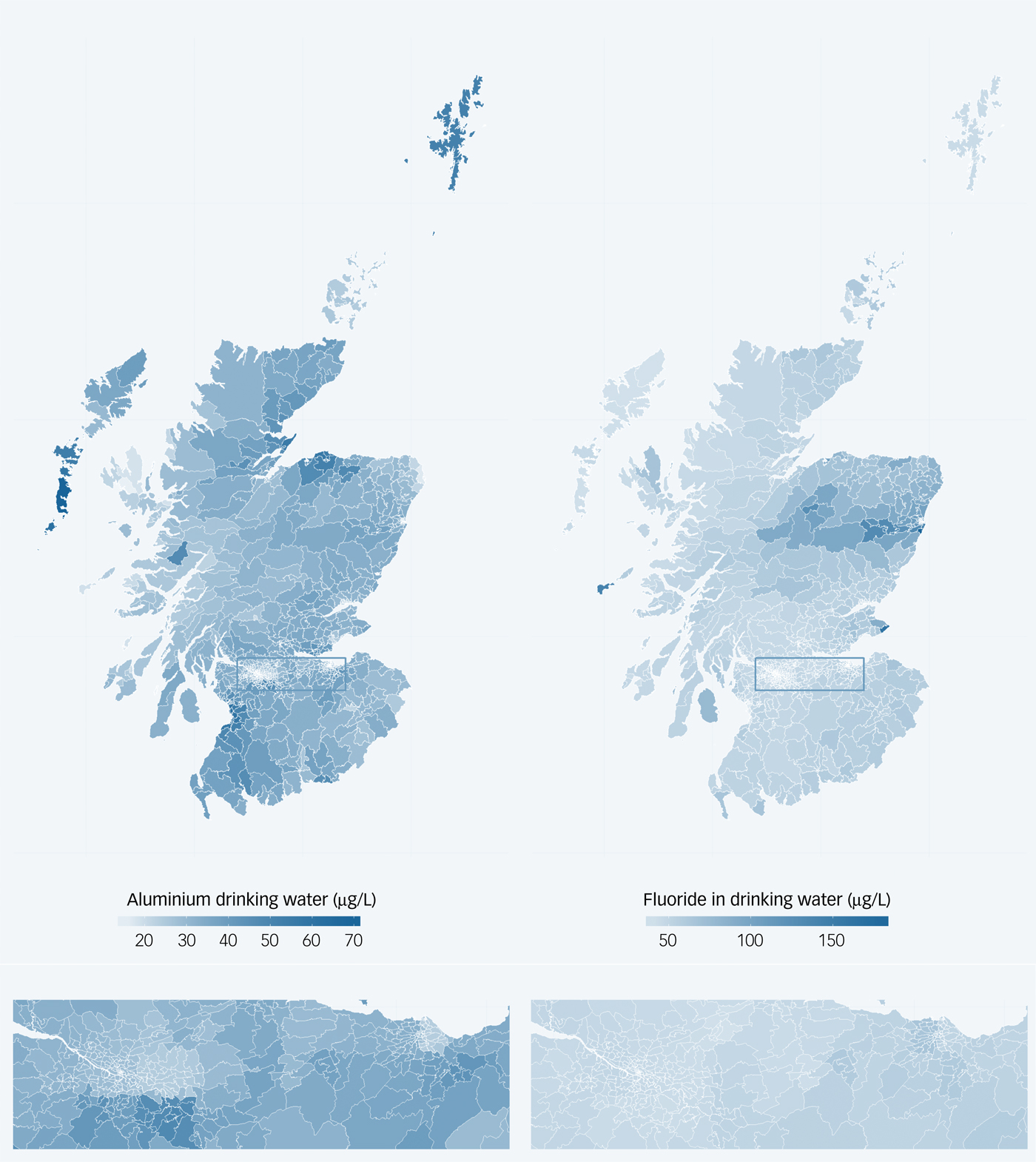
Fig. 1 Maps indicating mean levels of aluminium (left) and fluoride (right) in drinking water in Scotland, 2005–2012. Lower panel shows an enlarged view of the Central Belt of Scotland including Glasgow and Edinburgh.
Table 1 and Fig. 2 show the results of the Cox proportional hazards models. Higher mean aluminium levels in drinking water were associated with an increased risk of dementia in women (adjusted hazard ratio per s.d. increase 1.09, 95% CI 1.03–1.15, P < 0.001) and men (adjusted hazard ratio per s.d. increase 1.12, 95% CI 1.03–1.21, P = 0.004). Dementia risk was increased in all quartiles compared with the lowest, but Fig. 2 suggests no dose-response pattern of association. Higher mean fluoride levels in drinking water were associated with an increased risk of dementia in women (adjusted hazard ratio per s.d. increase 1.34, 95% CI 1.28–1.41, P < 0.001) and men (adjusted hazard ratio per s.d. increase 1.30, 95% CI 1.22–1.39, P < 0.001) in a stepwise pattern. Dementia risk was more than doubled in this highest fluoride quartile compared with the lowest. Similar to our previous report linking early-life cognition with dementia, the aluminium-adjusted hazard ratio of dementia per one s.d. decrease in IQ at age 11 years was 1.10 (95% CI 1.04–1.16) in women and 1.00 (95% CI 0.92–1.08) in men.Reference Russ, Hannah, Batty, Booth, Deary and Starr16 Living in the most deprived 15% of areas (in late-middle age or later life) was not associated with an increased risk of dementia compared with the least deprived 85% in women (aluminium-adjusted hazard ratio 0.99, 95% CI 0.86–1.14, P = 0.89) or men (aluminium-adjusted hazard ratio 0.93, 95% CI 0.75–1.16, P = 0.53). Adjusting for SIMD rank in addition to IQ at age 11 years did not alter our conclusions (Supplementary Table 2). There was no statistical interaction between aluminium and fluoride levels when both were included in a model together.
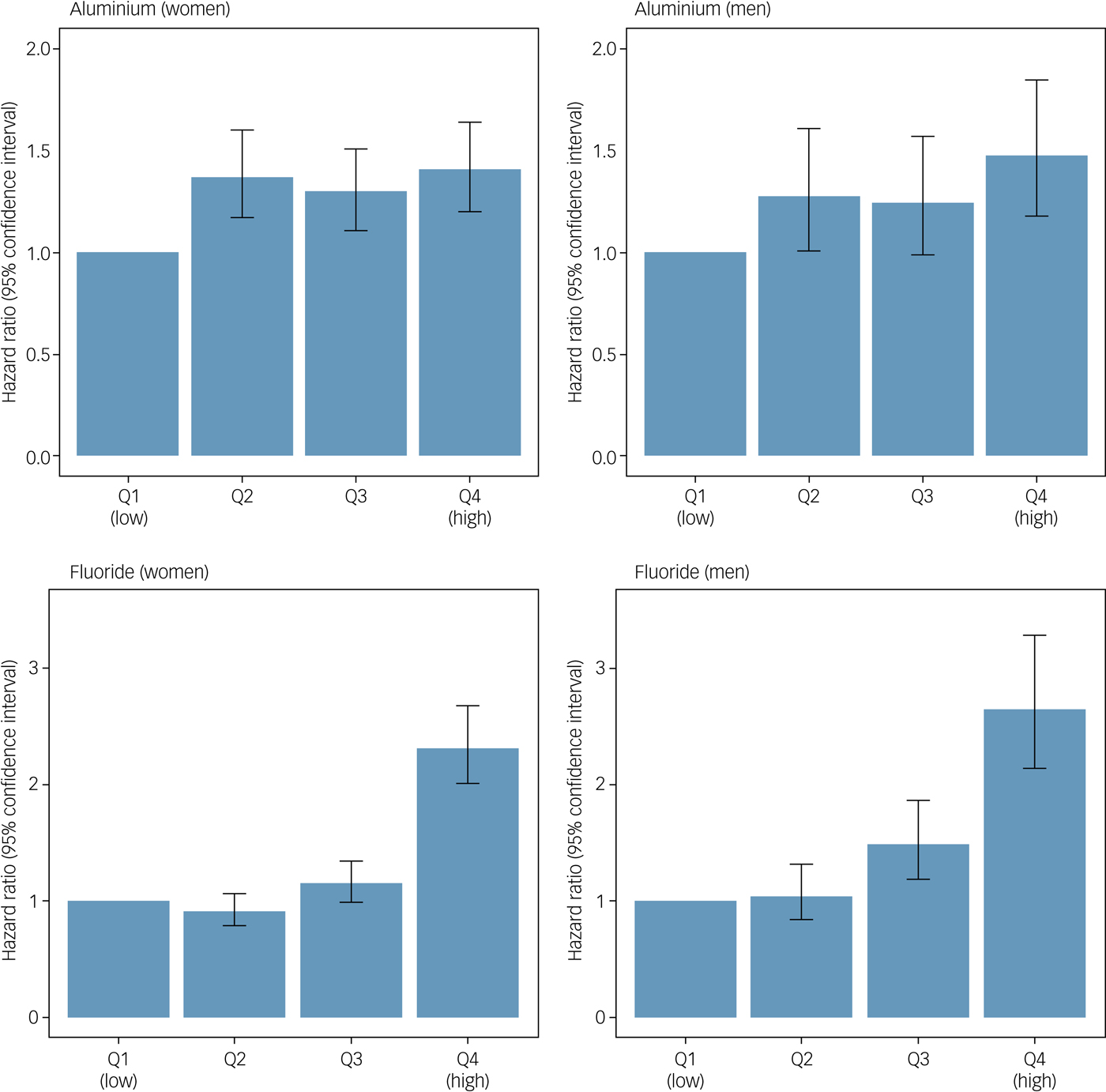
Fig. 2 IQ-adjusted hazard ratios and accompanying 95% confidence intervals for the association between mean aluminium and fluoride levels in drinking water and dementia in men and women. Q, quartile.
Table 1 Adjusted hazard ratios and accompanying 95% confidence intervals for the association between mean aluminium and fluoride levels in drinking water and dementia in men and women
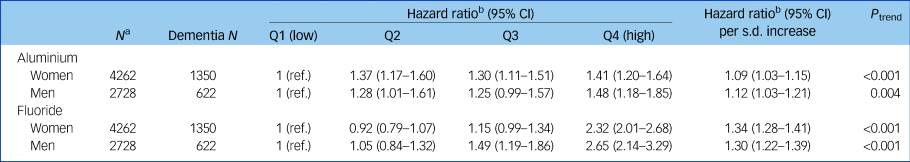
Cut-off points for aluminium quartiles (Q) were 30.8, 35.5 and 41.1 µg/L. Cut-off points for fluoride quartiles were 44.4, 48.7 and 56.3 µg/L.
a. Scottish Mental Survey 1932 participants who survived to 2005 (the start of the exposure period).
b. Hazard ratio adjusted for age 11 mental ability.
Discussion
We observed an association between the mean levels of aluminium and fluoride in drinking water and risk of dementia in women and men, but found no evidence for an interaction between the two. The pattern of association was different with evidence of a dose-response association for fluoride in women and men, but a flatter association of raised risk in all quartiles compared with the lowest quartile of aluminium concentrations. Further adjusting for area-level deprivation did not affect our results.
Comparison with the literature
The link between aluminium and dementia has a long and controversial history. The World Health Organization has stated that ‘[o]n the whole, the positive relationship between aluminium in drinking-water and Alzheimer's disease, which was demonstrated in several epidemiological studies, cannot be totally dismissed’ (p. 312).7 This report highlighted confounding and aluminium intake from other sources (aluminium from drinking water is only about 5% of total intake) as being important factors not frequently dealt with in the literature. We recently reviewed the literature on environmental risk factors for dementia, including aluminium in a variety of forms.Reference Killin, Starr, Shiue and Russ4 This review identified one cohort study and 12 cross-sectional analyses investigating the relationship between aluminium in drinking water and dementia. The only study assigned a high quality rating (1925 participants) found that consumption of aluminium in drinking water in excess of 0.1 mg per day doubled an individual's risk of dementia (n = 461) and tripled their risk of Alzheimer's disease (n = 364).Reference Rondeau, Jacqmin-Gadda, Commenges, Helmer and Dartigues9 With 6990 participants, of whom 1972 developed dementia, our study is substantially larger. The remaining studies (all cross-sectional) showed varying results (seven positive, five no effect), but a trend was noted that larger studies were more likely to observe a positive association between aluminium levels and dementia risk. The four studies identified in the review that examined occupational exposure to aluminium were generally small and gave mixed results. Accidental contamination of drinking water with aluminium sulphate caused cerebral dysfunction which adds weight to the possibility that lower levels of aluminium may carry a health risk.Reference Altmann, Cunningham, Dhanesha, Ballard, Thompson and Marsh17
Only one study was identified which investigated the association between fluoride and dementia.Reference Still and Kelley18 This cross-sectional US study linked annual county-level incidence of dementia (calculated from 160 hospital case records) to fluoride concentrations in public water supplies. In contrast to the direction of our findings, the county with the highest levels of fluoride in drinking water (4.18 mg/L) had the lowest annual incidence of dementia.
The levels of both aluminium and fluoride measured in Scotland are relatively low compared to the guidelines set by the World Health Organization. Therefore, the fact that we nevertheless observed a dose-response association between aluminium and fluoride levels in drinking water and dementia risk that was not explained by childhood IQ or area-level deprivation is particularly interesting. This suggests that there may be no safe levels of these substances when it comes to dementia risk.
The mechanisms of aluminium-related neurotoxicity are multiple and complex, but oxidative stress has been highlighted as being of particular importance.Reference Verstraeten, Aimo and Oteiza19
Limitations and strengths of the study
There are a number of limitations to this study that must be borne in mind, several of which have been discussed previously.Reference Russ, Gatz, Pedersen, Hannah, Wyper and Batty2, Reference Doubal, Ali, Batty, Charidimou, Eriksdotter and Hofmann-Apitius15 First, the linkage to electronic medical and mortality records was incomplete because of emigration, death before the start of the electronic records, and the probabilistic methodology used; less than half of the original 87 498 participants in the SMS1932 were identified in later life (43%). However, this compares favourably with the response in similar studies, for example 56% in Cognitive Function and Ageing Study-II.Reference Matthews, Arthur, Barnes, Bond, Jagger and Robinson20 Comparing IQ scores – the only baseline data available – in those who were traced and those who were not revealed only trivial differences.
Second, the dementia outcome is open to criticism. It was not feasible to follow up thousands of participants across the whole of Scotland and so ‘passive’ anonymised follow-up by record linkage was used. However, this relies on the accuracy and completeness of the records used and we have examined these in these Scottish data. The mortality data alone missed approximately 28% of people with a robust diagnosis of dementia from a tertiary-referral memory clinicReference Russ, Batty and Starr21 and the hospital admission data missed about 46% of people with a robust dementia diagnosis agreed by consensus.Reference Russ, Parra, Lim, Law, Connelly and Starr22 In the former study, there were also no differences in area-level deprivation or premorbid IQ (estimated by the National Adult Reading Test) at baseline between people who had dementia correctly recorded and those who did not (unpublished results available from the author on request), suggesting that there was no bias in reporting related to socioeconomic position or intelligence. Combining multiple sources, as in this study, will reduce the chance of missing individuals.Reference Russ, Gatz, Pedersen, Hannah, Wyper and Batty2 Furthermore, when examining associations between putative risk factors and outcomes, missing some cases should not alter an observed association, and indeed, this is the approach to follow-up being taken by the UK Biobank study (N = 500 000).Reference Sudlow, Gallacher, Allen, Beral, Burton and Danesh23 However, this methodology does not easily allow disaggregation of dementia into the individual diseases that cause this syndrome (Alzheimer's dementia, vascular dementia, dementia with Lewy bodies, etc.) because so many records use generic dementia codes rather than disease-specific ones.
Third, water data were only available for the period 2005–2012. Thus, the sample who had survived to the start of the exposure period was substantially reduced from baseline. The sample sites were distributed widely across Scotland (Supplementary Fig. 1) and the number of sites was approximately constant each year (Supplementary Table 1). The spatial interpolation used to estimate values for areas where no measurement occurred introduces uncertainty but, in fact, few participants would live far from a sampling site because samples were coterminous with areas of population density. Related to this exposure period is that we know nothing of the participants' exposure to drinking water before 2005, i.e. for the first 84 years of their lives. It seems reasonable to assume a low level of exposure to aluminium and fluoride during this period, but we cannot provide further justification for this assumption. Given that neurodegeneration starts decades before the clinical onset of dementia symptoms, it may be exposure many years before dementia diagnosis that is important, but we have no information about this.
Fourth, levels of aluminium and fluoride in drinking water vary substantially over the exposure period (Supplementary Table 1 and Supplementary Fig. 3). Aluminium levels show a general decline over the whole period. Indeed, this fact, combined with our findings, might suggest that a decline in levels of aluminium in drinking water could be a further partial explanation for the decrease in dementia rates observed in Europe and North America.Reference Wu, Fratiglioni, Matthews, Lobo, Breteler and Skoog24 Fluoride levels are higher at the start and end of the exposure period; the mean value represents a cumulative exposure but the variability of measurements over time highlights a limitation of the present analyses. Within postcode sectors, there was similar variation. The within-area correlation between 2005 and 2012 values is r = 0.49 (P < 0.001) for aluminium and r = 0.012 (P < 0.001) for fluoride. In addition, participants were only located at one point in time and it was assumed that they did not move during the study period, which may not be valid. However, our study was longitudinal in design, which is more robust than a cross-sectional study, particularly when considering Bradford Hill's temporality criterion when considering whether the observed association might be causal.Reference Bradford Hill25
Finally, a criticism of much of the literature in this area is a lack of consideration of confounding. There were very few data recorded at baseline in the SMS1932. We adjusted for childhood IQ because this has previously been linked with dementia.Reference Doubal, Ali, Batty, Charidimou, Eriksdotter and Hofmann-Apitius15, Reference Calvin, Batty, Der, Brett, Taylor and Pattie26–Reference Whalley, Starr, Athawes, Hunter, Pattie and Deary28 Furthermore, higher fluoride concentrations in water have been linked with lower childhood intelligence in multiple studies.Reference Duan, Jiao, Chen and Wang29 We were additionally able to adjust models for SIMD to take into account relative deprivation, albeit at an area level. On the other hand, the fact that this is a narrow age cohort (all born in 1921) means that the sample will be more homogenous than a broader-aged sample. For example, there will be no cohort effects complicating our findings.
Implications
Aluminium is widely used in water treatment to reduce organic matter and to improve other water parameters and is also influenced by water acidity.7 Low fluoride levels in drinking water are beneficial for teeth but high levels are harmful.7 Thus, both these substances are widely present in drinking water, albeit at levels considered acceptable. However, our findings suggest that even these relatively low levels of aluminium and fluoride are associated with deleterious effects on dementia risk, which should be weighed against their beneficial uses. We must be circumspect in the conclusions we draw from this study, not least because only limited account could be taken of potential confounders. However, this is clearly an area which deserves further investigation, given the substantial and growing global public health impact of dementia.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.287.
Funding
This work was supported by Alzheimer Scotland through the Marjorie MacBeath bequest, which funded T.C.R.'s salary for 1 year from 2015–2016.
Acknowledgements
We are grateful to David Grzybowski at the Drinking Water Quality Regulator for Scotland for providing the water data. Syntax to produce the maps in R was adapted from that posted on Timo Grossenbacher's website (https://timogrossenbacher.ch/).






eLetters
No eLetters have been published for this article.