Introduction
The tropical disease schistosomiasis is caused by blood-dwelling parasites of the Schistosoma genus. The major disease-causing species in humans are Schistosoma mansoni, Schistosoma haematobium and Schistosoma japonicum (Colley et al., Reference Colley, Bustinduy, Secor and King2014). With almost 800 million people at risk of infection, schistosomiasis is the second major parasitic disease after malaria (Steinmann et al., Reference Steinmann, Keiser, Bos, Tanner and Utzinger2006).
The various species of Schistosoma reside in blood vessels at different locations in the body, and these specific locations determine pathology. Schistosoma mansoni and S. japonicum reside in the mesenteric plexus, and chronic infection results in the development of intestinal or hepatosplenic schistosomiasis, affecting intestine, liver and spleen (Colley et al., Reference Colley, Bustinduy, Secor and King2014). In contrast, S. haematobium resides in the perivesicular plexus. Infection with this species results in urogenital schistosomiasis, affecting mainly the urogenital tract, although hepatic disease may occur in severe infections (Cheever et al., Reference Cheever, Kamel, Elwi, Mosimann and Danner1977; Colley et al., Reference Colley, Bustinduy, Secor and King2014).
In addition to intestinal and hepatic disease, the blood coagulation system is affected in schistosomiasis mansoni patients (Mebius et al., Reference Mebius, van Genderen, Urbanus, Tielens, de Groot and van Hellemond2013). These individuals are prone to bleeding as a result of coagulation-factor deficiencies, and often have reduced platelet counts (reviewed by Tanabe, Reference Tanabe2003). Several factors are presumed to contribute to the loss of coagulation factors and platelets. First, the consumption of coagulation factors through the continuous activation of coagulation may be involved, since markers of active coagulation, such as thrombin–antithrombin (TAT) complexes, and markers of fibrinolysis, such as D-dimers, are both elevated in these patients. This activation of coagulation can be induced by the parasite itself, but it is also assumed that immune responses to schistosomes play a role in the development of haemostatic abnormalities in hepatosplenic schistosomiasis mansoni – for example, through stimulation of monocyte-tissue-factor expression (Amer & Amer, Reference Amer and Amer2002). Second, the majority of coagulation factors are synthesized by the liver. Therefore, impaired liver function, resulting in reduced hepatic synthesis or clearance of activated coagulation factors, could contribute to the bleeding phenotype. It remains challenging to unravel the contribution of the parasite and liver damage to the development of haemostatic abnormalities in hepatosplenic schistosomiasis mansoni patients, since these factors cannot be separated in hepatosplenic disease.
This pilot study aims to elucidate the contribution of the parasite to the development of coagulation abnormalities in Schistosoma infections by studying haemostatic parameters in schoolchildren infected with S. haematobium. Since S. haematobium infections primarily cause urogenital schistosomiasis, strict selection on S. haematobium-infected individuals without hepatosplenomegaly limits the influence of impaired hepatic function and allows investigation of the effect of only the parasitic infection on the development of coagulation abnormalities.
Materials and methods
Participants and sample collection
Ten schoolchildren with non-hepatosplenic schistosomiaisis haematobium and four healthy controls without S. haematobium infection were recruited at the Centre de Recherches Médicales de Lambaréné (CERMEL), Lambaréné, Gabon. The study was approved by the CERMEL Institutional Ethics Committee (CEI, CEI-MRU 002 26/1/2016). Participants were schoolchildren without hepatosplenic complaints from Zilé-PK15 areas – S. haematobium-endemic areas surrounding Lambaréné (Ateba Ngoa et al., Reference Ateba Ngoa, Zinsou and Kassa2014). The presence of possible asymptomatic other infectious diseases that could affect haemostasis, such as malaria, were not investigated, but if present among the included participants these asymptomatic infections are expected to be evenly distributed between participants with and without urinary schistosomiasis. Informed consent was obtained from the parents or legal guardians of all children, and all infected individuals received treatment with praziquantel after sample collection. Venous blood was collected in a tube containing 1:10 volume of anticoagulant 3.2% sodium citrate solution. Whole blood was analysed with the Pentra 60 analyser (Horiba, Kyoto, Japan). Citrated platelet-poor plasma was obtained by twofold centrifugation of citrated blood at 2000×g for 15 min. Citrated plasma was aliquoted and stored at −80°C.
Determination of S. haematobium infection
Circulating anodic antigen (CAA) levels were determined in 20 µl citrated plasma with the wet reagent SCAA20 (standard serum/plasma CAA test on 20 µl trichloroacetic acid (TCA) extract) lateral flow assay, as described by Corstjens et al. (Reference Corstjens, De Dood and Kornelis2014). Samples were treated with 4% TCA to remove interfering components. CAA levels were calculated with a four-parametric curve-fitting method, using a standard dilution curve of the TCA-soluble fraction of schistosome adult worm antigen (AWA-TCA), which contains approximately 3% (w/w) CAA. The lower detection limit of the SCAA20 was 150 pg/ml AWA-TCA (=5 pg CAA/ml). Participants were considered positive when CAA levels were above 5 pg/ml. In addition, for all participants a midstream urine sample was collected during the daytime and at least 10 ml urine was passed through a 12.0 µm polyamide N-filter (Millipore, Billerica, MA, USA). Schistosoma haematobium eggs were detected by microscopic examination.
Enzyme-linked immunosorbent assay
Levels of D-dimer were measured in plasma with the Technozym D-dimer ELISA kit (Stago BNL, Leiden, The Netherlands) according to the manufacturer's instructions. Levels of von Willebrand Factor (VWF) antigen (VWF:ag), active VWF, ADAMTS-13 antigen (ADAMTS-13:ag), osteoprotegerin (OPG) and TAT complexes were measured in plasma with ELISA. Ninety-six-well or 384-well white Maxisorp microtiter plates (Thermo Scientific, Roskilde, Denmark) were coated overnight at 4°C with polyclonal rabbit-anti-human VWF (0.775 µg/ml; A0082; DAKO, Carpinteria, CA, USA), AU/VWFa-11 nanobody (Hulstein et al., Reference Hulstein, de Groot, Silence, Veyradier, Fijnheer and Lenting2005) (1.98 µg/ml), D053 (0.5 µg/ml; Sanquin, Amsterdam, The Netherlands), mouse-anti-human OPG/TNFRSF11B (1 µg/ml; R&D Systems, Minneapolis, MN, USA) or sheep-anti-human Thrombin (1 µg/ml; Stago BNL, Leiden, The Netherlands) in coating buffer (40 mm sodium carbonate, 35 mm sodium bicarbonate, 3 mm sodium azide, pH 9.6). Plates were washed four times with washing buffer (phosphate-buffered saline (PBS; ThermoFisher Scientific, Waltham, MA, USA), 0.05% Tween20, pH 7.4) between all incubation steps, and blocked for one hour at room temperature with blocking buffer (PBS, 1% bovine serum albumin (BSA), pH 7.4). Citrated plasma and standards were diluted in blocking buffer: 125-fold for VWF:ag ELISA, five- and tenfold for active VWF ELISA, 40-fold for ADAMTS-13:ag ELISA and fourfold for OPG and TAT ELISAs, and incubated for one hour at room temperature. Used standards were serial dilutions of normal pooled plasma (Sanquin, Amsterdam, The Netherlands) with a known concentration of VWF:ag for VWF:ag and ADAMTS-13:ag, normal pooled serum with known concentrations of TAT and OPG for TAT and OPG ELISAs and VWF-depleted plasma (Affinity Biologicals, Ancaster, Canada) supplemented with recombinant R1341Q-VWF for active VWF ELISA. Following sample incubation, the plates were incubated at room temperature for one hour with the following detection and secondary antibodies diluted in blocking buffer: peroxidase-conjugated rabbit-anti-human VWF (0.325 µg/ml), peroxidase-conjugated rabbit-anti-human VWF (1.2 µg/ml), biotinylated polyclonal sheep-anti-human ADAMTS-13 (0.25 µg/ml; R&D Systems, Minneapolis, MN, USA) and streptavidin-mono-horseradish peroxidase (HRP) (0.83 µg/ml; DAKO, Carpinteria, CA, USA), biotinylated goat-anti-human OPG/TNFRSF11B (100 ng/ml; R&D Systems, Minneapolis, MN, USA) and streptavidin-mono-HRP (0.25 µg/ml), and peroxidase-conjugated sheep-anti-human antithrombin (0.5 µg/ml; Stago BNL, Leiden, The Netherlands). ELISAs were developed with SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific, Waltham, MA, USA) and luminescence was detected with the Synergy2 ELISA reader (BioTek, Winooski, VT, USA). Antigen concentrations were calculated with a five-parametric curve-fitting method, using a dilution curve of the standard. The lower detection limits of the ELISAs were: 6.8 ng/ml (VWF:ag), 16 ng/ml (active VWF), 1.6% (ADAMTS-13:ag), 10.6 pg/ml (OPG) and 2.8 pM (TAT).
Ristocetin co-factor activity assay
Citrated plasma was diluted sevenfold in imidazole buffer (100 mm imidazole, 100 mm NaCl, pH 7.4). A standard dilution curve was prepared of diluted normal pooled plasma. Then, 200 µl BC von Willebrand Reagent (Siemens Healthcare, Erlangen, Germany) was added to the 70 µl sample in a 96-well clear Maxisorp microtiter plate. Following 10 min incubation at 37°C and 20 min incubation at room temperature, to allow platelet aggregates to precipitate, 100 µl of the mixture was transferred to a new plate and non-aggregated platelets were measured at 350 nm. Ristocetin co-factor activity (VWF:RCo) was calculated with a five-parametric curve-fitting method, using a dilution curve of the standard. The lower detection limit of the assay was 3% VWF:RCo.
ADAMTS-13 activity measurement
ADAMTS-13 activity was measured in a kinetic assay using the fluorescence resonance energy transfer substrate VWF73 (FRETS-VWF73; Peptide Institute, Osaka, Japan) as previously described (Kokame et al., Reference Kokame, Nobe, Kokubo, Okayama and Miyata2005). Samples were tenfold diluted in Tris buffer (5 mm Tris, 25 mm calcium chloride, 0.005% Tween20, pH 6.0). FRETS-VWF73 was added to a final concentration of 2 µm and fluorescence (λex 340 nm, λem 450 nm) was measured in a 96-well black microtiter plate (Thermo Scientific, Roskilde, Denmark) every 30 s for one hour at 30°C with a Spectramax M2e device (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Analyses were done using PRISM software (GraphPad, San Diego, CA, USA, version 6.01). Infected and non-infected control groups were compared using the Mann–Whitney U-test and P ≤ 0.05 was considered statistically significant.
Results and discussion
We included ten schoolchildren with schistosomiasis haematobium and four healthy children from S. haematobium-endemic, rural areas surrounding Lambaréné (Gabon) (table 1). Infection status was based on CAA levels in plasma (Corstjens et al., Reference Corstjens, De Dood and Kornelis2014) combined with the detection of viable eggs in urine, since the detection of eggs in urine as the only method to confirm infection has a low sensitivity (Colley et al., Reference Colley, Bustinduy, Secor and King2014). As expected, a positive correlation was found between the number of eggs detected by urine filtration and the concentration of CAA in plasma (not shown). Hepatomegaly was absent in all studied individuals and splenomegaly was absent in nine out of ten infected and in all non-infected individuals; therefore, all infected children were characterized as strictly urogenital schistosomiasis haematobium.
Table 1. Participant characteristics.
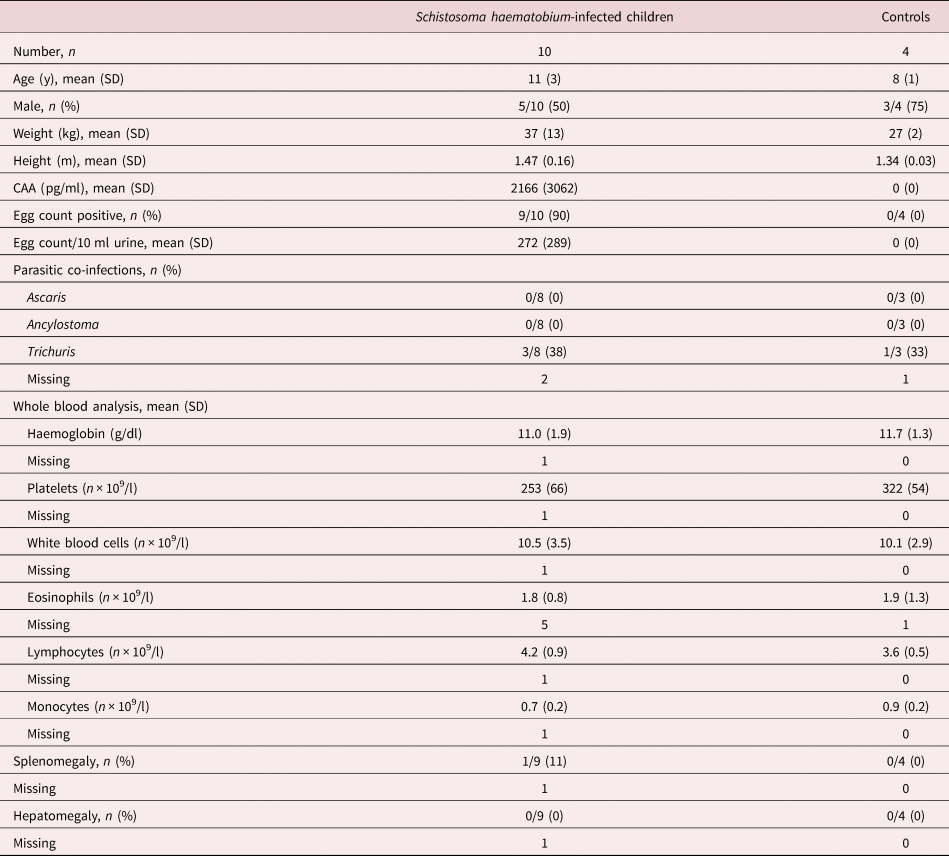
Data are number with percentage or mean with SD. CAA, circulating anodic antigen; SD, standard deviation.
In hepatosplenic schistosomiasis mansoni, levels of VWF:ag are highly elevated and inversely correlate with platelet counts, as patients experience thrombocytopenia (Correia et al., Reference Correia, Domingues and Lacerda2009). To determine the effect of Schistosoma infection on VWF levels in urinary schistosomiasis, we determined VWF:ag, active VWF and VWF ristocetin cofactor activity (VWF:RCo) in our study participants. VWF is secreted by endothelial cells and circulates in a globular conformation in which the platelet-binding A1-domain is inaccessible (Hulstein et al., Reference Hulstein, de Groot, Silence, Veyradier, Fijnheer and Lenting2005). Under blood flow, VWF unfolds and adopts an active platelet-binding conformation (active VWF) that can be detected using the AU/VWFa-11 nanobody (Hulstein et al., Reference Hulstein, de Groot, Silence, Veyradier, Fijnheer and Lenting2005). Besides flow, ristocetin can also be used to unfold VWF and study its binding to platelets (VWF:RCo). Levels of VWF:ag and active VWF were significantly increased in infected children compared to non-infected controls (P = 0.002 and P = 0.004, respectively; see fig. 1a, b). The elevated VWF:ag levels indicate either increased secretion or reduced breakdown of VWF. The proportion of VWF that is active (percentage of active VWF) is slightly decreased in infected children compared to non-infected individuals, indicating that increased conformational activation of circulating VWF is not responsible for the observed increase in active VWF (P = 0.024; fig. 1c). In contrast to increased VWF:ag and active VWF levels, VWF:RCo was low or absent in all individuals (P = 0.6234; fig. 1d). As all individuals had normal platelet counts ((253 ± 66) × 109/l and (322 ± 54) × 109/l in infected and non-infected individuals, respectively (P = 0.1063)) and plasma from Dutch controls showed normal VWF:RCo activity (data not shown), this observation cannot be explained by low platelet counts or poor performance of the VWF:RCo assay. Reduced or absent platelet aggregation on ristocetin in Africans compared to Europeans has been reported previously (Dupuy et al., Reference Dupuy, Fleming and Caen1978; Buchanan et al., Reference Buchanan, Holtkamp and Levy1981). Reduced platelet function was attributed to a plasma inhibitor of ristocetin-induced platelet aggregation (Dupuy et al., Reference Dupuy, Fleming and Caen1978; Buchanan et al., Reference Buchanan, Holtkamp and Levy1981), though genetic polymorphisms in VWF could also affect the ristocetin-based activity assay (Flood et al., Reference Flood, Gill and Morateck2010). It is, however, unclear whether these accounted for the observed low VWF:RCo activity in our study. In summary, in urinary schistosomiasis haematobium, VWF:ag and active VWF levels are elevated and do not correlate with platelet counts as thrombocytopenia is absent.

Fig. 1. Haemostatic parameters in Schistosoma haematobium-infected schoolchildren. VWF:ag levels (a), active VWF levels (b), ADAMTS-13:ag levels (e), OPG levels (g), TAT levels (h) and D-dimer levels (i) were measured with ELISA in citrated plasma of S. haematobium-infected schoolchildren and non-infected controls. Percentage of active VWF (c) was calculated with the determined active VWF and VWF:ag levels. VWF:RCo (d) was determined with the BC von Willebrand Reagent. ADAMTS-13 activity (f) was determined with the FRETS-VWF73 assay. Dotted lines indicate normal ranges (a, d) or cut-off values (g–i). Statistical analysis was performed with a Mann–Whitney U-test. P ≤ 0.05 was considered statistically significant. Abbreviation: ns, not significant.
Changes in levels of VWF:ag and active VWF could reflect defects in the VWF-degrading protease ADAMTS-13 (Hulstein et al., Reference Hulstein, de Groot, Silence, Veyradier, Fijnheer and Lenting2005), but could also indicate endothelial activation (Lip & Blann, Reference Lip and Blann1997). To study the functionality of ADAMTS-13, both ADAMTS-13:ag levels and ADAMTS-13 activity were determined. ADAMTS-13:ag levels and ADAMTS-13 activity were normal in both infected and non-infected children (P = 0.4116 and P = 0.5774, respectively; see fig. 1e, f), so no defects in VWF-degradation are present.
Next, endothelial activation was determined using OPG levels as a marker for inflammation-mediated endothelial activation (Zannettino et al., Reference Zannettino, Holding and Diamond2005). OPG is not directly involved in coagulation, as OPG is a cytokine of the TNF receptor superfamily involved in several physiological processes, among which are angiogenesis, osteogenesis and inflammation. Elevated levels of OPG were observed in the S. haematobium-infected children versus non-infected controls (P = 0.036; fig. 1g, dotted line indicates cut-off value). Simultaneous elevation of both VWF:ag and OPG during schistosomiasis haematobium indicates inflammation-mediated endothelial activation and is in line with the reported co-localization of these markers in endothelial Weibel Palade bodies and their simultaneous secretion upon endothelial cell stimulation (Zannettino et al., Reference Zannettino, Holding and Diamond2005).
Several mechanisms may explain activation of the vessel wall. Secretion of OPG is stimulated by the inflammatory cytokines TNF-α and IL-1β (Zannettino et al., Reference Zannettino, Holding and Diamond2005). In hepatosplenic schistosomiasis mansoni patients, TNF-α production by peripheral blood mononuclear cells (PBMC) is elevated and plasma levels of soluble TNF receptor I and II are increased (Mwatha et al., Reference Mwatha, Kimani and Kamau1998), suggesting the contribution of inflammatory cytokines produced during infection to the observed increased OPG levels. However, no correlation between serum levels of TNF-α and infection status or disease severity is observed in schistosomiasis haematobium (Bustinduy et al., Reference Bustinduy, Sutherland and Chang-Cojulun2015). Passage of schistosome eggs through the vessel wall or egg-derived materials could also induce endothelial activation (File, Reference File1995). Furthermore, endothelial adhesion of adult S. mansoni through their ventral sucker could damage the endothelium (Silva et al., Reference Silva, Morel and Noël1998) and the presence of the adult worm pair in the small veins could alter blood flow, leading to endothelial activation (Mebius et al., Reference Mebius, van Genderen, Urbanus, Tielens, de Groot and van Hellemond2013).
The observation that endothelial activation occurs during urinary schistosomiasis is of importance, as elevated levels of VWF, OPG and endothelial activation are associated with thrombosis and cardiovascular disease (Jono et al., Reference Jono, Ikari, Shioi, Mori, Miki, Hara and Nishizawa2002; Conway et al., Reference Conway, Pearce, Chin, Hart and Lip2003). Ongoing coagulation has been demonstrated in hepatosplenic schistosomiasis mansoni, reflected by increased levels of TAT and D-dimer (Tanabe, Reference Tanabe2003). Therefore, we investigated whether the observed endothelial activation in urinary schistosomiasis haematobium results in a procoagulant phenotype in urinary schistosomiasis. In contrast to hepatosplenic schistosomiasis mansoni patients, TAT and D-dimer levels were generally low in our study population and we could not demonstrate differences in urinary schistosomiasis patients compared to the non-infected individuals (P = 0.4116 and P = 0.7524, respectively; fig. 2h, i, dotted line indicates cut-off value), indicating that there is no ongoing coagulation or fibrinolysis in these individuals. However, five of the infected children had TAT levels above the normal threshold, without elevated D-dimer levels. Coagulation activation may have occurred in these individuals leading to low-level thrombin activation, followed by subsequent inhibition of the formed thrombin by antithrombin, leading to the formation of TAT without fibrin formation, which explains the absence of D-dimers in these individuals.
Here, we report the first study on the direct effects of S. haematobium on haemostatic abnormalities in urinary schistosomiasis haematobium patients without hepatosplenic complaints. Despite the small sample size of this pilot study, our observations indicate that S. haematobium directly alters the activation status of the vessel wall. This is not unexpected as the relatively large size of the adult worm pairs in the blood vessel will provoke substantial turbulence and increased shear stress along the vessel wall – processes known to induce endothelial activation (Mebius et al., Reference Mebius, van Genderen, Urbanus, Tielens, de Groot and van Hellemond2013). In contrast to reports on coagulation abnormalities in hepatosplenic schistosomiasis mansoni, ongoing coagulation and fibrinolysis, which is reflected by elevated levels of TAT and D-dimer, was absent in urinary schistosomiasis patients that thus lack apparent physiological changes in their haemostasis. In schistosomiasis mansoni, the reported coagulopathy may be aggravated as a result of impaired liver function (Tanabe, Reference Tanabe2003). The direct effects of the parasite on the vessel wall are also of interest, as endothelial activation and elevated levels of VWF are linked to thrombosis. Therefore, we hypothesize that direct activation of the endothelium by S. haematobium may be involved in the initiation of coagulation abnormalities in schistosomiasis. Interestingly, a VWF-cleaving peptidase was recently discovered in S. mansoni, which suggests that schistosomes have strategies to counteract endothelial activation and elevated levels of VWF, which are unfavourable for both the parasite and its host, as this can lead to thrombosis (Mebius et al., manuscript in preparation). Larger studies on endothelial activation in schistosomiasis haematobium patients with various disease severities are required to confirm our observations, and to elucidate the mechanisms involved in schistosomal-induced endothelial activation and the consequences of this endothelial activation on haemostasis.
Acknowledgements
The authors thank Claudia J. de Dood for the production of the lateral flow test materials and the excellent analysis of the plasma samples with the SCAA20 assay. The authors also thank the research lab team from CERMEL for their assistance in plasma collection.
Financial support
This work was supported by the Netherlands Organization for Scientific Research (NWO), the Erasmus Postgraduate School Molecular Medicine (MolMed; Erasmus Graduate Programme Infection & Immunity, NWO file number 022.005.032) and by a European and Developing Countries Clinical Trials Partnership (EDCTP) Senior Fellowship (project number TA.11.40200.025).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.




