Introduction
Reducing Emissions from Deforestation and forest Degradation (REDD) is an increasing part of the international community's efforts to tackle global climate change, and also a key mechanism to create an incentive for forest protection (Clements, Reference Clements2010). The inclusion of issues around conservation, sustainable development and forest restoration alongside carbon storage, led to its rebranding as REDD+ (Parker et al., Reference Parker, Mitchell, Trivedi, Mardas and Sosis2009; Venter & Koh, Reference Venter and Koh2012).
REDD+ addresses an important cause of carbon emissions: the world's forests hold an estimated 289 gigatonnes of carbon but 13 million ha per year (0.325% of total) is lost or degraded (FAO, 2010). The loss and degradation of tropical forests in particular is happening rapidly and is responsible for an estimated 12% of anthropogenic carbon emissions (van der Werf et al., Reference van der Werf, Morton, DeFries, Olivier, Kasibhatla and Jackson2009). Even existing protected areas, which contain 18.5% of humid tropical forest carbon, are not immune to forest loss: an estimated 1.75 million ha of forest in protected areas were lost between 2000 and 2005 (Scharlemann et al., Reference Scharlemann, Kapos, Campbell, Lysenko, Burgess and Hansen2010). Currently REDD+ primarily uses bilateral and multilateral agreements as well as the global voluntary carbon market, valued at USD 379 million in 2013, to ensure that the incentive for forest protection is greater than that for destruction (Parker et al., Reference Parker, Mitchell, Trivedi, Mardas and Sosis2009; Peters-Stanley et al., Reference Peters-Stanley, Gonzalez and Yin2013). Successful REDD+ development and implementation requires a set of important prerequisites to be in place, such as clear and secure tenure rights to land, forests and carbon, strong local and national level forest governance, free, prior and informed consent of communities, and the political will to forego some of the opportunity costs from short-term and purely extractive land-uses in the knowledge that not all ecosystem services provided by healthy, functioning carbon-rich forests are financially quantifiable (Angelsen et al., Reference Angelsen, Brockhaus, Sunderlin and Verchot2012). In addition, a sufficiently strong demand for the purchase of emission reductions in national and international markets is essential if project developers, investors, forest country governments and communities are to continue contributing resources, time and effort into developing REDD+ activities (GCP, IPAM, FFI, UNEP FI & UNORCID, 2014).
REDD+ and biodiversity conservation
REDD+ protects forests primarily for their carbon value (UNFCCC, 2008 p.3) using internationally accepted definitions of ‘forest’ and ‘deforestation’ that are based on percentage tree cover (Schoene et al., Reference Schoene, Killmann, von Lüpke and LoycheWilkie2007; Sasaki & Putz, Reference Sasaki and Putz2009; van der Werf, Reference van der Werf, Morton, DeFries, Olivier, Kasibhatla and Jackson2009). Maintaining tree cover in carbon-rich forests will have strong positive effects on biodiversity by preventing habitat loss. There is a congruence of areas rich in carbon and biodiversity on a global scale (Strassburg et al., Reference Strassburg, Kelly, Balmford, Davies, Gibbs and Lovett2010; Cavanaugh et al., Reference Cavanaugh, Gosnell, Davis, Ahumada, Boundja and Clark2014), and funds from REDD+ can also flow to existing forest reserves and protected areas where lack of finance restricts forest protection (Burgess et al., Reference Burgess, Bahane, Clairs, Danielsen, Dalsgaard and Funder2010; Scharlemann et al., Reference Scharlemann, Kapos, Campbell, Lysenko, Burgess and Hansen2010). However, the overlap between carbon and biodiversity may not always be apparent at finer scales (Paoli et al., Reference Paoli, Wells, Meijaard, Struebig, Marshall and Obidzinski2010) and in cases where it is not, REDD+ implementation may have more modest benefits for biodiversity conservation.
Without including conservation as a core objective there is no guarantee that biodiversity within a seemingly intact forest will persist (Schoene et al., Reference Schoene, Killmann, von Lüpke and LoycheWilkie2007). Phelps et al. (Reference Phelps, Webb and Adams2012) suggest that stronger evidence for the links between biodiversity and carbon storage is needed before it can be considered by the international community. Here we explore the available evidence to determine whether by overlooking the integral role of biodiversity in maintaining healthy forest ecosystems, we could be compromising long-term carbon storage.
Biodiversity loss and its implications
Tropical forests are complex ecosystems, underpinned by small-scale processes and species interactions that, if altered, can often have much wider effects on the diversity and functioning of the forest as a whole. Individual or groups of species may play important roles in pollination, seed dispersal or nutrient cycling and their loss can result in decreased tree recruitment (Forget & Jansen, Reference Forget and Jansen2007; Wang et al., Reference Wang, Sork, Leong and Smith2007; Brodie et al., Reference Brodie, Helmy, Brockelman and Maron2009; Holbrook & Loiselle, Reference Holbrook and Loiselle2009) and changing forest species composition (Wright et al., Reference Wright, Hernandez and Condit2007). Managing forests solely for carbon storage does not automatically take into account the complex interactions and interdependence of plant and animal organisms living within them (Bunker et al., Reference Bunker, DeClerck, Bradford, Colwell, Perfecto and Phillips2005).
REDD+ protects a forest from clear felling and fragmentation and should prevent destructive forest fires (but see Aragão & Shimabukuro, Reference Aragão and Shimabukuro2011; Barlow et al., Reference Barlow, Patty, Gardnera, Ferreira, Aragão and Carmenta2012). However, it is possible for high value trees of one species to be selectively removed or for other threats that do not affect tree cover in the short term to be overlooked. The concept of the empty forest (Redford, Reference Redford1992) is now well recognized; preventing the removal of trees is not always enough to protect other species within a forest. For example, forest patches set aside from clear felling, with no protection, lose more faunal species than strictly protected areas nearby (Canale et al., Reference Canale, Peres, Guidorizzi, Gatto and Kierulff2012).
How hunting affects forest carbon stocks
After habitat loss the greatest threat to mammals and birds in tropical forests is hunting (Wilkie et al., Reference Wilkie, Bennett, Peres and Cunningham2011). Hunting pressure for both commercial and subsistence use are increasing in many areas as a result of increasing human populations, greater demand for wild meat, and new technologies improving the global reach of wildlife trade networks (Harrison, Reference Harrison2011; Wilkie et al., Reference Wilkie, Bennett, Peres and Cunningham2011).
There is evidence of a link between defaunation as a result of hunting and short-term changes in forest diversity and species composition (e.g. Nñnez-Iturri & Howe, Reference Nuñez-Iturri and Howe2007; Wright et al., Reference Wright, Hernandez and Condit2007; Vanthomme et al., Reference Vanthomme, Bellé and Forget2010; Kurten, Reference Kurten2013), which has been cited as a possible cause for decreased carbon storage (Brodie & Gibbs, Reference Brodie and Gibbs2009; Jansen et al., Reference Jansen, Muller-Landau and Wright2010). In particular, the loss of seed dispersers is thought to be an important cause of long-term changes in forest composition (Forget & Jansen, Reference Forget and Jansen2007; Wang et al., Reference Wang, Sork, Leong and Smith2007; Brodie et al., Reference Brodie, Helmy, Brockelman and Maron2009; Holbrook & Loiselle, Reference Holbrook and Loiselle2009).
Hunting for meat often selectively targets larger-bodied species (Redford, Reference Redford1992; Peres & Palacios, Reference Peres and Palacios2007; Wilkie et al., Reference Wilkie, Bennett, Peres and Cunningham2011; Vidal et al., Reference Vidal, Pires and Guimarães2013), and subsistence hunting has been shown to target important tree seed dispersers including primates, birds, ungulates, scatter-hoarding rodents and frugivorous fish (Forget & Jansen, Reference Forget and Jansen2007; Wang et al., Reference Wang, Sork, Leong and Smith2007; Terborgh et al., Reference Terborgh, Nuñez-Iturri, Pitman, Valverde, Alvarez and Swamy2008; Holbrook & Loiselle, Reference Holbrook and Loiselle2009; Anderson et al., Reference Anderson, Nuttle, Rojas, Pendergast and Flecker2011).
The effects of hunting on different tree species and forests will vary depending on the relative importance of hunted vertebrates as dispersers. An estimated 50–70% of tropical forest trees are dispersed by vertebrates (Howe & Smallwood, Reference Howe and Smallwood1982), with recent studies from individual forests estimating this to be 70–90% (Maisels et al., Reference Maisels, Keming, Kemei and Toh2001; Wright et al., Reference Wright, Hernandez and Condit2007; Brodie et al., Reference Brodie, Helmy, Brockelman and Maron2009; Poulsen et al., Reference Poulsen, Clark and Palmer2013). Some tree species may have important or even obligate relationships with vertebrate seed dispersers or pollinators (e.g. elephants as obligate seed dispersers of African forest trees, Campos-Arceiz & Blake, Reference Campos-Arceiz and Blake2011; and tapirs as the most efficient dispersers for the tree Maximiliana maripa in Brazil, Fragoso et al., Reference Fragoso, Silvius and Correa2003), and populations of such trees may be affected if hunting removes these species from their ecosystems.
Most seed disperser relationships are, however, non-obligate (Campos-Arceiz & Blake, Reference Campos-Arceiz and Blake2011). When vertebrates are removed by hunting, other vertebrates may functionally replace them (Elmqvist et al., Reference Elmqvist, Folke, Nyström, Peterson, Bengtsson, Walker and Norberg2003; Muller-Landau, Reference Muller-Landau2007). Other forest studies show limited functional replacement when large species are lost (Vanthomme et al., Reference Vanthomme, Bellé and Forget2010) and smaller-bodied seed dispersers may show reduced efficiency (Peres & Palacios, Reference Peres and Palacios2007; Holbrook & Loiselle, Reference Holbrook and Loiselle2009); they may only carry seeds over much shorter distances, are not capable of dispersing larger seeds or may only disperse seeds of a small number of tree species (Peres & Palacios, Reference Peres and Palacios2007; Holbrook & Loiselle, Reference Holbrook and Loiselle2009; Campos-Arceiz et al., Reference Campos-Arceiz, Traeholt, Jaffar, Santamaria and Corlett2012).
Hunting-induced species declines are likely to lead to a decrease in the recruitment of large trees as larger-bodied dispersers are lost, although this impact may in theory be offset by a decline in large seed predators (Muller-Landau, Reference Muller-Landau2007; Wright et al., Reference Wright, Hernandez and Condit2007). A decline in the dispersal of large tree seeds has been linked to a shift within a forest towards the dominance of wind-dispersed plants such as lianas, which are unlikely to be affected by hunting pressure (Wright et al., Reference Wright, Hernandez and Condit2007). Whether a result of hunting or a combination of factors, there is evidence of increasing dominance of lianas in tropical forests (reviewed by Schnitzer & Bongers, Reference Schnitzer and Bongers2011).
In western Amazonia liana dominance increased by up to 4.6% per year during 1980–2000, accompanied by an increase in total basal area and density of lianas (Philips et al., Reference Phillips, Vásquez Martínez, Arroyo, Baker, Killeen and Lewis2002). Lianas grow quickly and are able to outcompete slower growing trees for light, water and soil nutrients (Philips et al., Reference Phillips, Vásquez Martínez, Arroyo, Baker, Killeen and Lewis2002; Wright et al., Reference Wright, Hernandez and Condit2007), but their thin stems and low wood density mean that they sequester and store relatively little carbon compared to the trees they replace (van der Heijden & Philips, Reference van der Heijden and Phillips2009). In addition, lianas may infest certain species preferentially, causing a shift in species composition to faster growing trees, which often store less carbon in the long term (van der Heijden & Philips, Reference van der Heijden and Phillips2009). This may result in a seemingly healthy forest having a greatly reduced capacity for carbon storage: one model predicts a decrease of up to 34% in the carbon storage capacity of a tropical forest in Panama (total carbon stock) following extinctions and subsequent liana infestation (Bunker et al., Reference Bunker, DeClerck, Bradford, Colwell, Perfecto and Phillips2005). Higher liana density has also been linked to an increase in tree seed predation, as lianas provide a means of access for rodents and other small mammals from the forest floor to the canopy (Kilgore et al., Reference Kilgore, Lambert and Adler2010).
Other potential threats to carbon stocks
Invasions by exotic plants and animals could potentially affect carbon stocks but the evidence available for tropical ecosystems is still insufficient to be conclusive (Peltzer et al., Reference Peltzer, Allen, Lovett, Whitehead and Wardle2010). Studies focusing on temperate forests have found that invasive species can reduce carbon storage and sequestration by increasing decomposition rates or removing native trees, dispersers or pollinators through direct predation (Roubik, Reference Roubik1978; Peltzer et al., Reference Peltzer, Allen, Lovett, Whitehead and Wardle2010). Similarly, die-back of trees in temperate ecosystems because of introduced pathogens is well documented (Thompson et al., Reference Thompson, Mackey, McNulty and Mosseler2009). The few studies that exist for tropical ecosystems suggest significant variability in outcomes, even on a local scale. For example, invasive trees in Hawaii were found to reduce the above ground biomass of lowland rainforests but increase it in a nearby highland forest (Asner et al., Reference Asner, Hughes, Varga, Knapp and Kennedy-Bowdoin2009).
Biodiversity should provide a certain level of ‘biological insurance’ that strengthens the stability and resilience of a tropical forest, with diversity within functional groups being especially important (Bunker et al., Reference Bunker, DeClerck, Bradford, Colwell, Perfecto and Phillips2005; Thompson et al., Reference Thompson, Mackey, McNulty and Mosseler2009; Miles et al., Reference Miles, Dunning, Doswald and Osti2010). Thus a loss of biodiversity may increase the risks to forests from other threats such as climate change (Thompson et al., Reference Thompson, Mackey, McNulty and Mosseler2009).
Under projected climate change scenarios, tropical forests may experience more frequent disturbances, such as fires, disease outbreaks and invasions of non-native species. If a REDD+ project is to continue to be effective in the long term, the forest it protects must survive these disturbances without extensive tree mortality or long-term degradation (Miles et al., Reference Miles, Dunning, Doswald and Osti2010). Although there is still a lack of direct evidence for this in tropical forests, Miles et al. (Reference Miles, Dunning, Doswald and Osti2010) note that, as research in other ecosystems has overwhelmingly demonstrated a positive correlation between biodiversity and resilience, a precautionary approach should be adopted, extending this likely relationship to tropical forests.
Discussion
The interrelationships between biodiversity and carbon sequestration and storage in tropical forests are summarized in Fig. 1. Recent evidence suggests that REDD+ will lead to the protection of areas of high biodiversity on a global scale (Cavanaugh et al., Reference Cavanaugh, Gosnell, Davis, Ahumada, Boundja and Clark2014). However, on a local scale we argue that the maintenance of biodiversity in these sites should not always be assumed over the long term. Successful REDD+ implementation is mostly based on proven emission reductions from avoided deforestation and degradation monitored through percentage tree cover and carbon stocks but several threats can reduce biodiversity without changing tree cover or carbon stocks in the short term, with hunting being the most widespread and best understood (Wilkie et al., Reference Wilkie, Bennett, Peres and Cunningham2011). Invasive species, selective logging and overexploitation of particular species may also occur, although the effects on tropical forest composition and biodiversity are under-researched, making it difficult to draw firm conclusions.
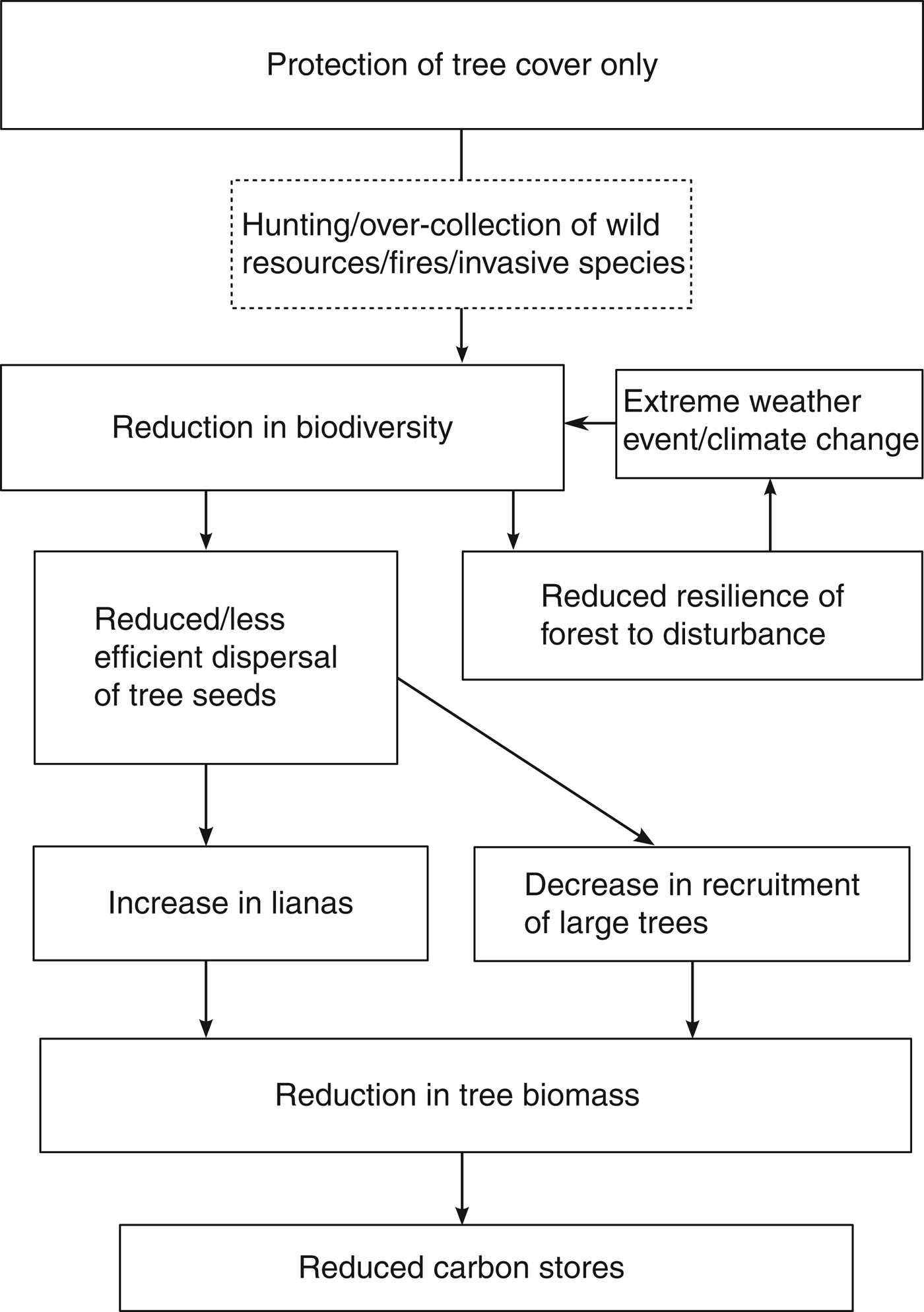
Fig. 1 The interrelationships between biodiversity and carbon sequestration and storage in tropical forests.
Hunting is an important problem in this context as it tends to target large-bodied species, especially those that disperse tree seeds (e.g. Forget & Jansen, Reference Forget and Jansen2007; Holbrook & Loiselle, Reference Holbrook and Loiselle2009), and ultimately results in an over-representation of wind-dispersed plants such as lianas (Wright et al., Reference Wright, Hernandez and Condit2007). Combined, the loss of large trees and the increase in lianas could lead to a decrease in carbon sequestration and storage in the long term (Bunker et al., Reference Bunker, DeClerck, Bradford, Colwell, Perfecto and Phillips2005; van der Heijden & Philips, Reference van der Heijden and Phillips2009).
Further to these direct effects, biodiversity declines may further affect the efforts of REDD+ projects by making a forest less able to withstand damage or degradation caused by climate change (Thompson et al., Reference Thompson, Mackey, McNulty and Mosseler2009). This could effectively lead to a feedback loop in which biodiversity decline leads to further species losses, resulting in forest becoming degraded more quickly. As both the exact impacts of climate change and the resilience that biodiversity may provide are unknown in many tropical forests, a precautionary approach should be required (Miles et al., Reference Miles, Dunning, Doswald and Osti2010).
We acknowledge that the relative importance of biodiversity conservation within REDD+ project development is increasing and welcome the recent efforts made by international standards that do not focus on biodiversity to streamline certification procedures with others that do (VCS & CCB, 2013). We also note recent shifts of other international standards towards more explicit reference to biodiversity and the inclusion of biodiversity measures in project monitoring targets (Plan Vivo Foundation, 2013).
As the most widespread threat, hunting needs to be monitored and efforts to reduce unsustainable hunting should be a priority, especially where it targets important functional groups such as seed dispersers or pollinators. It has been argued that overly complicated and expensive biodiversity monitoring protocols may reduce the uptake of REDD+ (Phelps et al., Reference Phelps, Webb and Adams2012). The financial implications of including biodiversity measures in REDD+ monitoring targets are not our primary focus here and, although biodiversity monitoring does represent an additional cost, this is generally a fraction of that involved in overall project development. Ensuring that a small number of locally appropriate indicator species (preferably including large-bodied seed dispersers) are selected and taking advantage of cost-effective tools appropriate for use by local community members are two ways of keeping these costs low. The financial implications, and more importantly the sustainability, of participatory biodiversity monitoring always compare favourably with conventional monitoring by professional ecologists. Ultimately, if biodiversity loss results in REDD+ projects reducing their impact in the long term, basic biodiversity monitoring may be too important to ignore.
Acknowledgements
We thank Josh Kempinski, Zoë Cullen and Matthew Linkie from Fauna & Flora International for input to this article, and support from Esther Bertram, Georgina Magin and the Global Trees Campaign.
Biographical sketches
Amy Hinsley uses interdisciplinary methods to study the international wildlife trade. Before joining the Durrell Institute of Conservation and Ecology she was a member of the Science team at Fauna & Flora International. Abigail Entwistle has a background in bat ecology and has worked in international conservation for nearly 20 years. Her research interests include the use of flagship species and approaches to determining conservation impact. Dorothea Pio is involved in the development and implementation of several REDD+ projects in Indonesia. Before joining Fauna & Flora International she worked for UNESCO supporting protected area management in Sumatra. She has a background in tropical forest ecology and conservation and has studied how extinctions induced by climate change may impact the tree of life in Southern Africa.



