Introduction
The early Neoproterozoic Mackenzie Mountains Supergroup in northwest Canada hosts exceptionally preserved macrofossils interpreted to represent multicellular eukaryotes, including probable macroalgae (Hofmann and Aitken, Reference Hofmann and Aitken1979; Hofmann, Reference Hofmann1985; Maloney et al., Reference Maloney2021, Reference Maloney, Schiffbauer, Halverson, Xiao and Laflamme2022). During the Neoproterozoic Era (1000–539 Ma), eukaryotic algae evolved and proliferated through marine ecosystems, becoming the dominant primary producers by at least the Cryogenian (~720–635 Ma; Brocks et al., Reference Brocks, Jarrett, Sirantoine, Hallmann, Hoshino and Liyanage2017; Sánchez-Baracaldo et al., Reference Sánchez-Baracaldo, Raven, Pisani and Knoll2017; Isson et al., Reference Isson2018). This algal diversification is interpreted to represent a major step-wise change in the makeup of Neoproterozoic paleoenvironments by restructuring benthic habitats and biogeochemical cycles (Del Cortona et al., Reference Del Cortona2020), which are thought to have played an important role in setting the stage for the emergence of diverse animals (Brocks, Reference Brocks2018).
To understand the drivers of the critical change in Earth's biosphere, it is important to examine and document fossil algal diversity (LoDuca et al., Reference LoDuca, Bykova, Wu, Xiao and Zhao2017). However, the current record from the Proterozoic is limited due to taphonomic biases associated with the fossilization of soft tissues (Muscente et al., Reference Muscente2017; Maloney et al., Reference Maloney, Schiffbauer, Halverson, Xiao and Laflamme2022). Recently, calls for detailed investigations of the fossil record during poorly documented time intervals (Cohen and Macdonald, Reference Cohen and Macdonald2015; Bykova et al., Reference Bykova, LoDuca, Ye, Marusin, Grazhdankin and Xiao2020) have highlighted the sparse records of the Tonian (e.g., Pang et al., Reference Pang, Tang, Chen, Wan, Niu, Yuan and Xiao2018; Xiao and Tang, Reference Xiao and Tang2018) and Cryogenian (e.g., Ye et al., Reference Ye, Tong, Xiao, Zhu, An, Tian and Hu2015) periods. These intervals are of particular interest because Proterozoic macroalgae experienced a significant morphological diversification during this time (Xiao and Tang, Reference Xiao and Tang2018; Bykova et al., Reference Bykova, LoDuca, Ye, Marusin, Grazhdankin and Xiao2020; Tang et al., Reference Tang, Pang, Yuan and Xiao2020).
The extensive Proterozoic stratigraphy in Arctic Canada provides an ideal locality to target Tonian eukaryotic fossils. Previous studies have yielded numerous exceptional fossil localities (Hofmann and Aitken, Reference Hofmann and Aitken1979; Butterfield, Reference Butterfield2000; Cohen and Macdonald, Reference Cohen and Macdonald2015; Loron et al., Reference Loron, François, Rainbird, Turner, Borensztajn and Javaux2019), which include poorly constrained forms such as the Little Dal macrobiota (Hofmann and Aitken, Reference Hofmann and Aitken1979; Hofmann, Reference Hofmann1985) and the Rusty and Wynniatt assemblages (Butterfield, Reference Butterfield2005a, Reference Butterfieldb); vase-shaped (Strauss et al., Reference Strauss, Rooney, MacDonald, Brandon and Knoll2014; Cohen et al., Reference Cohen, Irvine and Strauss2017a) and scale-like (Cohen and Knoll, Reference Cohen and Knoll2012) microfossils from the Fifteenmile Group; Shaler Supergroup fungal microfossils (Loron et al., Reference Loron, François, Rainbird, Turner, Borensztajn and Javaux2019); red algal microfossils of the Bylot basins (Butterfield, Reference Butterfield1990; Knoll et al., Reference Knoll, Wörndle and Kah2013; Gibson et al., Reference Gibson2018); and purported sponge fossils from the Little Dal Group (Turner, Reference Turner2021).
Recent studies of the Dolores Creek Formation, the basal unit of the Tonian Mackenzie Mountains Supergroup, have yielded 300+ fossils representing three distinct size classes (Maloney et al., Reference Maloney2021) that have yet to be formally described. Here we provide a detailed formal description of three new fossils from the Dolores Creek Formation, including a new species, Archaeochaeta guncho new genus new species, and likely examples of the oldest known Vendotaenia sp. We also discuss distinctive morphological characteristics that can be used to aid in identifying Proterozoic macroalgae, compare the morphology of the new fossils with other known Proterozoic life, and consider the consequences of these morphological advancements on Neoproterozoic ecosystems.
Geologic setting
The fossil-bearing beds of the Dolores Creek Formation outcrop at the headwaters of Hematite Creek River, a tributary of the Bonnet Plume River in the Wernecke Mountains near the Yukon–Northwest Territories (NWT) border in northwestern Canada (Fig. 1). The 950–900 Ma Dolores Creek Formation is the oldest of three formations in the Hematite Creek Group, which in turn is the oldest group in the Tonian Mackenzie Mountains Supergroup (950–775 Ma). Broadly equivalent Tonian strata across Arctic Canada include the Fifteenmile Group in western Yukon (Halverson et al., Reference Halverson, Macdonald, Strauss, Smith, Cox and Hubert-Théou2012; Macdonald et al., Reference Macdonald, Halverson, Strauss, Smith, Cox, Sperling and Roots2012) and the Shaler Supergroup in northern NWT (Rainbird et al., Reference Rainbird, Jefferson and Young1996). In the Wernecke Mountains, the Dolores Creek Formation unconformably overlies the Mesoproterozoic Pinguicula Group, which is a mixed siliciclastic and carbonate succession with a maximum age of 1380 Ma (Medig et al., Reference Medig, Thorkelson and Dunlop2010, Reference Medig, Thorkelson, Turner, Davis, Gibson and Marshall2012). The Black Canyon Creek Formation conformably overlies the Dolores Creek Formation and represents the shallowing-upward transition into tidally influenced deposits characterized by meter-scale carbonate–shale cycles (Turner, Reference Turner2011).

Figure 1. Fossil locality. (1) Stratigraphy log of the Mackenzie Mountains Supergroup with radiometric (purple and orange star) and stratigraphic age constraints. (2) Measured section where fossils were recovered (Yukon, Canada, 64°41′17.6′′N; 133°14′30.3′′W), scale in meters. (3) Fossil interval enlarged with individual fossil horizons labeled (OP1, SP1, SP2, RP1, SP3, RP2), scale in meters; x axis shows relative grain sizes from mud/shale to coarse sand. (4) Map of the Proterozoic inliers, including the Wernecke, Mackenzie Mountains, and Windermere supergroups that span the Yukon and Northwest Territories border in northwestern Canada, with a black rectangle indicating the study area. Gp.= Group; Fm. = Formation; Sta. = Statherian Period; Eta = Etagochile Formation; Sh. Ran. = Shatter Ridge Formation; Abr. Pl. = Abraham Plains Formation; Cryo = Cryogenian Period; E = Ediacaran Period; Winder. = Windermere supergroup; Mt. Land. = Mount Landreville Formation; Pass Mtn. = Pass Mountain Formation; SG = supergroup.
The strata of the Dolores Creek Formation consist of fine-grained siliciclastic rocks (shale to siltstone) interbedded with microbial dolostone interpreted as marginal marine to offshore deposits (Turner, Reference Turner2011; Maloney et al., Reference Maloney2021). The type section of the Dolores Creek Formation characteristically comprises ~300 m of strata but reaches a thicknesses of ~1,000 m in the southernmost studied sections, where it is informally divided into a lower and upper unit that together represent a single regressive sequence (Gibson et al., Reference Gibson2019). The lower unit comprises ~600 m of predominantly shales and siltstones with interbedded interclast breccias and wackestones interpreted as gravity-flow deposits (“debrites”). Increasing carbonate content within the debrites, and the occurrence of stromatolitic olistoliths up-section, hint at progradation of a shelf margin and presage the overlying unit, which consists of biostromes of columnar stromatolites interbedded with organic-rich shales. The strata within the lower Dolores Creek Formation where the fossils abruptly appear are interpreted as shelf-margin deposits controlled by a fault escarpment that formed in response to an extensional episode during the formation of the Hematite Creek Basin (Turner, Reference Turner2011; Gibson et al., Reference Gibson2019).
The first in situ carbonate unit from the Dolores Creek Formation is a minor microbially laminated bed ~50 m below the fossil beds, while the first semi-continuous stromatolitic biostromes occur ~20 m above the fossil interval (Fig. 1). The fossils occur within gravity-flow deposits that record the downslope movement and deposition of sediment from the photic zone at the platform margin (Maloney et al., Reference Maloney2021). The fossils appear along bedding planes throughout slabs interpreted to record rapid burial events on the basis of the organization of the thin beds of differing grain sizes interbedded with floatstone (carbonate debrites). These depositional processes contributed to the exceptional preservation of these organisms by emplacing them within (presumably) anoxic, sulfate-reducing conditions (Cai et al., Reference Cai, Schiffbauer, Hua and Xiao2012; Schiffbauer et al., Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014; Maloney et al., Reference Maloney, Schiffbauer, Halverson, Xiao and Laflamme2022).
Materials and methods
More than 340 individual fossil specimens from 17 in situ slabs collected from six distinct horizons, and five slabs with exceptionally preserved fossils recovered from float, were investigated (Figs. 1–5). Slabs contain 3–40 total specimens, with 1–22 per bedding surface. Some slabs were susceptible to fracturing, which exposed additional bedding surfaces that contained fossils. Such surfaces were included during data collection. In slabs with a high density of overlapping specimens, individual fossils were at times difficult to discern and in turn were excluded from both the total specimen count and the length measurements. Specimens were observed under a stereoscope. Whenever possible, morphometric data—including total organism length, cell height and width, cell-wall thickness, differentiated cells, specimen features (true and false branching, twisting/deformation, overlapping, tapering ends), and specimen shape (straight, slightly curved, U-shaped, V-shaped, J-shaped, or S-shaped)—were collected using the open-source program ImageJ (Schneider et al., Reference Schneider, Rasband and Eliceiri2012). Three distinct size classes of fossils were defined on the basis of widths (Fig. 6). The widest specimens (n = 19, width = 1.0–1.7 mm) are interpreted to represent Vendotaenia sp. The medium-sized specimens (n = 304, width = 0.20–0.85 mm) show significant morphological detail and are attributed to a newly defined form, Archaeochaeta guncho n. gen. n. sp. Specimens of the narrowest size class (n = 90, width = 0.03–0.06 mm) were abundant throughout the section and found on every slab collected, although their small size and lack of detail precludes any definitive taxonomic identification and phylogenetic interpretation.

Figure 2. Archaeochaeta guncho n. gen. n. sp. (1, 2) ROMIP66169 fossil slab showing the distribution of macroalgal fossils on each side and the locations of enlarged specimens in (3–6). (3) Holotype specimen 59.18 with an elongated holdfast (white arrowhead with black outline), longitudinal striations (black arrowhead, inset), and double septa (white arrowhead with gray outline). (4) Specimen 59.22, longitudinal striations (black arrowhead, inset) and double septa (white arrowhead with gray outline, inset). (5) Paratype specimen 59.28, elongated holdfast (white arrowhead with black outline) and longitudinal striations (black arrowhead). (6) Specimen 59.9 with individual cells. (7) Idealized sketch showing morphological traits and morphometric measurements. Scale bars = 1 mm.

Figure 3. Three-dimensional (3D) preservation in Archaeochaeta guncho n. gen. n. sp. (1) ROMIP66170, 3D preservation of longitudinal striations (black arrowhead) in SEM image. (2) ROMIP66169, 2D preservation of longitudinal striations (black arrowhead) in SEM image. (3) ROMIP66170, 3D preservation of several fossils in SEM image. (4–6) Light microscope photos showing 3D preservation of ROMIP66170, showing fossil borders (dotted lines), longitudinal striations (black arrowhead), and a thin filament of unnamed species (white arrowhead). Scale bars = 1 mm.
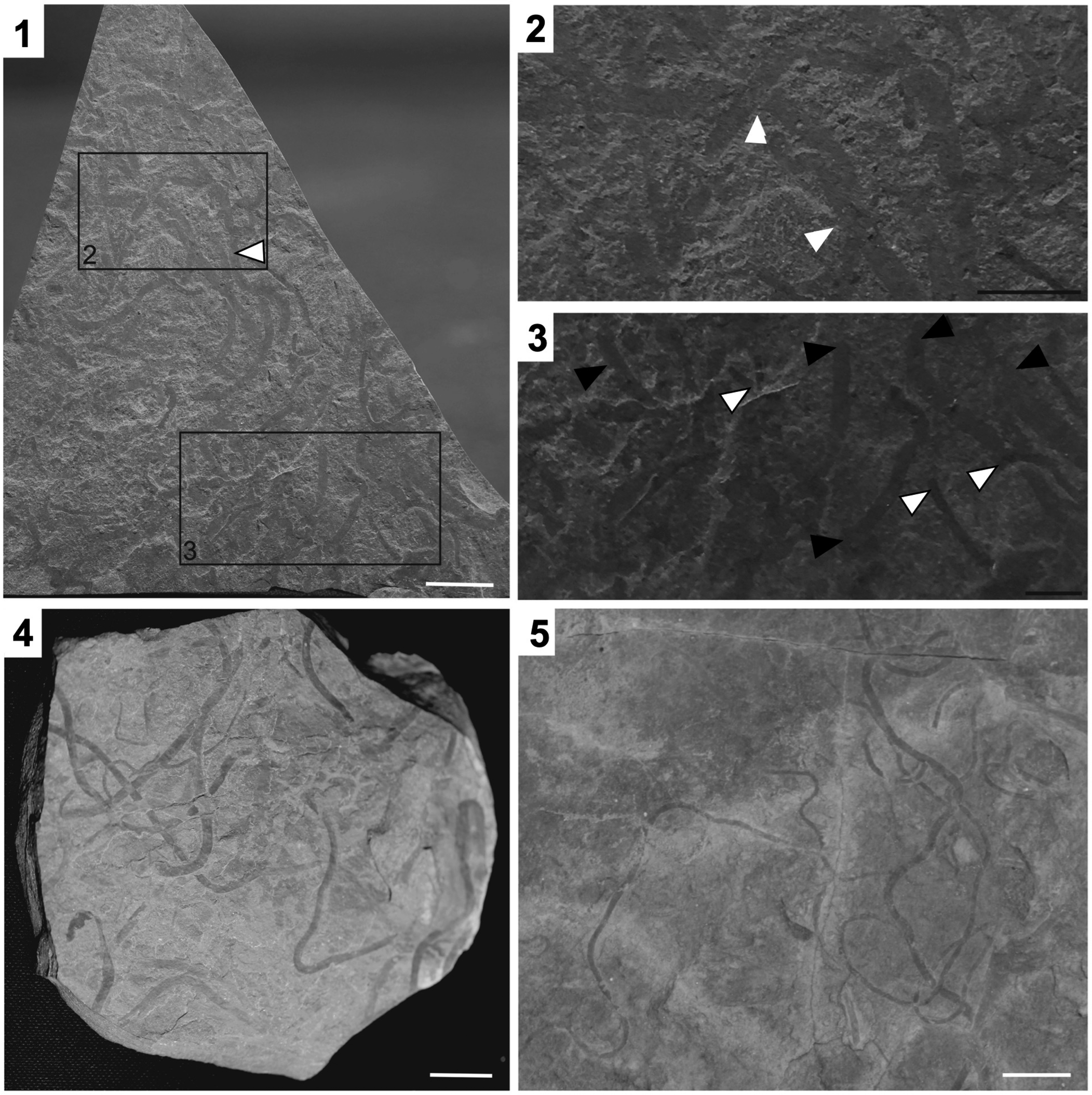
Figure 4. Vendotaenia sp. (1) Fossil slab ROMIP66283 with larger fossils assigned to Vendotaenia sp. (example indicated with white arrowhead) while smaller fossils are Archaeochaeta guncho n. gen. n. sp. (2) Vendotaenia sp. (labeled rectangle in (1)) showing overlapping specimens (white arrowheads). (3) Archaeochaeta guncho n. gen. n. sp. (white arrowhead with black outline) and Vendotaenia sp. (black arrowhead) in area marked by labeled rectangle in (1), demonstrating size difference between the two taxa. (4, 5) Vendotaenids from the Ediacaran Feldschuhhorn Member of the Nama Group, Namibia, for morphological comparison. (1, 4, 5) White scale bars = 1 cm; (2, 3) black scale bars = 0.5 mm.

Figure 5. Unnamed Dolores Creek macrofossils. (1, 3, 6, 7, 9) Examples of true branching (black arrows). (2, 4, 5, 8) Examples of non-branching thalli. (5, 7) Smaller macrofossil adjacent to Archaeochaeta guncho n. gen. n. sp. (white arrows) to demonstrate size difference between the two taxa. (10) Idealized sketch showing morphological traits. (2, 5–7) White scale bars = 1 mm; (1, 3, 4, 8, 9) black scale bars = 0.5 mm. All specimens from slab ROMIP66167.

Figure 6. Frequency distribution of fossil width measurements by taxon, showing three separate size classes: the smallest size class ranges from 0.03 to 0.06 mm (unnamed taxon), the medium size class ranges from 0.20 to 0.85 mm (Archaeochaeta guncho n. gen. n. sp.), and the large size class ranges from 1.0 to 1.7 mm (Vendotaenia sp.). Note the scale on the x axis is different for the unnamed taxon compared with the two other size classes.
Selected specimens of the two smallest size classes (n = 12) were targeted for additional analyses using scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (EDS) and tomographic X-ray microscopy (μCT) at the University of Missouri X-ray Microanalysis Core Laboratory (see Maloney et al., Reference Maloney, Schiffbauer, Halverson, Xiao and Laflamme2022). A Zeiss Sigma 500 variable-pressure system equipped with dual, co-planar Bruker XFlash spectrometers was used to conduct the SEM and EDS analyses at identical beam and chamber conditions (20 keV beam accelerating voltage, 40 nA high current mode, 60 μm aperture, and 20 Pa chamber pressure with a 99.999% nitrogen atmosphere). Z-contrast imaging was conducted using a high-definition five-segment backscatter detector, and a cascade current detector was used for secondary electron imaging in low vacuum. EDS elemental mapping was used to characterize the composition of the specimens, with both spectrometers used in tandem (360 seconds live time, 120 μm aperture). A single specimen preserved in three dimensions was further analyzed through μCT (Zeiss Xradia 510 Versa). The operating conditions were as follows: 80 kV source voltage, 7 W source power, LE3 (low energy) filter, 0.4× objective, 4.5 sec exposure, 2001 projections at 360°, and voxel size 11.09 μm.
Repository and institutional abbreviation
Types, figured, and other specimens are reposited with the Royal Ontario Museum (ROMIP) in Toronto, Canada (ROMIP66160–ROMIP66170; ROMIP66282–ROMIP66292).
Systematic paleontology
The size distribution of the fossil assemblage was investigated to determine whether these fossils include distinct species. When fossil widths are compared, three distinct size classes emerge (Fig. 6) regardless of preservational quality (see Supplemental Data 1). Although the affinities of many Proterozoic fossils remain controversial, a subset of exceptional Dolores Creek Formation fossils have clear morphological characteristics that aid in their interpretation as eukaryotic macroalgae with a possible green algal affinity (Maloney et al., Reference Maloney2021); thus, the International Code of Nomenclature for Algae, Fungi, and Plants (Turland et al., Reference Turland2018) is followed in this paper. The largest size class ranges from 1.0 to 1.7 mm, and its specimens are attributed to Vendotaenia Gnilovskaya, Reference Gnilovskaya1971 on the basis of their ribbon-like morphology and comparable size. The middle size class has the most complex morphology preserved, with holdfasts, longitudinal striations, and large cells with a thallus width ranging from 0.20 to 0.85 mm. The smaller fossils range in width from 0.03 to 0.06 mm and show dichotomous branching. Given that these smaller fossils lack any morphologically distinct characters, we have not assigned them to a specific taxonomic rank and instead provide a description of the fossils as well as comparisons with other Proterozoic fossils.
Kingdom Archaeplastida Adl et al., Reference Adl2005
Phylum Viridiplantae Cavalier-Smith, Reference Cavalier-Smith1981
Division Chlorophyta Reichenbach, Reference Reichenbach1828
Genus Archaeochaeta new genus
Type species
Archaeochaeta guncho n. gen. n. sp. by monotypy.
Diagnosis
As per species.
Occurrence
Dolores Creek Formation, Mackenzie Mountains Supergroup, near the headwaters of Hematite Creek (64°41′17.6″N; 133°14′30.3″W), Wernecke Mountains, Yukon Territory, Canada; Tonian (Maloney et al., Reference Maloney2021).
Etymology
From Greek, archaeo, meaning “ancient,” with reference to the geological age of this genus, and chaeto, meaning “hair or bristle,” owing to its morphological comparison to the extant green algal genus Chaetomorpha Kützing, Reference Kützing1845.
Holotype
Specimen 59.18 on ROMIP66169, illustrated in Figure 2.2, 2.3.
Paratype
Specimen 59.28 on ROMIP6616, illustrated in Figure 2.1, 2.5.
Diagnosis
Multicellular, uniseriate, unbranching thallus of uniform width. Individual cells are rectangular (width greater than length) with two (double) septa between adjacent cells and rib-like cell-wall ornamentation parallel to the thallus length. Ellipsoidal to globose holdfasts rarely present.
Occurrence
Dolores Creek Formation, Mackenzie Mountains Supergroup, near the headwaters of Hematite Creek, Wernecke Mountains, Yukon Territory, Canada; Tonian (Maloney et al., Reference Maloney2021).
Description
Uniseriate filament of multiple individual cells. Thallus length ranges from 1.0 to 32.7 mm (n = 304 specimens on 20 fossil slabs) with sharp terminations at one or both ends of the filaments. Thallus widths are consistent along the entire length, with an average width of 0.67 mm (ranges from 0.27 to 0.80 mm, n = 304). Each well-preserved cell contains a recalcitrant cell wall with rib-like cell-wall ornamentation (Figs. 2.3–2.7, 3) resembling longitudinal striations in modern green algae (Gontcharov and Watanabe, Reference Gontcharov and Watanabe1999; Maloney et al., Reference Maloney2021).
Each filament is composed of cells with transverse cell walls or septa (perpendicular to thallus length) preferentially preserved compared with the lateral cell walls (parallel to thallus length; Fig. 2.3–2.7). Lateral cell walls are on average 0.06 mm thick (n = 40) while transverse cell walls are 0.10 mm thick (n = 410). Individual cells are rectangular (presumably originally cylindrical), ranging from 0.2 to 1.0 mm long and 0.3 to 0.7 mm wide. The presence of double septa rather than a single thick transverse cell wall is suggested by the occurrence of a clear gap between two adjacent cells (Fig. 2.3–2.7). Holdfasts are rare, but when found they are elliptical to globose and are located at the terminus of the thallus (Fig. 2.1–2.3, 2.5, 2.7). Holdfasts range from 0.19 to 0.98 mm (mean = 0.53 mm, n = 6) in the longest dimension and from 0.19 to 0.58 mm (mean = 0.40 mm, n = 6) in the shortest dimension. However, these unequal dimensions could represent a preservational bias of an originally spherical structure.
Etymology
The fossils reported herein were recovered from the traditional territory of the First Nation of Na-Cho Nyak Dun. The species epithet guncho is derived from Northern Tutchone, the language of the First Nation of Na-Cho Nyak Dun (Billy and Wheeler, Reference Billy and Wheeler1998; Ritter, Reference Ritter2015), meaning “big worm.” The name references the large size and visible segment-like morphology of the organism.
Materials
A total of 304 specimens from the Dolores Creek Formation, Mackenzie Mountains Supergroup, near the headwaters of Hematite Creek, Wernecke Mountains, Yukon Territory, Canada; Tonian (Maloney et al., Reference Maloney2021).
Remarks
Specimens are fragmented (likely transported downslope via gravity flows; Maloney et al., Reference Maloney2021), and therefore the measured lengths best represent minimum estimates. The thallus is unbranching, with consistent width along its entire length. However, the preservational quality differs between transverse and lateral cell walls. The transverse cell walls (between cells) are generally 1.7 times the thickness of lateral cell walls (parallel to thallus length), which likely represents a taphonomic artifact created by the higher preservation potential of transverse double septa compared with cell walls.
Thallus architecture is important for higher-level classification. When specimens are found in dense accumulations and preserved as two-dimensional compressions (Maloney et al., Reference Maloney, Schiffbauer, Halverson, Xiao and Laflamme2022), it can be difficult to differentiate confidently between a single branching specimen and the overlap of two unbranching specimens of similar size (e.g., Fig. 2.4). We note that the thallus filaments have sharp terminations where they interact with another filament and that double septa are poorly preserved or even at times abruptly absent at the junction. We note that there is no cellular differentiation at the thallus junction or even any curvature change in the taphonomic “branching” angle. These features all point to a structurally simple uniserial unbranching thallus for Archaeochaeta.
Uniseriate filaments are common among green algae (South and Whittick, Reference South and Whittick1987), whereas the majority of modern brown algae possess parenchymatous cells, and red algae possess pseudoparenchymatous forms (Graham and Wilcox, Reference Graham and Wilcox2000). Cells in parenchymatous thalli divide in all directions, resulting in a three-dimensional algal construction rather than a one-dimensional filamentous structure (Barsanti and Gualtieri, Reference Barsanti and Gualtieri2014). By contrast, pseudoparenchymatous forms are tessellations of multiple filaments that are intertwined and/or branched, preserving the filamentous construction (Barsanti and Gualtieri, Reference Barsanti and Gualtieri2014). Some modern rhodophytes, such as compsopogonophyceans and bangiophyceans (Yoon et al., Reference Yoon, Nelson, Lindstrom, Boo, Pueschel, Qiu, Bhattacharya, Archibald, Simpson and Slamovits2016), possess a uniseriate filamentous construction in their thalli, but their filaments typically consist of uniquely arranged paired cells with multiseriate or corticated thalli (Krishnamurthy, Reference Krishnamurthy1962; Butterfield, Reference Butterfield2000). Given that A. guncho n. gen. n. sp. consists of a uniseriate, filamentous thalli with unidirectional growth, it is unlikely that it represents a red or brown alga; the large size, uniseriate filamentous construction with elongated holdfasts, and rib-like wall ornamentation all support a green macroalgal affinity (Maloney et al., Reference Maloney2021).
The comparatively large size of Archaeochaeta invites comparison to the Proterozoic macrofossil Grypania Walter et al., Reference Walter, Oehler and Oehler1976. However, Archaeochaeta has exceptionally preserved complex morphology with differentiated cell walls and holdfasts, while Grypania typically lacks such structures, although Sharma and Shukla (Reference Sharma and Shukla2009) have interpreted transverse markings as cell walls in specimens from India. Furthermore, Grypania is typically observed as coils whereas Archaeochaeta specimens are typically straight and only rarely curved, twisted, or folded over upon themselves, suggesting that the filaments had some degree of structural integrity or rigidity.
Eosolena Hermann in Hermann and Timofeev, Reference Hermann and Timofeev1985 from the late Mesoproterozoic Lakhanda biota (German and Podkovyrov, Reference German and Podkovyrov2009; Hermann and Podkovyrov, Reference Hermann and Podkovyrov2014) and the Mesoproterozoic Kotuikan biota (Vorob'eva et al., Reference Vorob'eva, Sergeev and Petrov2015) can be compared to Archaeochaeta given their shared filamentous construction with a holdfast (German and Podkovyrov, Reference German and Podkovyrov2009). However, Eosolena is notably smaller (<0.25 mm wide versus 0.27–0.80 mm; Vorob'eva et al., Reference Vorob'eva, Sergeev and Petrov2015). Archaeochaeta is also superficially similar to Segmentothallus asperus Hermann in Yankauskas et al., Reference Yankauskas, Mikhailova and Hermann1989 from the ca. 1000 Ma Lakhanda biota, but the latter too is much narrower (~30 μm; German and Podkovyrov, Reference German and Podkovyrov2009). The Mesoproterozoic–Tonian Lakhanda Group also includes “eosolenides tubular fossils,” an informal group of macroscopic organic-walled tubes that are slightly larger (0.4–1.0 mm) than the Dolores Creek macroalgae. Some eosolenides even have thin, straight, closely spaced fine fibro-lamellar striations, which are broadly comparable to the longitudinal striations observed within the cells of Archaeochaeta. Eosolenides segments are separated by membranes and vary from box-like (0.15–0.20 mm) to barrel-shaped (0.40–0.60 mm), the latter of which are similar in size to Archaeochaeta. However, the Archaeochaeta cells are wider than they are long whereas eosolenides segments are longer than they are wide.
The modern cyanobacterium Nostoc flagelliforme Harvey ex Molinari-Novoa et al. in Calvo-Pérez et al., Reference Calvo-Pérez, Molinari-Novoa and Guiry2016 (fat choy) is superficially similar to Archaeochaeta in that the colony of trichomes can reach up to 1 mm in diameter. However, the majority of the cells in N. flagelliforme are two orders of magnitude smaller (0.004–0.005 mm) than the cells within Archaeochaeta (Wang and Gu, Reference Wang and Gu1984). In addition, N. flagelliforme hosts larger cells (heterocysts) that typically occur throughout the thallus (Gao, Reference Gao1998), as opposed to the uniform cell size (except for the holdfast) of Archaeochaeta.
Genus Vendotaenia Gnilovskaya, Reference Gnilovskaya1971
Type species
Vendotaenia antiqua Gnilovskaya Reference Gnilovskaya1971, p. 375.
Vendotaenia sp.
Figure 4.1–4.3
Occurrence
Dolores Creek Formation, Mackenzie Mountains Supergroup, near the headwaters of Hematite Creek, Wernecke Mountains, Yukon Territory, Canada; Tonian.
Description
Specimens consist of a slender, ribbon-shaped thalli. Width: 1.01–1.69 mm; length: 4.96–40.8 mm (n = 19), with no variation along the length of the organism. Specimen length terminates sharply, and given the high density of specimens, numerous examples of overlap between specimens is noted (Fig. 4). Surficial features and cross-walls not evident.
Materials
Nineteen specimens from the Dolores Creek Formation, Mackenzie Mountains Supergroup, near the headwaters of Hematite Creek, Wernecke Mountains, Yukon Territory, Canada; Tonian.
Remarks
One fossil slab with 28 specimens (nine specimens of Archaeochaeta and 19 of Vendotaenia sp.; Fig. 4.1–4.3) was recovered. Vendotaenia sp. differs from Archaeochaeta in its much larger overall size and complete absence of morphological characters on the fossil surface, such as striations or cell walls. V. antiqua typically includes visible longitudinal striations (Gnilovskaya, Reference Gnilovskaya1971, Reference Gnilovskaya, Sokolov and Iwanowski1990), which are missing from our specimens, although this absence could be taphonomic. For example, Gaucher et al. (Reference Gaucher, Boggiani, Sprechmann, Sial and Fairchild2003, Reference Gaucher, Blanco, Chiglino, Poiré and Germs2008) and Becker-Kerber et al. (Reference Becker-Kerber2021) suggest that the longitudinal striations in Vendotaenia could result from the compression of an originally circular tube and caution with the use of this character for diagnostic purposes.
Vendotaenia sp. closely resembles tubular compression from the Vingerbreek and Felschuhhorn members of the Ediacaran Nama Group of Namibia (Fig. 4.4, 4.5). Cohen et al. (Reference Cohen2009) assigned the Felschuhhorn specimens to Vendotaenia antiqua due to their sinuous bending, fine longitudinal striations, and rare branching (Gnilovskaya, Reference Gnilovskaya1971; Germs et al., Reference Germs, Knoll and Vidal1986; Cohen et al., Reference Cohen2009). Vendotaenia sp. does superficially resemble the unassigned Vingerbreek population, falling within the size range of the Vingerbreek specimens (1.0–1.7 mm compared with 0.6–2.1 mm).
The morphology of Vendotaenia sp. warrants comparison with another filamentous fossil from the Mackenzie Mountains Supergroup, Daltaenia mackenziensis Hofmann, Reference Hofmann1985, which shares a similar size range (width 0.3–1.5 mm) and a similar preservation mode with Vendotaenia sp. (both preserved as carbonaceous or pyritized compressions). However, D. mackenziensis exhibits clear branching and lacks the bending and twisting apparent in Vendotaenia sp., suggesting a greater structural rigidity (Hofmann, Reference Hofmann1985). However, Vendotaenia sp. appears to be more rigid than Grypania from the Belt Supergroup, Montana (Walter et al., Reference Walter, Oehler and Oehler1976; Han and Runnegar, Reference Han and Runnegar1992) since Vendotaenia sp. lacks any coiling. Vendotaenia sp. can also be compared to Proterotainia Walter et al., Reference Walter, Oehler and Oehler1976, which shares a ribbon-like shape ranging from 0.6 to 2.0 mm wide and for which longitudinal striations are common (Walter et al., Reference Walter, Oehler and Oehler1976). Proterotainia is much longer (up to 125 mm) than Vendotaenia sp., but the fragmentary nature of all specimens from northwestern Canada makes it difficult to compare them on the basis of length (Walter et al., Reference Walter, Oehler and Oehler1976).
The phylogenetic affinity of Vendotaenia remains unresolved, and the taxon is poorly defined (Bykova et al., Reference Bykova, LoDuca, Ye, Marusin, Grazhdankin and Xiao2020). This genus was first interpreted as macroalgae (Gnilovskaya, Reference Gnilovskaya, Urbanek and Rozanov1983) on the basis of its morphological characteristics, including oogonia (the female sex organ of some algae and fungi) and cell walls (Cohen et al., Reference Cohen2009). The characteristic longitudinal striations in Vendotaenia are present in modern bacteria such as Thioploca Lauterborn, Reference Lauterborn1907 (Vidal, Reference Vidal1989), which tends to be an order of magnitude smaller (Cohen et al., Reference Cohen2009), and could instead represent the effects of compressing a three-dimensional tube into a two-dimensional film (Gaucher et al., Reference Gaucher, Boggiani, Sprechmann, Sial and Fairchild2003, Reference Gaucher, Blanco, Chiglino, Poiré and Germs2008; Becker-Kerber et al., Reference Becker-Kerber2021). An algal interpretation for Vendotaenia remains probable given that the brown alga Chorda Stackhouse, Reference Stackhouse1797, the green alga Enteromorpha Link, Reference Link and Von Esenbeck1820, and the red alga Nemalion Duby, Reference Duby1830 all represent potential modern analogs (Cohen et al., Reference Cohen2009).
Despite previous interpretations of Vendotaenia as a brown alga (Phaeophyceae; Gnilovskaya, Reference Gnilovskaya, Urbanek and Rozanov1983), we follow Cohen et al. (Reference Cohen2009), who proposed a green or red algal affinity as more likely, particularly considering that molecular clocks indicate a significantly younger (i.e., Mesozoic) origin for brown algae (Silberfeld et al., Reference Silberfeld, Leigh, Verbruggen, Cruaud, de Reviers and Rousseau2010; Bringloe et al., Reference Bringloe2020). By contrast, photosynthesizing eukaryotes, including red and green algal lineages, are hypothesized to have originated in the mid-Proterozoic, with the diversification of major clades of Archaeplastida in the Neoproterozoic (Eme et al., Reference Eme, Sharpe, Brown and Roger2014; Knoll, Reference Knoll2014; Yang et al., Reference Yang, Boo, Bhattacharya, Saunders, Knoll, Fredericq, Graf and Yoon2016; Hou et al., Reference Hou, Ma, Shi, Li, Yang, Xiao, Leliaert and Zhong2022). Notwithstanding limitations on interpretations imposed by taphonomy (Maloney et al., Reference Maloney, Schiffbauer, Halverson, Xiao and Laflamme2022), a green algal affinity for Vendotaenia is consistent with its overall large thallus size and longitudinal striations. Body fossils of both red (Butterfield, Reference Butterfield2000; Gibson et al., Reference Gibson2018) and green (Tang et al., Reference Tang, Pang, Yuan and Xiao2020) algae have been found in ca. 1 Ga rocks, which is also consistent with the interpretation of Vendotaenia as a green or red alga.
Unnamed species
Figure 5
Materials
Ninety specimens from the Dolores Creek Formation, Mackenzie Mountains Supergroup, near the headwaters of Hematite Creek, Wernecke Mountains, Yukon Territory, Canada; Tonian.
Occurrence
Dolores Creek Formation, Mackenzie Mountains Supergroup, near the headwaters of Hematite Creek, Wernecke Mountains, Yukon Territory, Canada; Tonian (Maloney et al., Reference Maloney2021).
Description
Ninety specimens of thin filamentous forms with widths ranging from 30 to 50 μm (Fig. 5) and preserved lengths ranging from 230 to 4,900 μm. Some of these specimens show asymmetrical branching (Fig. 5.1, 5.3, 5.7, 5.9), with a single specimen showing presumed dichotomous branching (Fig. 5.6). These specimens are found on the same bedding planes as Archaeochaeta guncho n. gen. n. sp. and were first reported as “smaller Dolores Creek macroalgae” by Maloney et al. (Reference Maloney2021).
Remarks
Simple ribbons can resemble trace fossils in the field. However, there are sharp terminations at the ends of the specimens that would not be expected in trace fossils and instead are likely a result of fragmentation during transport. Cross-cutting relationships are common in trace fossils and not observed in these specimens. Most specimens show minimal distortion of their overall shape, suggesting that the filaments had a degree of structural integrity. A subset of specimens, however, are deformed into J- and C-shaped curves, suggesting these organisms were deformed plastically during transport and burial. This evidence, along with their consistent size, provides support for the biogenicity of the specimens.
The phylogenetic affinity of these specimens remains unresolved due to a lack of morphologically diagnostic characters. The Dolores Creek specimens are poorly preserved and extensively pyritized, which is known to limit the morphological detail in organisms lacking hard parts (Briggs et al., Reference Briggs, Raiswell, Bottrell, Hatfield and Bartels1996; Petrovich, Reference Petrovich2001; Schiffbauer et al., Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014). These fossils contain very limited organic carbon and cannot be extracted by acid dissolution. Continued investigations may help resolve their affinity, but until more diagnostic specimens are recovered, we adopt a conservative approach in their taxonomic treatment.
The size and general morphology of these organisms are similar to the green algal fossil Proterocladus Butterfield in Butterfield et al., Reference Butterfield, Knoll and Swett1994 reported from Svalbard and North China (Tang et al., Reference Tang, Pang, Yuan and Xiao2020). These organisms also resemble extant and fossil false-branching cyanobacteria, such as Ramivaginalis Nyberg and Schopf, Reference Nyberg and Schopf1984, Pseudodendron Butterfield in Butterfield et al., Reference Butterfield, Knoll and Swett1994, “unnamed form 2” in Vorob'eva et al. (Reference Vorob'eva, Sergeev and Petrov2015), and “dichotomously branching filamentous form” in Nagovitsin et al. (Reference Nagovitsin, Rogov, Marusin, Karlova, Kolesnikov, Bykova and Grazhdankin2015). Ramivaginalis is morphologically similar to the smaller Dolores Creek macrofossils with a branching nonseptate structure (Nyberg and Schopf, Reference Nyberg and Schopf1984). However, the Dolores Creek form taxon is an order of magnitude larger (widths of 30–50 μm versus 4–9 μm; Sergeev et al., Reference Sergeev, Sharma and Shukla2012). Pseudodendron is another filamentous, branching fossil that is similar in size to the unnamed Dolores Creek specimens, but it is characterized by longitudinal striations, and its branch junctions are reinforced by sheaths (Butterfield et al., Reference Butterfield, Knoll and Swett1994; Nagovitsin et al., Reference Nagovitsin, Rogov, Marusin, Karlova, Kolesnikov, Bykova and Grazhdankin2015). Pseudodendron is an interesting comparison because it has been interpreted as multiseriate and false branching, similar to modern Schizothrix-type cyanobacteria. Nevertheless, the poor preservation of the Dolores Creek forms precludes conclusive assignment to this genus. Unnamed form 2 from the ca. 1500 Ma Kotuikan Formation of Siberia (Vorob'eva et al., Reference Vorob'eva, Sergeev and Petrov2015) is a dichotomously branching thallus that contains numerous vesicles. It is similar in size to the Dolores Creek form taxon (~20 μm in diameter) but can be distinguished by the spherical vesicles within its branching tubular thallus (Vorob'eva et al., Reference Vorob'eva, Sergeev and Petrov2015). In addition, unnamed branching fossils from the Tonian Kulady and Khastakh formations in Siberia (Nagovitsin et al., Reference Nagovitsin, Rogov, Marusin, Karlova, Kolesnikov, Bykova and Grazhdankin2015, figs. 9Q–R) resemble the Dolores Creek unnamed filaments. These fossils can be differentiated on the basis of the typical dichotomous branching in the unnamed branching fossils that is exceedingly rare (if even real) in the unnamed species presented here.
Discussion
Detailed investigation of the macrofossil assemblage from the basal Mackenzie Mountains Supergroup reveals at least three distinct organisms. These fossils, along with green algal fossils from North China (Tang et al., Reference Tang, Pang, Yuan and Xiao2020) and probable green algae from the Congo Basin (Sforna et al., Reference Sforna2022), suggest that green algae had colonized marine environments by the early Tonian and likely had profound impacts on their ancient environments by reorganizing seafloor habitats and influencing biogeochemical cycles. Macroalgae utilize bio-essential trace elements, including nitrogen, phosphorus, and metals (e.g., iron and zinc) that are understood to play an important role in the evolution of early life on Earth (Anbar and Knoll, Reference Anbar and Knoll2002; Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Knoll and Nowak, Reference Knoll and Nowak2017; Isson et al., Reference Isson2018). These organisms were also photosynthetic, producing oxygen in shallow marine environments and possibly creating oxygen oases in an ocean with anoxic deep waters (Lyons et al., Reference Lyons, Diamond, Planavsky, Reinhard and Li2021; H. Wang et al., Reference Wang, Liu, Li, Feng, Tang, Cheng and Algeo2021; Wang et al., Reference Wang2022).
Interpreting Proterozoic algae
Early algal fossils remain poorly documented and are fraught by uncertainty. Fossilization of cellular-level tissues can occur only under exceptional taphonomic circumstances. Microfossils and simple macrofossils were understood to dominate the early Neoproterozoic (Xiao and Dong, Reference Xiao, Dong, Xiao and Kaufman2006; Xiao and Tang, Reference Xiao and Tang2018) until more recent discoveries of relatively complex macroalgae (Tang et al., Reference Tang, Pang, Yuan and Xiao2020; Maloney et al., Reference Maloney2021). These fossils have challenged our understanding of algal ecosystems (e.g., Brocks et al., Reference Brocks, Jarrett, Sirantoine, Hallmann, Hoshino and Liyanage2017) and can help calibrate molecular clocks of algal evolution (e.g., Hou et al., Reference Hou, Ma, Shi, Li, Yang, Xiao, Leliaert and Zhong2022).
Enigmatic, long-ranging macroscopic fossils—such as Chuaria Walcott, Reference Walcott1899 and Grypania Walcott, Reference Walcott1899, both dating back to the Paleoproterozoic (2500–1600 Ma; Hofmann and Jinbiao, Reference Hofmann and Jinbiao1981; Han and Runnegar, Reference Han and Runnegar1992; Schneider et al., Reference Schneider, Bickford, Cannon, Schulz and Hamilton2002), as well as Neoproterozoic vendotaenids (Gnilovskaya, Reference Gnilovskaya1971, Reference Gnilovskaya, Sokolov and Iwanowski1990; Hofmann and Rainbird, Reference Hofmann and Rainbird1994; Cohen et al., Reference Cohen2009; Ye et al., Reference Ye, Tong, Xiao, Zhu, An, Tian and Hu2015)—have also been interpreted as algae (Walter et al., Reference Walter, Oehler and Oehler1976; Vidal, Reference Vidal1989). However, given their simple morphologies and taphonomic limitations, these interpretations have been questioned (Sun, Reference Sun1987; Steiner, Reference Steiner1996; Sharma and Shukla, Reference Sharma and Shukla2009). The ca. 1560 Ma Gaoyuzhuang biota of North China includes large carbonaceous compressions that have also been interpreted as algae (Zhu et al., Reference Zhu, Zhu, Knoll, Yin, Zhao, Sun, Qu, Shi and Liu2016), but in the absence of cellular preservation or other morphologically informative characters, their phylogenetic placement within broader algae remains uncertain.
There are several other Proterozoic fossils whose algal affinities remain unresolved that are superficially similar to Archaeochaeta, including the submillimeter eosolenid tubular fossils from late Mesoproterozoic strata in Siberia (German and Podkovyrov, Reference German and Podkovyrov2009) and early Neoproterozoic strata in North China (Li et al., Reference Li, Chen, Pang, Zhou, Han, Yang, Lv, Wu, Wang and Yang2020). Proteroarenicola Wang, Reference Wang1982, Pararenicola Wang, Reference Wang1982, Sinosabellidites Zheng, Reference Zheng1980, and Parmia Gnilovskaya et al., Reference Gnilovskaya, Veis, Bekker, Olovyanishnikov and Raaben2000 are annulated tubular fossils reported from early Neoproterozoic strata in India (Sharma and Shukla, Reference Sharma and Shukla2012), North China (Dong et al., Reference Dong, Xiao, Shen, Yuan, Yan and Peng2008; Li et al., Reference Li, Chen, Pang, Zhou, Han, Yang, Lv, Wu, Wang and Yang2020), and Russia (Gnilovskaya, Reference Gnilovskaya1998). These organisms were first interpreted as worm-like metazoans (Sun et al., Reference Sun, Wang and Zhou1986) but have been reinterpreted as cyanobacteria (Sharma and Shukla, Reference Sharma and Shukla2012) or macroalgae (Dong et al., Reference Dong, Xiao, Shen, Yuan, Yan and Peng2008) according to whether the “proboscis-like” structure of Sun et al. (Reference Sun, Wang and Zhou1986) is interpreted as an akinete-like body (Sharma and Shukla, Reference Sharma and Shukla2012) or a discoidal holdfast structure (Dong et al., Reference Dong, Xiao, Shen, Yuan, Yan and Peng2008).
Although our interpretations are restricted by taphonomy, there is growing evidence that complex algal ecosystems were present in marine environments by the early Neoproterozoic (Fig. 7). The oldest macroscopic green alga is Proterocladus from the ca. 1000 Ma Nanfen Formation in North China (Tang et al., Reference Tang, Pang, Yuan and Xiao2020) and the 790 Ma Svanbergfjellet Formation in Spitsbergen, which was recovered along with Palaeastrum Butterfield in Butterfield et al., Reference Butterfield, Knoll and Swett1994, a colonial-coenobial multicellular green alga comparable to extant hydrodictyacean green algae (Butterfield et al., Reference Butterfield, Knoll and Swett1994). Ligand conjugated acids that form complexes by binding with metals, specifically nickel-bound geoporphyrin moieties, have been observed in Arctacellularia tetragonala Maithy, Reference Maithy1975 from ca. 1000 Ma strata in the Congo Basin. These porphyrins have been interpreted as derivatives of chlorophyll, which supports an algal affinity for these forms (Sforna et al., Reference Sforna2022). Fossils from the Paleoproterozoic Ruyang Group in North China have also been interpreted as green algae, including the organic-walled microfossils Dictyosphaera Xing and Liu, Reference Xing and Liu1973, Shuiyousphaeridium Yan and Zhu, Reference Yan and Zhu1992, and Gigantosphaeridium Agić et al., Reference Agić, Moczydłowska and Yin2015. Bangiomorpha pubescens Butterfield, Reference Butterfield2000, recovered from strata in the Bylot basins of northeastern Canada, is the oldest unequivocal red algal fossil (Butterfield, Reference Butterfield2000; Knoll et al., Reference Knoll, Wörndle and Kah2013; Gibson et al., Reference Gibson2018), although still older microfossils from the ca. 1.6 Ga Tirohan Dolomite in central India have also been interpreted as red algae (Bengtson et al., Reference Bengtson, Sallstedt, Belivanova and Whitehouse2017), while younger spores of possible red algal affinity have been reported from Cryogenian rocks in Mongolia (Cohen et al., Reference Cohen, Vizcaíno and Anderson2020). The record of diverse eukaryotic fossils from the Mesoproterozoic–Neoproterozoic transition is rapidly expanding and requires detailed descriptions of fossil morphology to understand their role within evolving ecosystems.

Figure 7. Summary of the evolution and diversification of eukaryotes in the Proterozoic Eon. Examples of documented fossils with age constraints include the cyanobacteria Eoentophysalis (Hofmann, Reference Hofmann1976; Hodgskiss et al., Reference Hodgskiss, Crockford, Peng, Wing and Horner2019), fungal microfossils Ourasparia (Loron et al., Reference Loron, François, Rainbird, Turner, Borensztajn and Javaux2019), red algal microfossils Bangiomorpha (Butterfield, Reference Butterfield2000; Gibson et al., Reference Gibson2018), green macroalgae Proterocladus (Butterfield et al., Reference Butterfield, Knoll and Swett1994; Tang et al., Reference Tang, Pang, Yuan and Xiao2020), Archaeochaeta from the Dolores Creek Formation (this paper), vase-shaped microfossils (Porter and Knoll, Reference Porter and Knoll2000; Strauss et al., Reference Strauss, Rooney, MacDonald, Brandon and Knoll2014; Porter and Riedman, Reference Porter and Riedman2016; Cohen et al., Reference Cohen, Irvine and Strauss2017a), phosphatic microfossils (Cohen and Knoll, Reference Cohen and Knoll2012; Cohen et al., Reference Cohen, Strauss, Rooney, Sharma and Tosca2017b), and Ediacaran-type biota (Narbonne and Aitken, Reference Narbonne and Aitken1995; Carbone et al., Reference Carbone, Narbonne, MacDonald and Boag2015). Carbon isotopes shown in gray curve from various sources (e.g., Karhu and Holland, Reference Karhu and Holland1996; Cox et al., Reference Cox, Halverson, Stevenson, Vokaty, Poirier, Kunzmann, Li, Denyszyn, Strauss and Macdonald2016; Hodgskiss et al., Reference Hodgskiss, Crockford, Peng, Wing and Horner2019): oxygen constraints (Lyons et al., Reference Lyons, Reinhard and Planavsky2014; Sperling et al., Reference Sperling, Wolock, Morgan, Gill, Kunzmann, Halverson, Macdonald, Knoll and Johnston2015), low middle Proterozoic primary productivity (Crockford et al., Reference Crockford, Hayles, Bao, Planavsky, Bekker, Fralick, Halverson, Bui, Peng and Wing2018; Hodgskiss et al., Reference Hodgskiss, Sansjofre, Kunzmann, Sperling, Cole, Crockford, Gibson and Halverson2020), supercontinent assembly and breakup (Li et al., Reference Li2008), and biomarkers (Brocks et al., Reference Brocks, Jarrett, Sirantoine, Hallmann, Hoshino and Liyanage2017). Dotted lines are unconstrained. Cryo = Cryogenian Period; Edia = Ediacaran Period; C = Cambrian Period; Pl = Paleozoic Era; PH = Phanerozoic Eon.
Morphological complexity
Algal complexity increased throughout the Neoproterozoic in two stepwise transitions: (1) the emergence of branching macroalgal forms in the early Tonian and (2) a significant increase in maximum size in the Ediacaran (Bykova et al., Reference Bykova, LoDuca, Ye, Marusin, Grazhdankin and Xiao2020). The new fossils described here represent important advancements. For example, Archaeochaeta guncho and Vendotaenia sp. are unusually large for an early Neoproterozoic biosphere typically dominated by organisms represented by microfossils (Cohen and Kodner, Reference Cohen and Kodner2021). Vendotaenia sp. resembles specimens of Vendotaenia antiqua from the Nama Group in strata that is almost 400 million years younger. Continued research will provide critical insight into the role of Vendotaenia in ancient oceans.
Thallus morphology diversified during the Tonian, as demonstrated by the first examples of dichotomous branching, delicate branching, and putative pseudomonopodial branching (Bykova et al., Reference Bykova, LoDuca, Ye, Marusin, Grazhdankin and Xiao2020). Examples include Longfengshania Du, Reference Du1982 from northwestern Canada (Hofmann, Reference Hofmann1985) and the Sinosabellidites–Protoarenicola–Pararenicola assemblage from North China (Dong et al., Reference Dong, Xiao, Shen, Yuan, Yan and Peng2008). The unnamed taxon described in the present study has a simple branching pattern (Fig. 5.1, 5.3, 5.7, 5.9), except for one specimen (Fig. 5.6) that suggests relatively more complex branching. However, taphonomic overprinting biases the retention of additional morphological characters necessary for proper phylogenetic assignment. Transport of the fossils by gravity flows aided in their preservation (Maloney et al., Reference Maloney2021) but also contributed to fragmentation of the fossilized specimens, possibly masking more-complex structures and branching. These morphological advancements could indicate ecosystem-level change driven by growing competition for resources and/or space between species (Wang et al., Reference Wang, Du, Komiya, Wang and Wang2015).
The morphological complexity of macroalgal holdfasts also increased throughout the Neoproterozoic, ranging from simple discoidal-globose holdfasts to differentiated rhizomes (Wang et al., Reference Wang, Wang, Tang and Zhao2020; X. Wang et al., Reference Wang, Wu, Wan, Niu, Zheng, Guan, Pang, Chen and Yuan2021). Tonian macroalgae have some of the earliest documented examples of holdfasts, including Tawuia Hofmann in Hofmann and Aitken, Reference Hofmann and Aitken1979 (see Xiao and Dong, Reference Xiao, Dong, Xiao and Kaufman2006), Lonfengshania Du, Reference Du1982 (see Hofmann, Reference Hofmann1985), and Protoarenicola Wang, Reference Wang1982 (see Du et al., Reference Du, Tian and Li1986; Dong et al., Reference Dong, Xiao, Shen, Yuan, Yan and Peng2008). Simple discoidal holdfasts are also common in specimens from the Miaohe biota (X. Wang et al., Reference Wang, Wu, Wan, Niu, Zheng, Guan, Pang, Chen and Yuan2021). The holdfasts observed in Lonfengshania and Protoarenicola (Du et al., Reference Du, Tian and Li1986; Dong et al., Reference Dong, Xiao, Shen, Yuan, Yan and Peng2008) have been described as “deformed globular” because they can be flattened during fossilization (Wang et al., Reference Wang, Wang, Tang and Zhao2020). Archaeochaeta guncho have elongated, globose holdfasts best classified as Gemmaphyton-type holdfasts (Wang et al., Reference Wang, Wang, Tang and Zhao2020), which are also found in branching algae Anhuiphyton Chen et al., Reference Chen, Xiao and Yuan1994 and Marpolia Walcott, Reference Walcott1919 from the Ediacaran Lantian biota (X. Wang et al., Reference Wang, Wu, Wan, Niu, Zheng, Guan, Pang, Chen and Yuan2021). To date, holdfasts have not been found in Vendotaenia sp., most likely due to their transport as part of density flows, but rounded holdfasts have been reported in Vendotaenia-like macroalgae from the Cryogenian Nantuo Formation in South China (Ye et al., Reference Ye, Tong, Xiao, Zhu, An, Tian and Hu2015). The evolution of holdfasts has been suggested to be driven by transition from stable firm substrates in the Proterozoic to soupy substrates in the Phanerozoic (X. Wang et al., Reference Wang, Wu, Wan, Niu, Zheng, Guan, Pang, Chen and Yuan2021). Simple discs would have been suitable to provide support on a substrate held firm by microbial mats, but a differentiated rhizome holdfast would have been necessary to remain anchored in a soupy substrate. In addition, destabilization of the substrate may have driven benthic macroalgae to firm, rocky substrates where more complex holdfasts were required (Xiao and Dong, Reference Xiao, Dong, Xiao and Kaufman2006).
Implications for eukaryotic diversity and ecosystems
The occurrence of centimeter-scale green algae such as A. guncho n. gen. n. sp. and Vendotaenia sp. in early Tonian strata has implications for the ecological expansion of algae and feedbacks between environmental change and algal diversification. The increase in macroalgal morphological disparity (Bykova et al., Reference Bykova, LoDuca, Ye, Marusin, Grazhdankin and Xiao2020), coupled with an increasing diversity of earliest Neoproterozoic macroalgal fossils (Tang et al., Reference Tang, Pang, Yuan and Xiao2020), points to an important ecological restructuring of ecosystems by the Tonian Period. Biomarkers from Cryogenian strata suggest that algae overtook cyanobacteria as the dominant primary producers in marine environments after the Sturtian snowball glaciation (Brocks et al., Reference Brocks, Jarrett, Sirantoine, Hallmann, Hoshino and Liyanage2017; Brocks, Reference Brocks2018), while other studies report a fundamental shift to eukaryotic-rich ecosystems in the late Tonian (Isson et al., Reference Isson2018; Zumberge et al., Reference Zumberge, Rocher, Love, Zumberge, Rocher, Love, Zumberge, Rocher and Love2020). Although the timings of these studies differ, they concur that ecological restriction of eukaryotes by nutrient limitation is a likely cause for the delayed rise of eukaryotic ecosystems (Brocks et al., Reference Brocks, Jarrett, Sirantoine, Hallmann, Hoshino and Liyanage2017; Isson et al., Reference Isson2018; Zumberge et al., Reference Zumberge, Rocher, Love, Zumberge, Rocher, Love, Zumberge, Rocher and Love2020). The hypotheses proposed in these biomarker studies will need to be reconciled with the recent reports of both green macroalgae (Tang et al., Reference Tang, Pang, Yuan and Xiao2020; Maloney et al., Reference Maloney2021) and red algal microfossils (Butterfield, Reference Butterfield2000; Cohen et al., Reference Cohen, Vizcaíno and Anderson2020) during the Mesoproterozoic–Neoproterozoic transition (Cohen and Kodner, Reference Cohen and Kodner2021). Resolving the taxonomy of Proterozoic macroalgal fossils will aid in understanding and assessing the potential causes of this 300 Myr age gap between the earliest reports of fossil eukaryotic algae (red and green lineages) and their delayed ecological dominance.
Global environmental events have been documented during this transition, including increased oxygenation of the ocean (Planavsky et al., Reference Planavsky, Tarhan, Bellefroid, Evans, Reinhard, Love and Lyons2015) and biogeochemical cycles (Bykova et al., Reference Bykova, LoDuca, Ye, Marusin, Grazhdankin and Xiao2020; Del Cortona et al., Reference Del Cortona2020), perhaps partially initiated by the emergence of large, photoautotrophic eukaryotes. Their timing suggests a link to the expansion of marine algae and eukaryotic-driven processes, including carbon fixation, filter feeding, and carnivory (Planavsky et al., Reference Planavsky, Tarhan, Bellefroid, Evans, Reinhard, Love and Lyons2015). These processes influence the habitability of an environment (Sánchez-Baracaldo et al., Reference Sánchez-Baracaldo, Raven, Pisani and Knoll2017; Del Cortona et al., Reference Del Cortona2020) and contribute to establishing oxygenated, nutrient-rich, shallow-marine ecosystems suitable for the emergence of complex animal life and behaviors.
Conclusion
Algae are eukaryotic primary producers that reorganized seafloor habits and biogeochemical cycles during the Neoproterozoic. Unfortunately, algal fossils are rarely recovered with enough morphological characteristics to establish formal systematic paleontology. Three unique size classes of Tonian fossils are reported from the 950–900 Ma Dolores Creek Formation in the Wernecke Mountains of Northwestern Canada. These are described as Vendotaenia sp., Archaeochaeta guncho, and an unnamed taxon. A. guncho is interpreted as a benthic green alga, adding to the fossil record of early Tonian chlorophytes. In addition, we consider the current interpretations of vendotaenids as probable green algae on the basis of their large size, limited preservation of holdfasts, and alignment with molecular clocks. The occurrence of benthic macroalgae in the same horizons as two likely photoautotrophs supports the proposal that algae were already playing an important ecological role in ecosystems by the early Tonian. The exceptionally preserved macroalgal fossil assemblage in the Mackenzie Mountains Supergroup provides key insights into increasing ecosystem complexity and influence of algae during the Tonian Period.
Acknowledgments
We are grateful to the First Nation of Na-Cho Nyak Dun for permitting us to conduct fieldwork on their traditional territory. We thank J. Johnny and C. Fraser for their guidance in naming Archaeochaeta guncho. This research was supported by a National Science and Engineering Research Council of Canada (NSERC) postgraduate scholarship, Queen Elizabeth II Graduate Scholarship in Science & Technology (QEII-GSST), Geological Society of America Graduate Research Grant, Northern Scientific Training Program, and a Chemical and Physical Sciences Research Visit Program (University of Toronto Mississauga) to K.M.M.; NSERC Undergraduate Student Research Award to D.P.M.; National Science foundation (NSF) IF 1636643 and NSF CAREER 1652351 to J.D.S.; NSERC Discovery (RGPIN2017-04025), an Agouron Grant, and logistical support from the Polar Continental Shelf Program to G.P.H.; a NASA exobiology Grant (80NSSC18K1086) to S.X.; a NSERC Discovery Grant (RGPIN435402) to M.L.
Declaration of competing interests
The authors declare none.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.hx3ffbgj9.










