Community-onset methicillin-resistant Staphylococcus aureus (CO-MRSA), in particular pulsed-field gel electrophoresis (PFGE) type USA300, became a primary cause of skin and soft tissue infections (SSTIs) in many urban centres in North America beginning in about 2003 [Reference David and Daum1]. In San Francisco, minimal CO-MRSA was detected in community-based surveillance samples in inner-city populations without healthcare exposure in the early 2000s [Reference Charlebois2]. Over the course of the decade, CO-MRSA markedly increased, with USA300 becoming the predominant outpatient MRSA strain [Reference Liu3]. Additionally, a significant proportion of MRSA cases identified within the hospital setting were attributable to the USA300 strain [Reference Liu3].
The epidemiology of MRSA within inner-city populations in Vancouver, Canada has mirrored that of San Francisco. CO-MRSA nasal colonization climbed rapidly, from none detected in 2000 to 13·6% in 2006 [Reference Al-Rawahi4]. By 2006, significant proportions of emergency-room SSTIs were attributable to Panton–Valentine leukocidin (PVL)-producing USA300 strains [Reference Hull5]. Further community-based screens of injecting drug users recruited from the Vancouver Supervised Injection Site in 2008 revealed growth of PVL-positive MRSA strains in 38·1% of culture-positive wounds [Reference Lloyd-Smith6]. Over 2008/2009, USA300 was found to be a prevalent cause of hospital-onset MRSA in Vancouver hospitals, also serving this inner-city population [Reference Wilmer7].
Recent data from the Veterans Affairs (VA) Surveillance system as well as the US Military Health System suggest that the CO-MRSA epidemic may have peaked in the USA [Reference Hidron, Moanna and Rimland8–Reference Stenehjem, Stafford and Rimland10]. We hypothesized that CO-MRSA rates at Providence Health Care (PHC) acute Vancouver hospitals may have similarly begun to decline.
PHC includes one academic tertiary-care hospital (435 beds) serving a large inner-city, impoverished population, and a community hospital (101 beds). Cases of MRSA bacteraemia, as well as deep and superficial wound cultures (excluding tissue or bone biopsies) were extracted from the PHC laboratory database for the period 1 January 2003 to 31 December 2011. Over this period, emergency department (ED) physicians' practice was to obtain cultures of all cutaneous abscesses or skin and soft tissue wounds that could be cultured. Cellulitis, in the absence of purulent material, was not typically cultured. This practice did not change over the study period. For each case, the first MRSA isolate per patient annually was included for review, with subsequent isolates in a calendar year excluded. Location of collection was then reviewed, with only ED isolates included for study. These isolates were assumed to be CO-MRSA cases, as the patients had acquired infection within 72 h of hospital admission. Rates of MRSA bacteraemia and wound infection were subsequently calculated per 10 000 ED visits per year. ED visits included multiple visits per case over the course of a year, if applicable. For ED wounds in 2007, 2009 and 2011, the rate of trimethoprim-sulfamethoxazole (TMP-SMX)-susceptible MRSA, which has been highly correlated with PVL-positive MRSA isolates in our population [Reference Lloyd-Smith6], was calculated per 10 000 ED visits. As a substudy, available MRSA bacteraemia isolates from 2007 and 2008 retrospectively underwent PVL PCR testing. Laboratory methods, including PCR testing, are described elsewhere [Reference Lloyd-Smith6]. The Spearman rank correlation test and coefficient (r) was used to determine the strength as well as the direction of the association between infection rates and calendar year. All tests were two-tailed and were performed at a significance level of α = 0·05.
In total, 378 cases of MRSA bacteraemia were identified from the laboratory information system during the study period after excluding repeat episodes and non-ED isolates per patient (Table 1). The rate of ED bacteraemia cases peaked at 7·8 cases/10 000 ED visits in 2005, and decreased to 3·3 cases/10 000 ED visits in 2011 (r = − 0·72, P = 0·03 for trend) (Fig. 1). In 2007, 47/55 (85·5%) ED bacteraemia cases were tested by PVL PCR and 40 (85·1%) were found to be positive. In 2008, 29/37 (78·4%) such cases were similarly tested and 20 (69·0%) proved to be PVL positive.
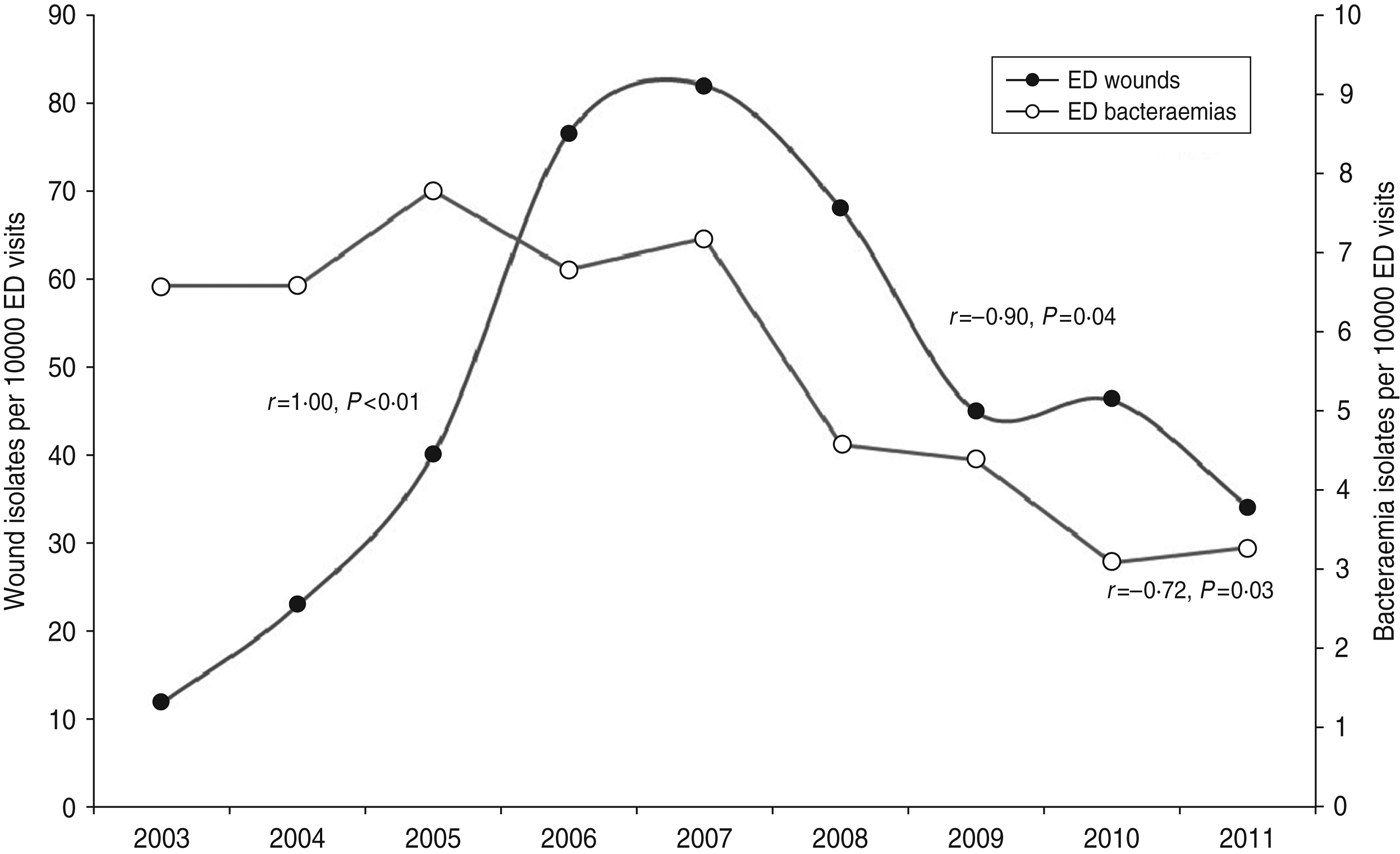
Fig. 1. Rates of emergency department (ED) wound and bacteraemia isolates, 2003–2011.
Table 1. Absolute number and rates of emergency department (ED) MRSA wound and bacteraemia isolates from 2003 to 2011
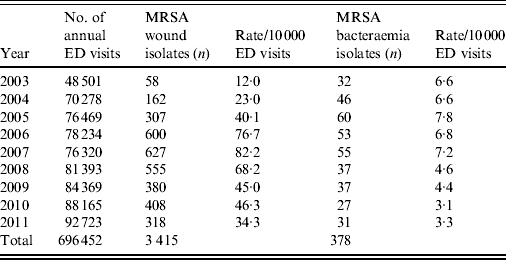
Overall, 3415 MRSA wound isolates were identified during the study period. By 2007, the ED MRSA-positive wound rates had peaked at 82·2 cases/10 000 ED visits (r = 1·00, P < 0·01 for trend) (Fig. 1). Subsequently, rates declined significantly to 34·3 cases/10 000 ED visits in 2011 (r = − 0·90, P = 0·04 for trend). For ED wounds, rates of TMP-SMX susceptible MRSA isolates declined significantly from 79·5 cases/10 000 ED visits in 2007 to 43·0 in 2009 to 32·4 cases/10 000 ED visits in 2011 (r = 0·85, P < 0·01 for trend). During this period, there was no significant change in the proportion of MRSA wound isolates susceptible to TMP-SMX (96·8% in 2007 vs. 95·5% in 2009 vs. 94·3% in 2011).
Rates of MRSA infections have changed significantly within two Vancouver acute-care hospitals over the last decade. We identified a peak in CO-MRSA cases presenting to our EDs in 2007, as evidenced by large numbers of clinical MRSA wound isolates detected by the laboratory. Rates of MRSA wound and bacteraemia cases have subsequently declined significantly in our population. We believe that the demonstrated upswing and recent decline in MRSA rates represents changes in the amount of USA300 MRSA isolates within our population. Significant proportions of the ED bacteraemia isolates tested positive for PVL by PCR in 2007 and 2008, which probably represents the dissemination of USA300 within a vulnerable population. The presence of PVL has been shown to correlate exclusively with the USA300 PFGE pattern within PHC [Reference Wilmer7]. The more recent decline in MRSA rates is again probably attributable predominantly to changes in CO-MRSA, as demonstrated by the significant decrease in the rate of TMP-SMX susceptible wound isolates presenting to the ED from 2007 to 2011, with TMP-SMX susceptibility being highly correlated with PVL-positive MRSA in our population [Reference Lloyd-Smith6]. A previous study at our centre demonstrated that USA300 isolates, which were all PVL positive, were more likely to be TMP-SMX susceptible, compared to other MRSA strains (98% vs. 28%) [Reference Hull5].
Changes in our MRSA rates reflect those seen in North America over the last decade. Outpatient rates of community-associated MRSA infection increased sevenfold over 1999–2006 in the USA, and subsequently became a significant cause of MRSA infections within the hospital setting [Reference Liu3, Reference Klein, Smith and Laxminarayan11]. Since 2005, hospital-associated rates of invasive MRSA have declined in several centres in the USA [Reference Kallen12]. The Atlanta VA Medical Center has recently described an overall reduction in CO-MRSA infections [Reference Stenehjem, Stafford and Rimland10]. They defined CO-MRSA infections as those with clinical culture obtained with 48 h of admission, if not present on admission. CO-MRSA infections peaked in their population in 2007 with 5·45 MRSA infections/1000 veterans in care, and consistently declined to 3·14 MRSA infections/1000 veterans in care in 2011 (P < 0·001) [Reference Stenehjem, Stafford and Rimland10]. The majority of these cases were attributable to SSTIs. The incidence of CO-MRSA bacteraemias decreased from 0·68/1000 veterans in care in 2006 to 0·21/1000 veterans in care in 2011 (P < 0·001). Decreasing incidence of MRSA infections was also observed specifically in HIV-infected individuals followed through the HIV Atlanta VA Cohort Study, where the incidence of CO-MRSA peaked in 2007 at 51·0 episodes/1000 patients and declined by 2009 [Reference Hidron, Moanna and Rimland8]. Our results demonstrate a similar increase in CO-MRSA over this period, with a peak of 82·2 wound isolates/10 000 ED visits in 2007, before declining to 34·3 cases/10 000 ED visits in 2011.
A recent study of the US Military Health System demonstrated significant decreases in the annual incidence of CO-MRSA bacteraemia cases between 2005 to 2010, from 1·7 to 1·2/100 000 person-years (P = 0·005). Additionally, they demonstrated a peak in the proportion of community-onset SSTIs secondary to MRSA at 62% in 2006, followed by significant decrease to 52% by 2010 (P < 0·001) [Reference Landrum9].
The reason for the decrease in CO-MRSA clinical infections in patients presenting to our institution, and throughout North America, remains unclear. Improved infection control strategies may account for decreases in nosocomial transmission and infection, but this should not influence community transmission of the MRSA. S. aureus strains have been demonstrated to have natural variation within populations over the last five decades, with sudden decreases in certain clonal types without a clear aetiology [Reference Oliveira, Tomasz and de Lencastre13]. One might speculate that herd immunity or another biological factor has reached critical levels in our inner-city at-risk population, limiting further spread of the organism [Reference Gupta, Ferguson and Anderson14] but further work is required to investigate these possibilities.
Our study has several limitations. The data examined were laboratory-based and thus may have underestimated true MRSA rates, as cases were dependent on primary physicians collecting wound swabs or blood cultures. However, diagnostic practices within the hospital did not change markedly over the study period, so this would not account for the observed decreases in MRSA rates. Additionally, although we had limited access to clinical information, our tertiary centre serves a large, impoverished inner-city population, in whom intravenous drug use and HIV infection are prevalent [Reference Palepu15]. Moreover, as ED isolates were assumed to be CO-MRSA, this may also have underestimated the rate of CO-MRSA as many inpatient specimens were probably collected within 72 h of admission, and thus would also meet the epidemiological definition of CO-MRSA. Similarly, we cannot rule out the possibility that some isolates may have originated from previous hospitalization of patients as these data were not captured in the study. Further molecular testing with a larger sample is required to confirm that the decline in CO-MRSA bacteraemia and wound infections are a consequence of decreasing prevalence of the USA300 lineage.
In conclusion, this study has documented the prevalence of MRSA within two acute-care facilities in Vancouver, which displayed a peak in the rate of MRSA bacteraemia and wound isolates in the mid-2000s that subsequently declined over the last 5 years. The rate of CO-MRSA wound isolates detected from ED specimens increased substantially from 2003 to 2007, contributing greatly to the overall rate of MRSA which was probably attributable to the emergence of USA300 within a susceptible inner-city population. The subsequent decline in CO-MRSA isolations may reflect a decrease in the burden of USA300-related infections in the community superimposed on progressive declines in acquisition of other MRSA strains. Further studies in other populations are required to confirm whether this represents the beginning of a significant change in the epidemiology of CO-MRSA in North America.
ACKNOWLEDGEMENTS
The authors thank James Chan for his assistance with data management, and Anna Wong for her assistance in data extraction.
DECLARATION OF INTEREST
None.




