Many health concerns arise with menopause in ageing women. These include deleterious changes in plasma lipids(Reference LaRosa1), accumulation of fat mass (FM) in the abdominal compartment(Reference Ijuin, Douchi and Oki2) and accelerated resorption of bone tissue(Reference Kanis3, Reference Barrett-Connor4). As such, the prevalence of the metabolic syndrome(Reference Ren and Kelley5), type 2 diabetes(Reference Rosano, Vitale and Silvestri6), CVD(Reference Mosca, Linfante and Benjamin7, Reference Alexander and Clearfield8) and osteoporotic fractures(Reference Cummings, Black and Rubin9) is particularly high in postmenopausal women.
In postmenopausal women, synergistic actions between hormonal therapy (HT) and exercise have been suggested, particularly with regard to bone mineral density (BMD)(Reference Villareal, Binder and Yarasheski10), body composition(Reference Evans, Van Pelt and Binder11, Reference Sipilä, Taaffe and Cheng12), muscle function(Reference Sipilä, Taaffe and Cheng12) and insulin sensitivity(Reference Evans, Van Pelt and Binder11). Potential mechanisms by which oestrogens could influence body composition are unclear, but it is believed that oestrogens may modulate lipid oxidation in muscle(Reference Huss, Torra and Staels13), which could limit TAG accumulation and maintain muscle quality(Reference Hackney, Muoio and Meyer14). In line with that, HT generally helps to preserve TAG infiltration in muscles(Reference Phillips, Rook and Siddle15). Unfortunately, increased risks of health-related hazards have been associated with HT(Reference Rossouw, Anderson and Prentice16). Thus, investigators have suggested possible alternatives to combine oestrogenic compounds with exercise. Dietary supplementation of isoflavones, a class of phyto-oestrogens found in soyabeans, has recently been used in this perspective; their oestrogenic properties appear to reach the level of statistical significance with a consumption as low as 50 mg/d(Reference Cassidy, Bingham and Setchell17, Reference Shutt and Cox18).
Beneficial effects of isoflavones have been suggested with regard to several health concerns in postmenopausal women. Improvements in the blood lipid profile have been observed in men and women when isoflavones were contained in a food matrix, such as a soya protein beverage(Reference Anderson, Johnstone and Cook-Newell19, Reference Zhan and Ho20), but not observed with isoflavone capsules(Reference Taku, Umegaki and Ishimi21). Isoflavones and genistein, in particular, appear to decrease fasting glucose in postmenopausal women whose fasting glucose is higher than 5·56 mmol/l(Reference Ho, Chen and Ho22). In addition, reduction in blood concentrations of bone resorption biomarkers observed with an isoflavone supplement suggests that it could help prevent the loss in BMD(Reference Yamori, Moriguchi and Teramoto23–Reference Uesugi, Watanabe and Ishiwata25). Finally, a study from our group has reported increases in appendicular lean body mass (LBM) in sarcopenic obese postmenopausal women after a 6-month isoflavone supplementation(Reference Aubertin-Leheudre, Lord and Khalil26). Altogether, there is evidence suggesting that isoflavone supplementation could be beneficial for postmenopausal women to improve CVD risk factors and possibly contribute to body composition management.
Studies conducted to evaluate the potential synergistic action of these oestrogenic compounds with exercise have reported mixed results. In ovariectomised mice, Wu et al. (Reference Wu, Wang and Chiba27) have found that combining isoflavones and exercise leads to improvements similar or greater to oestrogen therapy in all variables of interest such as FM, LBM, BMD and plasma lipids. Conversely, HT consistently produced significantly better results than either exercise or isoflavones taken separately.
In human subjects, only a few studies have been conducted, lasting 4–12 months in duration, using either capsules of isoflavones(Reference Aubertin-Leheudre, Lord and Khalil28–Reference Wu, Oka and Tabata31) or soya protein beverages(Reference Evans, Racette and Van Pelt32, Reference Maesta, Nahas and Nahas-Neto33), with exercise programmes including fast walking(Reference Wu, Oka and Higuchi30, Reference Wu, Oka and Tabata31), aerobic training(Reference Aubertin-Leheudre, Lord and Khalil28, Reference Evans, Racette and Van Pelt32) or resistance training(Reference Orsatti, Nahas and Nahas-Neto29, Reference Maesta, Nahas and Nahas-Neto33). In fact, in a population of Asian women, Wu et al. (Reference Wu, Oka and Tabata31) have found a significant main effect after 12 months of both fast walking and isoflavones on abdominal FM and hip BMD but have found no interaction between exercise and isoflavone interventions. Shorter-duration studies with resistance training and aerobic exercises have similarly reported that improvements in fitness(Reference Orsatti, Nahas and Nahas-Neto29, Reference Evans, Racette and Van Pelt32) and body composition(Reference Orsatti, Nahas and Nahas-Neto29, Reference Maesta, Nahas and Nahas-Neto33) pertained to exercise training, with no further improvements with isoflavone supplementation.
In a pilot project, Aubertin-Leheudre et al. (Reference Aubertin-Leheudre, Lord and Khalil28) took a direct interest in the combination of isoflavones and exercise on a broader spectrum of risk factors of CVD in Caucasian postmenopausal women(Reference Aubertin-Leheudre, Lord and Khalil28). This study showed interesting results, most notably a 5 % decrease in body weight over a 12-month period. Significant improvements were also observed in trunk FM, BMI and appendicular LBM. In contrast, the exercise-only group showed no significant improvement during the same period. However, the present study had several limitations, the most important being that exercise was introduced in the later 6 months of the intervention, after a first 6-month treatment of isoflavone supplementation. This particular design precludes any discrimination between the effects of exercise (6 months), isoflavones (12 months) or a potential synergistic action between the two.
The purpose of the present randomised double-blind controlled trial was thus to investigate whether 6 months of isoflavones combined with exercise could improve FM, LBM, BMD, RMR and risk factors of CVD (lipid profile, fasting glucose and fasting insulin). The effects of exercise and isoflavones will be assessed alone and in combination to investigate possible synergistic effects. We hypothesised that isoflavones and exercise would have a synergistic effect on body weight, BMI, trunk FM, appendicular LBM and BMD, while improvements in risk factors of CVD would be attributed to exercise.
Methods
Subjects
Postmenopausal women (n 100) aged between 50 and 70 years were recruited by the use of advertisements in a local newspaper. To be included in this double-blind controlled trial, women had to meet the following criteria: Caucasian; absence of menses for the past 12 months; overweight or obese (BMI 28–40 kg/m2 and waist circumference >88 cm); healthy; without major physical incapacity; without HT (off for ≥ 1 years); sedentary (no participation in a systematic/supervised exercise programme during the last 5 years); weight stable ( ± 2 kg) for the last 6 months; non-smoker; moderate drinker ( < 15 g alcohol/d); no medication that influences glucose or lipid metabolism.
Experimental protocol and study design
A telephone interview was conducted for initial screening. Eligible subjects were then provided a thorough explanation of the nature and goals of the study. Written informed consent was provided during the first visit to the Research Centre on Aging (Geriatric Institute of the University of Sherbrooke, CSSS-IUGS, Sherbrooke, QC, Canada). A 12 h fasting blood sample was then obtained, and measurements of body composition were performed. Resting heart rate (HR) and maximal HR were measured during a 30 min rest and a maximal effort test on a treadmill, respectively. The latter was performed on a separate day to establish exercise intensity and to get medical clearance for the subject's inclusion in the study. Then, the subjects were randomly assigned to the following four groups: (1) placebo (PLA; n 26); (2) isoflavones (ISO; n 26); (3) exercise and placebo (Ex+PLA; n 25); (4) exercise and isoflavones (Ex+ISO; n 23). Measurements were repeated after 6 months, with at least 3–5 d of rest after the last training session for subjects in the exercise groups. The study design is illustrated in Fig. 1. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the CSSS-IUGS. Written informed consent was obtained from all subjects.
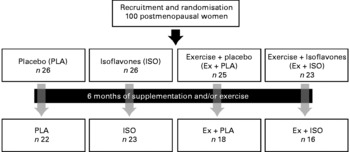
Fig. 1 Design of the study.
Isoflavone supplementation
Subjects received four capsules daily, containing either soya isoflavones or placebo (Arkopharma Limited, Carros, France). The 70 mg daily dose of isoflavones contained 44 mg of daidzein, 16 mg of glycitein and 10 mg of genistein. The placebo capsules contained cellulose only.
Exercise programme
The 6-month programme consisted of three exercise sessions per week. Each session lasted 1 h and consisted of 30 min of resistance training and 30 min of aerobic exercise. To be included in analyses, subjects had to attend to ≥ 85 % of all sessions. Resistance training included movements for all major muscle groups centred around three core exercises (leg press, bench press and lat pulldown), using several variations with free weights and selective plate machines (Life Fitness, Schiller Park, IL, USA). Intensity was increased on a monthly basis from 60 % of maximal strength (measured as 1-repetition maximum), for one set of twelve to fifteen repetitions during the first month, to 85 % during the sixth month, completing four sets of four to six repetitions. To ensure that overload was achieved, resistance increments were adjusted on a weekly basis for all exercises, under close supervision, as soon as the subject was able to complete the maximal volume of a given set/repetition scheme (i.e. 3 × 12 repetitions achieved for a given exercise with a prescription of 3 × 10–12). At the end of the 6-month intervention, 1-repetition maximum was re-assessed to confirm that subjects were exercising at an appropriate intensity level.
Aerobic exercise was performed on a cycle ergometer and a treadmill, using a protocol that closely followed the guidelines of the American College of Sports Medicine for sedentary adults(34). Training started at 40–50 % of HR reserve (HRRES; as described later) and increased up to 70–85 % of HRRES. HRRES was computed as follows: (HRRES = HRMAX − HRRESTING), using Karvonen's formula(Reference Karvonen, Kentala and Mustala35). Target HR was then established with the following equation: (HRRES × 0·7+HRRESTING), where ‘0·7’ stands for an arbitrary intensity of 70 % of HRRES(Reference Karvonen, Kentala and Mustala35).
After 3 months of training, continuous aerobic training was alternated with interval training. In other words, during a given week, a subject would perform continuous aerobic training on Monday and Friday and would perform interval training on Wednesday. During the following week, interval training would take place on Monday and Friday, while continuous aerobic training would be done on Wednesday. Interval training consists of alternating periods of high intensity (i.e. sprint) and periods of active recovery (i.e. cycling or walking slowly)(Reference Wisløff, Støylen and Loennechen36). During four rounds of 4 min high-intensity intervals, subjects were asked to maintain ≥ 90 % of HRMAX. Active recovery was maintained at 50–65 % of HRMAX for 3 min, which allows the completion of work periods at a higher intensity than that would be achievable in a steady-state, longer-duration aerobic exercise session.
HRRESTING was measured at baseline, after lying down for 30 min. HRMAX and VO2max were obtained on a separate day, during an incremental test to exhaustion on a treadmill, using the Balke protocol, as described previously(Reference Balke and Ware37). The measure of VO2max was considered valid when two of the following three criteria were met: (1) a respiratory quotient >1·10; (2) a HR greater than or equal to the age-predicted maximum (220 age); (3) no further increase in VO2, despite regular increases in treadmill inclination. This evaluation aimed principally to get medical clearance and to guide aerobic exercise prescription; it was consequently performed in the exercise groups only. On an average, subjects had a baseline VO2max of 22·8 (sd 4·2) ml/kg per min.
All training sessions were closely supervised by a kinesiologist. Training loads were adjusted on a weekly basis for all exercises in order to match progression of each subject in terms of muscular strength and cardiovascular endurance. All subjects were instructed not to change their physical activity and nutritional habits outside of the programme.
Dropouts and side effects
Of the 100 subjects, seventy-nine completed the study. Withdrawals included 67 % of subjects in the exercise groups. Reasons for dropping out were personal (n 11), professional (n 6) and health-related (n 2; conditions unrelated to the study). Because of reported side effects (hot flashes) that they attributed to isoflavone supplementation, two women discontinued their participation; however, it was later found that both of them received a placebo. Many women in the exercise groups reported an increase in hot flashes during the initial weeks. Hot flashes were reported on exercise days and could be considered as an acute effect of training. In addition, the situation appeared to resolve after a few sessions. Possible explanations for this effect include an increase in RMR and acute hormonal effects of exercise such as growth hormones and catecholamines. These hypotheses aside; we are not aware of any conclusive studies showing a clear effect of exercise on menopausal symptoms, especially vasomotor symptoms(Reference Daley, MacArthur and Mutrie38). No pattern was observed between reported hot flashes and supplementation status.
Anthropometrics and body composition measurements
Body weight ( ± 0·2 kg) was determined by an electronic scale (SECA707, Hamburg, Germany). Standing height was measured using a wall stadiometer (Takei, Tokyo, Japan) with the subject in stockinged feet. BMI was then calculated as body mass (kg)/height (m2). Waist and hip circumferences ( ± 0·1 cm) were measured using a tape measure, respectively, at the narrowest circumference of the trunk and at the greater trochanter. FM, LBM and BMD of the lumbar spine (L2–L4) and left hip (total hip, femoral neck, greater trochanter and Ward's triangle) were assessed in a supine position using dual-energy X-ray absorptiometry (GE Prodigy Lunar, Madison, WI, USA). Our laboratory CV for repeated measures of FM, LBM and BMD in ten adults (measured 1 week apart) are 0·9, 0·4 and 0·1 %, respectively.
Blood collection and biochemical analyses
Blood samples were obtained in the morning, after a 12 h fasting period. Plasma lipid profile (HDL-cholesterol, LDL-cholesterol, total cholesterol and TAG), plasma glucose and insulin were analysed at the Sherbrooke University Hospital Center (CHUS, Sherbrooke, QC, Canada). Insulin sensitivity was assessed indirectly with the homeostasis assessment model (HOMA)(Reference Matthews, Hosker and Rudenski39), a homeostasis model of β-cell function, computed as: (fasting glucose (mmol/l)) × (fasting insulin (mU/l))/22·5.
Resting metabolic rate and respiratory quotient
RMR was measured using a breathing mask by indirect calorimetry (CCM/D; Medgraphics Corporation, St Paul, MN, USA). RMR was assessed after a 12 h fast, in the early morning. Measurement lasted 30 min, with the subject lying comfortably on her back. The first 15 min were used to allow the subject to familiarise with the equipment and environment and were thus discarded from analyses. RMR (kJ/d) and respiratory quotient (ratio of CO2 production to O2 consumption) were then calculated using the remaining 15 min. Test–retest measures of RMR in ten adults, with a 1-week interval, yielded a mean absolute CV of 2·1 % in our laboratory.
Dietary intake
Each subject was instructed to maintain normal dietary habits throughout the study. Subjects were provided with a 5 kg (11 lb) food scale and instructed on how to complete a 3 d dietary record (two weekdays and one weekend day). Daily energy intake and macronutrients intake were analysed using Nutrifiq software (Laval University, Sainte-Foy, QC, Canada).
Statistical analyses
The normality of the data distribution at baseline was assessed with the Kolmogorov–Smirnov test. Where an abnormal distribution was found, analyses were performed on a log-transformed data. Similarity of inter-group baseline values and inter-group differences in the percentage of change in each variable after the intervention were assessed with a one-way ANOVA, using Tukey's post hoc test if the difference was significant. A repeated-measures univariate model was used to assess principal main treatment effects of the interventions compared with baseline and to verify the presence of interactions between isoflavones and exercise. Energy intake was used as a covariate as a significant baseline difference was found between groups. Finally, post-intervention changes compared with baseline were assessed for each group with a Wilcoxon signed-rank test. P values of ≤ 0·05 were considered statistically significant. All analyses and graphs were performed using the SPSS 17.0 program (Chicago, IL, USA). Results are presented as means and standard deviations.
Results
Baseline comparisons
Subjects' characteristics are presented in Table 1. At baseline, subjects were 58·7 (sd 5·3) years old and overweight-to-obese, as evidenced by a BMI of 29·9 (sd 3·2) kg/m2. Analyses of dietary logs revealed an average daily intake of 8732·01 (sd 2297·02) kJ, composed of 46 (sd 8) % of carbohydrates, 36 (sd 7) % of lipids and 17 (sd 4) % of proteins. Subjects consumed approximately 1·1 g/kg of protein daily. None of the aforementioned variables changed throughout the 6-month intervention, with the exception of a modest but significant change in energy intake ( − 447·68 kJ/d; P = 0·041) in the ISO group. Furthermore, energy intake was slightly higher in the ISO group than in the Ex+PLA group at baseline (P = 0·005). The difference between the groups appeared to be predominantly related to lipids, with a greater intake in the ISO group than in the Ex+PLA group, both in absolute (P < 0·001) and in relative (P = 0·009) terms. This was the sole macronutrient difference among the groups. To control for any effect of the diet on variables of interest, energy intake was used as a potential confounder in statistical analyses, which did not modify the overall conclusions. In addition, at the end of the study, no inter-group difference could be found in energy intake, daily lipid consumption and percentage of energy coming from fat.
Table 1 Baseline characteristics
(Mean values and standard deviations)

PLA, placebo; ISO, isoflavones; Ex+PLA, exercise and placebo; Ex+ISO, exercise and isoflavones.
* P value presents inter-group differences at baseline, assessed with a one-way ANOVA.
† Mean values were significantly different (P < 0·05) from each other at baseline.
Body composition
Significant changes in waist circumference (P = 0·001; Fig. 2), hip circumference (P = 0·009), and total (P = 0·021; Fig. 2) and relative FM (P = 0·007; Table 2) were observed after 6 months of exercise. Exercise also had a significant effect on arm (P = 0·000), leg (P = 0·024) and appendicular (P = 0·020; Fig. 2) LBM, with trends for a significant effect on total LBM (P = 0·074) and trunk LBM (P = 0·080).

Fig. 2 Fat mass (a), appendicular lean body mass (b) and waist circumference (c) at baseline and after 6 months of intervention. Markers were clustered by measurement periods (baseline/post-intervention) and were spaced for a better visualisation of each group's standard deviation. Values are means, with standard deviations represented by vertical bars. (a) Main effects (P): (a) exercise (Ex), 0·021; isoflavones (ISO), 0·310; Ex × ISO, 0·935. (b) Ex, 0·001; ISO, 0·440; Ex × ISO, 0·134. (c) Ex, 0·001; ISO, 0·769; Ex × ISO, 0·702. □, Placebo (PLA); ○, ISO; ■, PLA+Ex; ●, ISO+Ex.
Table 2 Body composition
(Mean values and standard deviations)

PLA, placebo; ISO, isoflavones; Ex+PLA, exercise and placebo; Ex+ISO, exercise and isoflavones; L2–L4: lumbar spine bone mineral density.
* Significant pre/post-difference (P < 0·05), assessed by a Wilcoxon signed-rank test.
† Significant inter-group main effect (P < 0·05) assessed by a repeated measures ANOVA.
Table 2 also displays body composition variables that changed after the 6-month intervention within each group. Exercise groups experienced changes in the same body composition variables, reflecting the aforementioned main effects. However, the significant change in trunk FM that was observed in the Ex+ISO group (P = 0·026) did not reach significance in the Ex+PLA group (P = 0·133).
A significant effect of isoflavones was observed in leg FM% (P = 0·050). This effect is barely significant and should be interpreted with caution. Indeed, all groups surprisingly experienced similar pre/post-decreases in leg FM (P < 0·05; Fig. 3). The pre/post-change in leg LBM indicates a significant increase only in the Ex+ISO group (P = 0·031), with no significant changes in the ISO group (P = 0·661), the Ex+PLA group (P = 0·199) and the PLA group (P = 0·181). Therefore, the main effect of isoflavones on leg FM% may either be the result of chance, resulting from a random increase in leg LBM and a decrease in leg FM, or a significant effect that would need to be confirmed.

Fig. 3 Body composition of the legs at baseline and after 6 months of intervention. Markers were clustered by measurement periods (baseline/post-intervention) and were spaced for a better visualisation of each group's standard deviation. Values are means, with standard deviations represented by vertical bars. (a) Main effects (P): (a) exercise (Ex), 0·296; isoflavones (ISO), 0·416; Ex × ISO, 0·642. (b) Ex, 0·024; ISO, 0·329; Ex × ISO, 0·587. (c) Ex, 0·020; ISO, 0·050; Ex × ISO, 0·906. □, Placebo (PLA); ○, ISO; ■, PLA+Ex; ●, ISO+Ex.
Finally, no treatment effect was observed in BMD, neither for exercise nor for isoflavones. Only one significant change was observed compared with baseline, with a decrease in total hip BMD for the ISO group (P = 0·034).
Metabolic profile
Variables pertaining to the metabolic profile are presented in Table 3. A trend for an exercise effect was observed towards RMR (P = 0·052), but pre/post-changes in the same variable displayed a trend only for the Ex and ISO group (P = 0·079). No main effects were observed in lipid profile, fasting glucose, fasting insulin or HOMA. However, a significant increase in LDL-cholesterol was observed in both the PLA group (P = 0·050) and the Ex and PLA group (P = 0·044), with a trend for an increase in TAG (P = 0·096) in the latter. While HOMA (P = 0·02) and insulin (P = 0·009) improved in the ISO group, trends for increases were observed in fasting glucose in the Ex and ISO group only (P = 0·058).
Table 3 Metabolic profile
(Mean values and standard deviations)

PLA, placebo; ISO, isoflavones; Ex+PLA, exercise and placebo; Ex+ISO, exercise and isoflavones; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; Glucose, fasting glucose; Insulin, fasting insulin; HOMA, homeostasis assessment model.
* Significant pre/post-difference (P < 0·05), assessed by a Wilcoxon signed-rank test.
Discussion
The present study examined the combined effects of exercise and soya isoflavones on body composition and clinical risks of CVD in overweight-to-obese postmenopausal women. After 6 months of treatment, exercise intervention led to improvements in waist and hip circumferences, total FM and appendicular LBM. Exercise led to overall improvements in body composition as evidenced by significant effects on total and regional FM%. Although, no actual interactions were observed between isoflavones and exercise, trunk FM decreased and LBM increased significantly only in the group combining exercise and isoflavones.
Hence, by adding isoflavones to exercise, it appears that slight benefits could be observed in both LBM and abdominal FM. Indeed, the Ex and ISO group was the only group to display a significant pre/post-decrease in trunk FM as well as an increase in leg LBM (Table 2). However, the actual ‘absolute’ difference between the two exercise groups was rather small, with averages of 0·2 and 0·5 kg in leg LBM, respectively, for the Ex and PLA group and the Ex and ISO group, and − 0·45 and − 0·65 kg in trunk FM, respectively. Such small differences should be interpreted with caution, as similar studies report no additive effects between isoflavones and exercise on body composition(Reference Aubertin-Leheudre, Lord and Khalil28, Reference Wu, Oka and Higuchi30, Reference Wu, Oka and Tabata31, Reference Maesta, Nahas and Nahas-Neto33).
As in Aubertin-Leheudre et al. (Reference Aubertin-Leheudre, Lord and Khalil28), consequent to exercise, we observed a preferential gain of LBM in the appendicular regions in obese postmenopausal women. In the aforementioned study, exercise training was of aerobic nature and predominantly led to gains in leg LBM, whereas women in the present study achieved significant hypertrophy in the arms. This could be explained by the specificity of exercise stimuli since, in the present study, at least half of the resistance training exercises recruited the upper limbs. These findings suggest that ageing women are able to increase LBM as a consequence of exercise training, despite no nutritional changes.
Exercise modality appears to have an important effect on body composition changes. Indeed, Evans et al. observed a significant decrease in LBM with aerobic training only. Conversely, no significant effect was observed in adiposity, even after 9 months of intervention. In contrast, in a study using solely resistance training, Maesta et al. observed gains in LBM and loss in waist circumference in both exercise groups. Similarly, Orsatti et al. observed small but significant gains in LBM, and especially limb LBM, that were related to resistance training. Interestingly, a 12-month study using HT showed that oestrogen therapy did not benefit exercisers, as the hypertrophy stimulus achieved was similar in the exercise group with no HT(Reference Teixeira, Going and Houtkooper40, Reference Figueroa, Going and Milliken41).
Nevertheless, in the present study, an isoflavone supplement appeared to decrease significantly leg FM% (P = 0·05). As suggested in Fig. 3, this may be the combined effect of a loss in leg FM, common to all groups, and a more stable leg LBM compared with PLA. Although the effect appears to be small, increases in leg LBM after 6 months of supplementation with isoflavones have been reported elsewhere, in a population of sarcopenic obese women(Reference Aubertin-Leheudre, Lord and Khalil26) and support investigation of the question further.
No changes could be observed in BMD, which was also the case after 4 months of aerobic training in the study of Evans et al. (Reference Evans, Racette and Van Pelt32). In comparison, Wu et al. (Reference Wu, Oka and Tabata31) observed mainly a preventive effect on the loss in BMD after a 12-month period, at the hip, for walking exercise and isoflavones. A longer-duration study may therefore be necessary to observe significant results. Hence, while a few studies have observed improvements in BMD with the use of isoflavones(Reference Marini, Minutoli and Polito42, Reference Harkness, Fiedler and Sehgal43), most of them have suggested a preventive effect(Reference Lydeking-Olsen, Beck-Jensen and Setchell44). Early results of exercise and isoflavones on animal models should also be interpreted carefully and may not be relevant to humans, because treatment in mice was initiated hastily after a surgically induced menopause(Reference Wu, Wang and Chiba27). In these early studies, mice that received an isoflavone-rich diet and exercise had similar levels of BMD compared with sham-operated (pre-menopausal) or oestradiol-treated mice. These results therefore suggest a ‘preventive’ effect of the combined interventions on BMD. It is unclear, however, if isoflavone and exercise could revert loss in BMD – and thus increase BMD – in women who have been in their postmenopause for 8·5 years, considering that a possible rate of loss of 5 % in BMD may occur in the initial 5 years after menopause(Reference Xu, Wu and Yan45).
Similarly, no significant treatment effects were observed in the metabolic profile variables. Trends for an exercise effect on RMR are probably related to increases in appendicular LBM, as LBM is a strong determinant of energy metabolism(Reference St-Onge and Gallagher46). As for indirect indicators of insulin sensitivity, they appear to be unaffected by exercise and did not improve as a result of gains in appendicular LBM. In fact, a significant increase in fasting glucose was observed in the Ex+PLA group (P = 0·015), with a similar trend for the Ex+ISO group (P = 0·058). These results are difficult to interpret and may not reflect a pattern related directly to treatments. As values remain in the normal range, it may only reflect transient changes. It should, however, be stated that, by allowing a 3–5 d delay between the last exercise session and follow-up measurements, acute effects of exercise on glucose metabolism had probably vanished. Indeed, the effects of exercise on glucose metabolism have been reported to be particularly short-lived in elders, with a need to exercise every other day for sustained benefits(Reference DiPietro, Dziura and Yeckel47, Reference Goulet, Mélançon and Aubertin-Leheudre48).
In contrast, the ISO group showed improvements in fasting insulin (P = 0·009) and HOMA (P = 0·02), with no changes in fasting glucose. Previous studies have reported decreases in fasting insulin in postmenopausal women with 6 months of supplementation of 54 mg/d of genistein(Reference Villa, Costantini and Suriano49, Reference Crisafulli, Altavilla and Marini50). It is unclear, however, why any significant improvement in fasting insulin related to isoflavone supplementation should be absent in a group receiving the supplement in conjunction with exercise training. Interestingly, Maesta et al. (Reference Maesta, Nahas and Nahas-Neto33) similarly found that an improvement in lipid profile, while significant in a non-exercising group receiving a soya protein beverage, was absent in an exercise group that consumed the same beverage. Unfortunately, the authors did not propose an explanation for this result. Still, Villa et al. (Reference Villa, Costantini and Suriano49) have suggested that isoflavones may be more efficient in improving specific parameters, such as HDL-cholesterol, in hyperinsulinaemic subjects. Again, we stated earlier that isoflavones appeared to be more potent at decreasing fasting glucose in postmenopausal women who had baseline values greater than 5·56 mmol/l(Reference Ho, Chen and Ho22). In the present study, the Ex+ISO group appeared to have a slightly higher β-cell function than the ISO group (HOMA, respectively, of 1·56 (sd 0·89) and 1·42 (sd 1·15)). More in-depth analyses would be needed to investigate such a relationship.
The isoflavone formula used in the present study contained only small amounts of genistein (10 mg/d). Genistein has been found to exert beneficial effects on BMD(Reference Yamori, Moriguchi and Teramoto23–Reference Uesugi, Watanabe and Ishiwata25) and fasting glucose(Reference Ho, Chen and Ho22). While a formula similar to ours was used in other studies combining isoflavones and exercise(Reference Aubertin-Leheudre, Lord and Khalil28, Reference Wu, Oka and Higuchi30, Reference Wu, Oka and Tabata31), it is possible to hypothesise that a different formula may have yielded different results. Nevertheless, studies by Orsatti et al. (Reference Orsatti, Nahas and Nahas-Neto29) and Evans et al. (Reference Evans, Racette and Van Pelt32) have combined genistein and exercise, and have observed no synergistic effect with exercise, nor an independent effect of isoflavones. More studies are needed to clarify the effects of isoflavone formulations.
Although the present study could not highlight any significant changes in variables pertaining to the metabolic profile, it is possible that these effects could take place over the longer term. For example, it has been demonstrated that isoflavones may take up to 12 months in order to exert a main effect on trunk FM(Reference Wu, Oka and Tabata31). Therefore, longer-duration studies may be needed to evaluate important effects of isoflavones and exercise on abdominal FM, as these improvements are likely to have a profound impact on the metabolic profile and the health of postmenopausal women. Indeed, central adiposity is recognised as a risk factor of CVD(51). Affecting up to 50 % of postmenopausal women(Reference Park, Zhu and Palaniappan52), the metabolic syndrome is highly associated with risks of type 2 diabetes and CVD(51, Reference Collins, Rosano and Casey53). It is now well recognised that postmenopausal women tend to gain FM preferentially in the abdomen(Reference Tchernof, Poehlman and Despres54). Unfortunately, our measurements did not include a computed tomography scan and, therefore, we could not assess changes in subcutaneous and visceral adipose tissue. Nevertheless, the absence of overall changes in metabolic parameters in the present study, despite significant changes in adiposity, could be explained by the fact that body weight was not reduced. A minimal weight reduction of 5 % (preferentially 10 %) is generally recommended to improve the metabolic profile in overweight individuals(Reference Pi-Sunyer, Blackburn and Brancati55, Reference Villareal, Miller and Banks56), and weight reduction may be mandatory to improve LDL-cholesterol(Reference Lalonde, Gray-Donald and Lowensteyn57), TAG(Reference Fernandez, Metghalchi and Vega-López58) and insulin sensitivity(Reference Flechtner-Mors, Ditschuneit and Johnson59). In a sample similar to ours, such a decrease in body weight could represent at least 3·5 kg. Even when considering that this weight loss would not come entirely from adipose tissue, fat loss would still probably account for more than the 1·3 kg fat loss observed in our exercise groups. In addition, other studies have suggested that nutritional interventions may have an efficient impact on the lipid profile(Reference Jenkins, Kendall and Marchie60, Reference Jenkins, Kendall and Faulkner61) rather than exercise per se. This may partly explain why weight-loss intervention, generally driven by energy restriction, may be effective in improving lipid and metabolic profiles.
In conclusion, 6 months of exercise appear to bring favourable changes in LBM, total FM, waist circumference, hip circumference and FM% in overweight postmenopausal women. While the present results do not support directly an interaction between exercise and isoflavones, modest improvements in trunk FM and leg LBM appear to be achieved by the addition of isoflavones to exercise. The present findings suggest that studies should focus for a longer duration of intervention to assess potential synergistic actions of exercise and isoflavones, and that exercise modalities promoting both fat oxidation and muscle hypertrophy should be favoured.
Acknowledgements
The authors of the present study declare that they have no conflicts of interest. The Canadian Institute of Health Research took no part in any aspects of the present study, including elaboration, recruitment or interpretation of the results. The authors wish to thank all the participants in the present study, with a very special thanks to Martine Fisch, our research nurse, for her invaluable help in every aspect of the study. The contribution of authors is as follows: S. C. involved in the study design, data collection, analyses, figure preparation and manuscript preparation. E. R., E. C. and T. D. participated in the data collection and manuscript preparation. M. A.-L. and I. J. D., Senior writer, were responsible for the study design and manuscript preparation. The present study was funded by the Canadian Institute of Health Research. Trial Registration: NCT01048606. Universitaire de Sherbrooke Protocol Record IDionne_phyto_2008–2011, Exercise and phytoestrogens: effect on factors predisposing to CVD in postmenopausal women.








