INTRODUCTION
The xanthophylls lutein (L) and zeaxanthin (Z) are among 600 naturally occurring carotenoids that must be acquired via diet, predominantly through consumption of green leafy vegetables and colored fruits. Of the 30–40 carotenoids present in human sera, generally speaking, only L and Z cross the blood–retina barrier to form macular pigment (Bone, Landrum, & Tarsis, Reference Bone, Landrum and Tarsis1985). L and Z also preferentially accumulate in brain tissue, including frontal, occipital, and temporal cortices, as well as the cerebellum and pons, accounting for 66–77% of total brain carotenoid levels (Craft, Haitema, Garnett, Fitch, & Dorey, Reference Craft, Haitema, Garnett, Fitch and Dorey2004; Johnson et al., Reference Johnson, Vishwanathan, Johnson, Hausman, Davey, Scott and Poon2013; Vishwanathan, Kuchan, Sen, & Johnson, Reference Vishwanathan, Kuchan, Sen and Johnson2014). As isomers with identical chemical compositions and extremely similar structures (Krinsky, 2002), L and Z are often administered together in clinical trials (e.g., Chew et al., Reference Chew, Clemons, SanGiovanni, Danis, Ferris, Elman and Fish2014) and their effects are routinely considered conjointly in analyses (e.g., Vishwanathan, Iannaccone, et al., Reference Vishwanathan, Iannaccone, Scott, Kritchevsky, Jennings, Carboni and Johnson2014).
L and Z have demonstrated potential to benefit a range of neurodegenerative disorders, such as age-related macular degeneration, diabetic retinopathy, dementia, and Huntington’s disease (Arnal, Miranda, Barcia, Bosch-Morell, & Romero, Reference Arnal, Miranda, Barcia, Bosch-Morell and Romero2010; Binawade & Jagtap, Reference Binawade and Jagtap2013; Chew et al., Reference Chew, Clemons, SanGiovanni, Danis, Ferris, Elman and Fish2014; Feart et al., Reference Feart, Letenneur, Helmer, Samieri, Schalch, Etheve and Barberger-Gateau2016; Scanlon et al., Reference Scanlon, Connell, Ratzlaff, Foerg, McCartney, Murphy and Loughman2015; Wang, Shinto, Connor, & Quinn, Reference Wang, Shinto, Connor and Quinn2008). Results also appear to support a role of the macular carotenoids, not only in reducing the probability of age-related disease, but also in preventing many of the changes that tend to precede those diseases. For instance, preliminary data suggest a connection between L and Z levels, measured in serum and in retina, and executive cognitive functions, verbal fluency, attention, logical reasoning, psychomotor speed, perceptual speed, learning, memory, language, visual-spatial and constructional abilities, and global cognition (Akbaraly, Faure, Gourlet, Favier, & Beer, Reference Akbaraly, Faure, Gourlet, Favier and Berr2007; Feeney et al., Reference Feeney, Finucane, Savva, Cronin, Beatty, Nolan and Kenny2013; Renzi, Dengler, Puente, Miller, & Hammond, Reference Renzi, Dengler, Puente, Miller and Hammond2014; Vishwanathan, Iannaccone, et al., Reference Vishwanathan, Iannaccone, Scott, Kritchevsky, Jennings, Carboni and Johnson2014).
Dietary intake of green leafy vegetables high in L and Z buffers older adults from global cognitive decline, as demonstrated in longitudinal designs (Kang, Ascherio, & Grodstein, Reference Kang, Ascherio and Grodstein2005; Morris, Evans, Tangney, Bienias, & Wilson, Reference Morris, Evans, Tangney, Bienias and Wilson2006). Although most of the relevant data is cross-sectional, in an exploratory intervention trial in older women, docosahexaenoic acid and L supplementation were associated with improvements in verbal fluency, memory, and learning slope when compared to placebo (Johnson et al., 2008; although, see Chew et al., Reference Chew, Clemons, Agrón, Launer, Grodstein and Bernstein2015). Taken together, there is promising evidence suggesting that L and Z exert a positive effect on a range of cognitive outcomes in older adults.
L and Z have traditionally been measured via two primary methods: serum and retinal levels. Serum levels of L and Z, which are frequently combined for analyses due to their chemical and structural correspondence (e.g., Bone, Landrum, Dixon, Chen, & Llerena, Reference Bone, Landrum, Dixon, Chen and Llerena2000; Olmedilla-Alonso, Beltran-de-Miguel, Estevez-Santiago, & Cuadrado-Vives, Reference Olmedilla-Alonso, Beltrán-de-Miguel, Estévez-Santiago and Cuadrado-Vives2014; Vishwanathan, Iannaccone, et al., Reference Vishwanathan, Iannaccone, Scott, Kritchevsky, Jennings, Carboni and Johnson2014), are driven by recent dietary intakes and do not necessarily reflect long-term dietary behavior, unless dietary behavior is stable in the individuals measured (Beatty, Nolan, Kavanagh, & O’Donovan, Reference Beatty, Nolan, Kavanagh and O’Donovan2004). Some studies have found that serum L and Z levels remain generally constant with advancing age (e.g., Cardinault et al., Reference Cardinault, Gorrand, Tyssandier, Grolier, Rock and Borel2003) while others have observed modest increases (e.g., Olmedilla-Alonso et al., Reference Olmedilla-Alonso, Beltrán-de-Miguel, Estévez-Santiago and Cuadrado-Vives2014) likely related to corresponding increases in dietary intake (Johnson, Maras, Rasmussen, & Tucker, Reference Johnson, Maras, Rasmussen and Tucker2010). However, serum L and Z is lower in individuals with mild cognitive impairment and Alzheimer’s disease compared to cognitively healthy individuals (Nolan et al., Reference Nolan, Loskutova, Howard, Moran, Mulcahy, Stack and Beatty2014; Rinaldi et al., Reference Rinaldi, Polidori, Metastasio, Mariani, Mattioli, Cherubini and Mecocci2003). In the retina, L and Z preferentially accumulate in the macula as macular pigment (MP), and optical density of the macular pigment layer (MPOD) can be measured non-invasively using psychophysical techniques. The most common method of measuring MPOD, heterochromatic flicker photometry, has been well-validated (Hammond, Wooten, & Smollon, Reference Hammond, Wooten and Smollon2005) and may serve as a biomarker of L and Z concentrations in brain tissue (e.g., Vishwanathan, Iannaccone, et al., Reference Vishwanathan, Iannaccone, Scott, Kritchevsky, Jennings, Carboni and Johnson2014; Vishwanathan, Neuringer, Snodderly, Schalch, & Johnson, Reference Vishwanathan, Neuringer, Snodderly, Schalch and Johnson2013). Like serum levels, MPOD appears to be lower in individuals with cognitive impairment and dementia (Feeney et al., Reference Feeney, Finucane, Savva, Cronin, Beatty, Nolan and Kenny2013; Nolan et al., Reference Nolan, Loskutova, Howard, Moran, Mulcahy, Stack and Beatty2014; Renzi et al., Reference Renzi, Dengler, Puente, Miller and Hammond2014; Vishwanathan, Iannaccone, et al., Reference Vishwanathan, Iannaccone, Scott, Kritchevsky, Jennings, Carboni and Johnson2014). Unlike serum levels, one advantage of MPOD is that it represents a measure of L and Z already incorporated into central nervous system tissue and thus may be a better indicator of longer-term dietary intakes.
Although serum levels of L and Z are positively correlated with MPOD (typical r values are around 0.30; e.g., Renzi, Hammond, Dengler, & Roberts, Reference Renzi, Hammond, Dengler and Roberts2012), they represent distinct measures (e.g., Beatty, et al., Reference Beatty, Nolan, Kavanagh and O’Donovan2004; Burke, Curran-Celentano, & Wenzel, Reference Burke, Curran-Celentano and Wenzel2005). For example, one study assessed both serum L and Z and MPOD each month for 24 months and found that MPOD mean and variance were relatively stable, while serum concentrations were more variable; additionally, variations in MPOD and serum concentrations were not linearly related (Nolan et al., Reference Nolan, Stack, Mellerio, Godhinio, O’Donovan, Neelam and Beatty2006). Another study demonstrated that after discontinuing supplementation of L and Z, serum concentrations quickly returned to baseline, whereas changes in MPOD lasted for up to 100 days (Beatty et al., Reference Beatty, Nolan, Kavanagh and O’Donovan2004; Hammond et al., Reference Hammond, Johnson, Russell, Krinsky, Yeum, Edwards and Snodderly1997). These findings are consistent with the interpretation that serum concentrations of L and Z more closely reflect recent or “acute” dietary factors, whereas MPOD, which has a slower biological turnover, reflects more stable L and Z levels acquired over time.
The mechanisms underlying the relation of L and Z to cognitive functioning remain to be fully elucidated. In light of findings suggesting oxidative stress and inflammation play important roles in dementia and cognitive decline more broadly (Engelhart et al., Reference Engelhart, Geerlings, Meijer, Kiliaan, Ruitenberg, van Swieten and Breteler2004; Finkel & Holbrook, Reference Finkel and Holbrook2000; Pappolla et al., Reference Pappolla, Smith, Bryant-Thomas, Bazan, Petanceska, Perry and Refolo2002; Teunissen et al., Reference Teunissen, Van Boxtel, Bosma, Bosmans, Delanghe, De Bruijn and De Vente2003), it is possible that L and Z exert neuroprotective effects via their antioxidant and/or anti-inflammatory properties (Johnson, Reference Johnson2012). More specifically, L and Z quench reactive oxygen molecules to prevent free radical attack (Johnson, Reference Johnson2014) while altering inflammation-related gene expression (Bian et al., Reference Bian, Gao, Zhou, Qin, Taylor, Johnson and Shang2012), pro-inflammatory factor production (Li et al., Reference Li, Fung, Fu, Wong, Chan and Lo2012), and inflammatory cytokine signaling to buffer neural damage (Sasaki et al., Reference Sasaki, Ozawa, Kurihara, Noda, Imamura, Kobayashi and Tsubota2009). Carotenoids may also impact cognition by enhancing interneuronal communication through facilitation of signaling compound exchange at gap junctions (i.e., cell-to-cell channels) and by providing structural support to synaptic membranes (Krinsky, Mayne, & Sies, Reference Krinsky, Mayne and Sies2004; Stahl & Sies, Reference Stahl and Sies2001). Studies using animal models have supported the possibility of such mechanisms, demonstrating that carotenoid administration reduces neurodegeneration, ameliorates oxidative stress and inflammation, and improves cholinergic and mitochondrial dysfunction in brain regions relevant to cognitive aging, such as cerebral cortex and hippocampi (Arnal et al., Reference Arnal, Miranda, Barcia, Bosch-Morell and Romero2010; Binawade & Jagtap, Reference Binawade and Jagtap2013; Kuhad, Sethi, & Chopra, Reference Kuhad, Sethi and Chopra2008; Muriach et al., Reference Muriach, Bosch-Morell, Alexander, Blomhoff, Barcia, Arnal and Miranda2006; Nakashima et al., Reference Nakashima, Ohsawa, Konishi, Hasegawa, Kumamoto, Suzuki and Ohta2009). Importantly, these neuroprotective actions are associated with improvements in cognitive performance on tests of learning and memory (Binawade & Jagtap, Reference Binawade and Jagtap2013; Kuhad et al., Reference Kuhad, Sethi and Chopra2008; Nakashima et al., Reference Nakashima, Ohsawa, Konishi, Hasegawa, Kumamoto, Suzuki and Ohta2009).
The present study aimed to further illuminate mechanisms by which L and Z relate to cognitive functioning in older adults using functional magnetic resonance imaging (fMRI) of verbal memory performance. fMRI has demonstrated sensitivity to changes in neural activation in healthy and pathologically aging older adults on memory tasks as well as to the effects of memory-enhancing interventions, including both drug and antioxidant therapies (e.g., Bookheimer et al., Reference Bookheimer, Renner, Ekstrom, Li, Henning, Brown and Small2013; Dickerson et al., Reference Dickerson, Salat, Bates, Atiya, Killiany, Greve and Sperling2004; Gutchess et al., Reference Gutchess, Welsh, Hedden, Bangert, Minear, Liu and Park2005; Lorenzi et al., Reference Lorenzi, Beltramello, Mercuri, Canu, Zoccatelli, Pizzini and Frisoni2011; Saykin et al., Reference Saykin, Flashman, Frutiger, Johnson, Mamourian, Moritz and Weaver1999). To the authors’ knowledge, this represents the first investigation in which a neuroimaging technique was used to provide an in vivo assessment of the impact of L and Z on brain function.
According to the scaffolding theory of aging and cognition (STAC), cognitive functioning is preserved in the face of age-related neural insult through an ongoing process of reorganization of existing neural connections and recruitment of additional neural circuitry (Park & Reuter-Lorenz, Reference Park and Reuter-Lorenz2009). “Compensatory scaffolding” is considered to be an adaptive, dynamic process that occurs in response to intrinsic (e.g., biological aging) or extrinsic (e.g., task demands) neural challenges to maintain cognitive performance (Park & Reuter-Lorenz, 2009). It is reflected in neuroimaging studies as increased bilateral activation and/or overactivation in certain regions (e.g., prefrontal cortex; e.g., Cabeza et al., Reference Cabeza, Grady, Nyberg, McIntosh, Tulving, Kapur and Craik1997; Cabeza, Anderson, Locantore, & McIntosh, Reference Cabeza, Anderson, Locantore and McIntosh2002; Fera et al., Reference Fera, Weickert, Goldberg, Tessitore, Hariri, Das and Mattay2005; Haung, Polk, Goh, & Park, Reference Huang, Polk, Goh and Park2012). The notion that additional neural activity reflects a compensatory neurocognitive process is supported by reduced hemispheric asymmetry as the complexity of a given task increases (e.g., Banich, Reference Banich1998; Hillary, Genova, Chiaravalloti, Rypma, & DeLuca, Reference Hillary, Genova, Chiaravalloti, Rypma and DeLuca2006), while practice on a given task refines neural networks such that they become less dispersed and more specific (e.g., Petersen, van Mier, Fiez, & Raichle, Reference Petersen, Van Mier, Fiez and Raichle1998). With respect to verbal memory, neuroimaging findings have suggested that age-related cognitive changes, genetic vulnerability to disease, and early stages of neurodegenerative conditions such as dementia are associated with more pronounced and bilateral patterns of brain activity, suggestive of increased neurocognitive effort relative to healthy counterparts (e.g., Bookheimer et al., Reference Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta and Small2000; Cabeza et al., Reference Cabeza, Grady, Nyberg, McIntosh, Tulving, Kapur and Craik1997; Dickerson & Sperling, Reference Dickerson and Sperling2008).
In the present study, we evaluated whether L and Z levels are cross-sectionally related to neurobiological efficiency in community-dwelling older adults via promotion of more honed neural networks and reduced need for compensatory scaffolding in response to age-related neural deterioration. MPOD and serum concentrations of L and Z were assessed alongside performance on a verbal memory task in which participants were asked to learn and recall word pairs (Bookheimer et al., Reference Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta and Small2000). Lower MPOD and serum L and Z were hypothesized to predict greater compensatory recruitment during memory encoding and retrieval as evidenced by increased neural activity required to meet task demands (i.e., neurobiological inefficiency). For memory encoding, this pattern of activity was anticipated in medial temporal lobe, supramarginal and angular gyri, precuneus, dorsolateral and ventrolateral prefrontal cortex, anterior and posterior cingulate gyrus, Broca’s area, cerebellum, and premotor areas, consistent with regions-of-interest that have demonstrated involvement in verbal memory in other studies (e.g., Binder, Desai, Graves, & Conant, Reference Binder, Desai, Graves and Conant2009; Bookheimer et al., Reference Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta and Small2000; Cabeza & Nyberg, Reference Cabeza and Nyberg2000; Clément & Belleville, Reference Clément and Belleville2009).
With the exception of the cerebellum, brain activation was generally expected to be left-lateralized (Binder et al., Reference Binder, Desai, Graves and Conant2009; Cabeza & Nyberg, 2000). A similar frontotemporoparietal network was hypothesized for memory retrieval, although brain activity was expected to show greater tendency for right-lateralization and to evidence greater involvement of anterior prefrontal cortex and medial parieto-occipital areas, including retrosplenial cortex and cuneus (Cabeza & Nyberg 2000). Behaviorally, greater MPOD and serum L and Z were expected to predict enhanced cognitive performance on measures of word recall. Given that serum and retinal concentrations of L and Z and their isomers have been positively related in previous studies, we expected that both measures would follow the same general pattern of negative relation to brain activity and positive relation to behavioral measures of word recall. However, because serum concentrations represent acute L and Z levels and MPOD represents acquired levels, results were not expected to be identical in all hypothesized regions-of-interest.
METHOD
Participants
Data for the present study were derived from a larger intervention study evaluating the relationship between cognition and diet in community-dwelling older adults (65–86 years) recruited from the surrounding area via advertisements, flyers, and electronic media (e.g., listservs). Exclusion criteria included left-handedness, traumatic brain injury, age-related macular degeneration in either eye, gastric conditions with potential to interfere with L/Z absorption (gastric ulcer, gastric band or bypass, Crohn’s disease, ulcerative colitis), corrected visual acuity poorer than 20:40, MRI incompatibility, and/or evidence of dementia or other neurological disorder. Of the participants who were eligible for inclusion and for which neuroimaging, serum, and MPOD data were collected (N=50), 6 were unable to complete the MRI process (e.g., physical discomfort, fatigue, or obvious failure to follow task instructions) and behavioral data from one individual were lost due to technical malfunction, leaving a sample size of 43 for final analyses. Participants were compensated $100 for their time and effort. The study was approved by the University of Georgia Institutional Review Board for safety and ethical treatment of participants, and the tenets of the Declaration of Helsinki were adhered to at all times by all study personnel.
Measures
Wechsler test of adult reading
The Wechsler Test of Adult Reading (WTAR; The Psychological Corporation, 2001) was administered to estimate premorbid intellectual functioning (Wechsler, Reference Wechsler2001). The WTAR provides a Full-Scale Intelligence Quotient (FSIQ) estimate based on an algorithm that incorporates an examinee’s ability to pronounce a list of words as well as demographic (i.e., age, education, race, sex, and geographic region) variables.
Geriatric depression scale
Depressive symptomatology was assessed using the Geriatric Depression Scale (GDS; Yesavage et al., Reference Yesavage, Brink, Rose, Lum, Huang, Adey and Leirer1983). The GDS is a self-report instrument comprised of 30 items of yes/no format and is scored on a 0–30 scale, with higher scores indicating greater levels of depression.
Serum lutein and zeaxanthin
Details of serum analysis can be found elsewhere (Handelman, Shen, & Krinsky, Reference Handelman, Shen and Krinksy1992; Qin et al., Reference Qin, Yeum, Johnson, Krinsky, Russell and Tang2008). Briefly, a total of 7 mL of whole blood was collected via venipuncture by a certified phlebotomist. After collection, samples were immediately placed on ice and centrifuged cold for 15 min. Following centrifugation, serum was collected and aliquoted into 1-mL cryotubes and frozen at −80°C for analysis.
Frozen serum aliquots were thawed to a temperature of 23°C, and precipitated with ethanol. The fat soluble carotenoids were extracted from the aqueous suspension with n-hexane/chloroform. After centrifugation, an aliquot of the organic phase was evaporated to dryness, re-dissolved in mobile phase and prepared for injection using an autoinjection system. L/Z was analyzed using a Hewlett Packard/Agilent Technologies 1100 series high performance liquid chromatography (HPLC) system with a photodiode array detector (Agilent Technologies, Palo Alto, CA). A 5 μm, 200 A° polymeric C30 reverse-phase column (Pronto-SIL, MAC-MOD Analytical Inc., Chadds Ford, PA) was used to separate the analytes.
The HPLC mobile phase solvent A consists of methanol/tert-butyl methyl ether/water (83:15:2, v/v/v, with 1.5% ammonium acetate in the water) and solvent B is methanol/tert-butyl methyl ether/water (8:90:2, v/v/v, with 1% ammonium acetate in the water). The gradient procedure at a flow rate of 1 mL/min begins at 100% Solvent A for 2 min to 70% Solvent A over a 6-min linear gradient and held at 70% A for 3 min, then a 10-min linear gradient to 45% Solvent A and a 2-min hold at 45% Solvent A, then a 10-min linear gradient to 5% Solvent A, a 4-min hold at 5% Solvent A and, finally, a 2-min linear gradient back to 100% Solvent A. The system is held at 100% Solvent A for 10 min for equilibration back to initial conditions (Qin et al., Reference Qin, Yeum, Johnson, Krinsky, Russell and Tang2008).
Serum L and Z were analyzed separately via HPLC. To obtain a combined serum L+Z value, serum L levels (μmol/L) were added to serum Z levels (μmol/L). The combined L+Z value was used in all subsequent analyses. To assess the daily and long-term laboratory performance of the HPLC plasma analytics, dedicated control plasma was used following standardization with SRM 968 c (Standard Reference Materials, National Institute of Standards and Technology, Gaithersburg, MD).
Macular pigment optical density
Macular pigment optical density (MPOD) was evaluated using customized heterochromatic flicker photometry, as described previously (Stringham et al., Reference Stringham, Hammond, Nolan, Wooten, Mammen, Smollon and Snodderly2008). Briefly, a macular densitometer (Macular Metrics; Rehoboth, MA) was used to present participants with a 1° visual stimulus that consisted of two narrow-band LED-based light sources, peaking at 460 nm and 570 nm. The light sources were presented in square-wave, counter-phase orientation to present the appearance of flicker. Before measurement, each participant’s critical flicker fusion frequency was measured using only the mid-wave portion of the stimulus, so that the task could be customized to the individual viewer. Following determination of customized flicker sensitivity, participants were asked to fixate on a black dot displayed at the disk’s core.
The radiance of the lower (i.e., 460 nm) waveband was manipulated relative to the 570 nm component to assess the point at which flickering was no longer perceivable. This sequence was again conducted with a 2° target and fixation point at 7° nasally to allow a reference measurement in the parafovea (where MPOD approaches zero). The two loci (i.e., 30 min, derived from the 1° target, and 7° of retinal eccentricity) were then compared to provide the MPOD measurement at 30 min of retinal eccentricity.
Neuroimaging
fMRI task
Participants completed a block design verbal learning task involving unrelated word pairs (e.g., “UP” and “FOOT”) conceptually based on the Wechsler Memory Scale Verbal Paired Associates (Wechsler, Reference Wechsler2009) and similar to previous fMRI paradigms (e.g., Bookheimer et al., Reference Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta and Small2000, Reference Bookheimer, Renner, Ekstrom, Li, Henning, Brown and Small2013; Braskie, Small, & Bookheimer, Reference Braskie, Small and Bookheimer2009). The task was programmed using E-Prime software (version 1.2, Psychology Software Tools, Inc., Pittsburgh, PA) and presented through MRI compatible goggles (Resonance Technology Inc., Northridge, CA). Participants responded using a pair of 2-button Cedrus Lumina LU400 MRI compatible response pads (Cedrus, San Pedro, CA). The task consisted of 10 separate learning blocks, control blocks, retrieval blocks, and fixation blocks, presented sequentially in the aforementioned order (i.e., learning, control, retrieval, fixation) for every participant (see Figure 1).

Fig. 1 Verbal Learning Task. The above figure provides a visual schematic of the progression of the verbal learning task. The four blocks (i.e., learning, control, retrieval, and fixation) were presented a total of 10 times, resulting in a total acquisition time of 12 min, 24 s. Five different words pairs were presented in each learning block and 5 sets of XXXXX – YYYYY pairings were presented in each control block. During each retrieval block, participants were asked to recall the second word for five different word pairs.
In the learning blocks, the first word of each pair was presented alone on the left side of the screen (1 s) followed by presentation of the second word on the right side of the screen, such that both words were viewable simultaneously (2 s). There were a total of 10 word pairs, 5 of which were presented in each encoding block. Participants were instructed to respond with their right index finger whenever the second word in the pair appeared, to help verify attention during learning. During the retrieval portion of the task, participants were presented with the first word in each pair (3 s) and asked to mentally recall (to avoid head motion) the second word, consistent with procedures used in similar fMRI-adapted verbal learning paradigms (e.g., Bookheimer et al., Reference Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta and Small2000, Reference Bookheimer, Renner, Ekstrom, Li, Henning, Brown and Small2013). Participants were instructed to respond with their right index finger if they remembered the second word or to respond with their left index finger if they did not (maximum score: 50).
Learning and retrieval blocks were interspersed with a control task analogous to the learning block except that Xs (i.e., “XXXXX”) and Ys (i.e., “YYYYY”) were presented instead of word pairs. Participants responded with their right index finger whenever the Ys appeared on the screen. Fixation blocks consisted of a crosshair presented for 6 s. Immediately following the scan, participants were asked to engage in free recall (maximum score: 10) followed by cued recall (maximum score: 10) of the “second word” in each pair to help verify task engagement within the scanner.
MRI acquisition
A General Electric (GE; Waukesha, WI) 3 Tesla Signa HDx MRI system was used to acquire all scans. Structural scans were collected using a high-resolution three-dimensional T1-weighted fast spoiled gradient recall echo sequence [repetition time (TR)=7.5 ms; echo time (TE)=<5 ms; field of view (FOV)=256×256 mm matrix; flip angle=20°; slice thickness=1 mm; 154 axial slices; voxel size=0.94×0.94×1 mm] with a total acquisition time of 6 min, 20 s. This protocol covered the top of the head to the brainstem and collected 176 images.
Functional scans were aligned to the anterior commissure-posterior commissure line and collected axially using a T2*-weighted single shot echo planar imaging (EPI) sequence (TR =1500 ms; TE=25 ms; 90° RF pulse; acquisition matrix =64×64; FOV =220×220 mm; in-plane resolution, 220×64 mm; slice thickness =4 mm; 30 interleaved axial slices; voxel size =3.43×3.43×4 mm) with an acquisition time of 12 min, 24 s. The EPI sequence covered the cortical surface and a portion of the cerebellum, and consisted of 290 volumes. A pair of magnitude and phase images was acquired, lasting 1 min, 40 s each, for fieldmap-based unwarping (TR =700 ms; TE =5.0/7.2 ms; FOV =220×220 mm matrix; flip angle =30°; slice thickness =2 mm; 60 interleaved slices; voxel size =1.72×1.72×2 mm).
Data analysis
Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK) was used to process and analyze the data. Data were first converted from GE DICOM to NIFTI format with the dcm2nii conversion tool (Rorden, Reference Rorden2007). Preprocessing of functional data included slice time correction to adjust for the non-sequential, interleaved acquisition and realignment of functional images to the first image of the functional scan to correct for head movement. Fieldmaps were created to realign and unwarp images to account for phase and magnitude variations during the scan. Anatomical scans were co-registered to the first image of the functional scan followed by registration of anatomical and functional images to the Montreal Neurological Institute (MNI) template. The anatomical image was segmented to differentiate brain tissue (i.e., white matter and gray matter), cerebrospinal fluid, bone, non-brain soft tissue, and air. Deformation fields were created and applied to functional images to allow spatial normalization to MNI space. Finally, images were smoothed using a 6.75-mm FWHM Gaussian filter.
Following pre-processing, activation maps of encoding minus control task and retrieval minus control task were created using the General Linear Model (SPM12b). All trials were included in analyses, regardless of occasional failure to respond or self-perceived recall success. A statistical threshold of p<.05, family-wise-error (FWE) corrected, and a minimum of eight contiguous voxels were selected given optimal balance between Type I and Type II error rates (Lazar, Reference Lazar2008). Regression-based analyses were conducted to determine the relation of L and Z levels to voxel activity within hypothesized regions-of-interest during memory encoding and recall.
The relation of L and Z levels to behavioral measures of recall was also evaluated using regression analyses. More specifically, the number of self-reported successful retrievals across the entire task and on the final 10 retrieval trials only (the assumed point of maximal learning), as well as the number of verified cued successful retrievals measured immediately post-scan, were considered as dependent variables in three separate regressions.
Procedure
Following recruitment and screening for exclusion criteria (described above), data were collected across three testing sessions. During the first session, written informed consent was obtained and MPOD was measured. Participants completed the GDS and WTAR in the second session, as well as a handful of other measures that were part of the larger intervention study and not a focus of the present analyses. The third session was conducted at the University of Georgia Bio-Imaging Research Center, which houses the MRI scanner. Before being placed in the MRI environment, participants practiced an abbreviated version of the verbal learning task on a desktop computer to ensure understanding of task directions and provide the opportunity to ask questions. The MRI protocol included structural imaging first, followed by collection of phase and magnitude images, and finally acquisition of functional data during the verbal learning task. Participants were debriefed and thanked at the end of the testing session.
RESULTS
Sample Characteristics and Verbal Learning Behavioral Responses
Serum L+Z levels, MPOD, and demographic information including age, years of formal education, gender and racial composition, and estimated intellectual functioning are presented in Table 1. It is notable that the sample was entirely Caucasian and tended to be well educated.
Table 1 Descriptive statistics (N=43)

Note. o.d. represents the log ratio of transmitted light passing through the macula.
GDS=Geriatric Depression Scale; FSIQ=Full-Scale Intelligence Quotient from the Wechsler Test of Adult Reading; MPOD=macular pigment optical density; o.d.=optical density.
a Data only available for 42 participants.
Table 1 also provides descriptive statistics related to behavioral performance on the verbal learning task. While in the scanner, participants, on average, self-reported recall of the second word in the pair on 37 of the 50 total recall trials (74%). The group average self-reported (within scanner) recall during the last two recall blocks only (the assumed point of maximal learning) was 9 of the 10 total word pairs (90%). As expected, self-reported recall within the scanner significantly correlated with actual cued recall immediately following the scan (r=0.45; p=.002), differing from each other by less than two words, on average (mean difference=1.67; SD=2.00, 3 participants with discrepancy>4 words). The observed correlation provides evidence that participants were engaged in the task and actively attempting to learn and recall word pairs within the scanner. In addition, discrepancy scores between within-scanner recall and actual post-scan recall were unrelated to MPOD (p=.593) or serum L+Z (p=.073).
Age and education were considered as potential covariates in analyses but given nonsignificant zero-order bivariate correlations to MPOD (r=.17; p=.279, and r=.10; p=.521, respectively), serum L+Z (r=−.02; p=.926, and r=.07; p=.681, respectively), and behavioral measures of verbal learning, they were not included in the regression models.
Whole-Brain Analyses
Using a FWE corrected p<.05 and minimum of eight contiguous voxels, the encoding minus control contrast revealed widespread activation in regions commonly associated with verbal learning task performance, including left inferior frontal regions (Broca’s area), middle frontal gyri, hippocampus, precentral gyrus, and occipital lobe (see Figure 2). The recall minus control contrast similarly revealed diffuse activation in several regions including paracingulate gyri, insular cortex, middle frontal gyrus, and occipital lobe (see Figure 3).

Fig. 2 Panel (a) depicts whole-brain analyses of the encoding minus control contrast (independent of lutein and zeaxanthin levels) superimposed on a single-subject anatomical template in MNI space provided by MRIcron (http://www.mricro.com/mricron/install.html). To conserve space, only six slices were selected for visualization based on largest extent activation and thus not all voxel activity is represented. Panel (b) displays brain activation significantly related to lutein and zeaxanthin concentrations during encoding. Areas in green represent increased activation associated with lower MPOD levels, while areas in red represent increased activation associated with lower serum lutein and zeaxanthin. Only six slices were selected based on largest extent activation to showcase the relation of lutein and zeaxanthin to brain activity and thus not every significant cluster is displayed.

Fig. 3 Panel (a) depicts whole-brain analyses of the recall minus control contrast (independent of lutein and zeaxanthin levels) superimposed on a single-subject anatomical template in MNI space provided by MRIcron (http://www.mricro.com/mricron/install.html). To conserve space, only six slices were selected for visualization based on largest extent activation and thus not all significant voxel activity is represented. Panel (b) displays brain activation significantly related to lutein and zeaxanthin concentrations during retrieval. Areas in green represent increased activation associated with lower MPOD levels, while areas in red represent increased activation associated with lower serum lutein and zeaxanthin. Only six slices were selected based on largest extent activation to showcase the relation of lutein and zeaxanthin to brain activity and thus not every significant cluster is displayed.
Lutein and Zeaxanthin Levels and fMRI Performance
Behavioral
Serum L+Z and MPOD were unrelated to overt within-scanner behavioral performance on the verbal learning task. More specifically, MPOD was not a significant predictor of the number of self-reported successful retrievals across the entire paradigm (r=−.001; p=.993) nor across the last 10 trials only (i.e., the final block; r=.103; p=.511). Similarly, serum L+Z concentrations were not significantly related to word retrieval across the entire paradigm (r=.236; p=.127) nor on the final block (r=.275; p=.074). MPOD and serum L+Z levels also displayed nonsignificant relations to actual cued recall post-scan (r=.137; p=.380, and r=−.084; p=.591, respectively).
Functional imaging
Following the encoding minus control contrast (p<.05, FWE, minimal voxel cluster=8), regression-based analyses of blood-oxygen-level-dependent BOLD) signal demonstrated that lower MPOD levels were associated with significantly greater brain activity (i.e., neural inefficiency) in several regions relevant to verbal learning including left insular cortex, right middle temporal gyrus, left supramarginal gyrus, and left cerebellum (p<.01; see Table 2 and Figure 2). With respect to the recall minus control contrast (p<.05; FWE, minimal voxel cluster=8), lower MPOD was associated with increased activation in left inferior frontal gyrus, left insular cortex, left planum polare, right middle frontal gyrus, left cerebellum, and left and right occipital pole (p<.01; see Table 3 and Figure 3). As indicated in Tables 2 and 3, effect sizes for MPOD during encoding and retrieval ranged from r=.36 to r=.46.
Table 2 Relationship of lutein and zeaxanthin to brain activation during encoding (N=43)
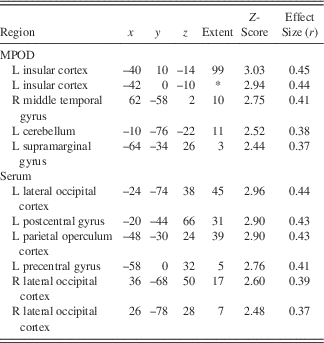
Note. The above table includes brain activity that was significantly and negatively associated with lutein and zeaxanthin levels during encoding of word pairs.MPOD=macular pigment optical density. x, y, and z coordinates are in MNI space (mm). L=left and R=right.*=cluster overlap with preceding row.
Table 3 Relationship of lutein and zeaxanthin to brain activation during recall (N=43)
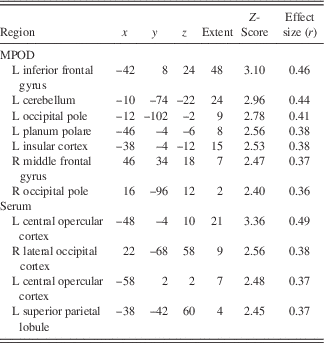
Note. The above table includes brain activity that was significantly and negatively associated with lutein and zeaxanthin levels during retrieval of word pairs. x, y, and z coordinates are in MNI space (mm).
MPOD=macular pigment optical density. L=left and R=right.
L+Z concentrations in serum similarly demonstrated a negative relationship with BOLD signal in several brain regions during encoding including left parietal operculum, occipital cortex bilaterally (superior division), left postcentral gyrus, and left precentral gyrus (p<.01; see Table 2 and Figure 2). During recall, less L+Z in serum was associated with increased activation in left central opercular cortex, left superior parietal lobule, and left lateral occipital cortex (p<.01; see Table 3 and Figure 3). As represented in Tables 2 and 3, effect sizes for L+Z in serum during encoding and retrieval ranged from r=.37 to r=.49.
DISCUSSION
L and Z are two carotenoids previously shown to accumulate in human brain tissue, improve cognitive functioning, and reduce risk of age-related degenerative diseases (e.g., Akbaraly et al., Reference Akbaraly, Faure, Gourlet, Favier and Berr2007; Johnson, Reference Johnson2014; Johnson et al., Reference Johnson, Vishwanathan, Johnson, Hausman, Davey, Scott and Poon2013; Nolan et al., Reference Nolan, Loskutova, Howard, Moran, Mulcahy, Stack and Beatty2014; Rinaldi et al., Reference Rinaldi, Polidori, Metastasio, Mariani, Mattioli, Cherubini and Mecocci2003; Vishwanathan, Iannaccone, et al., Reference Vishwanathan, Iannaccone, Scott, Kritchevsky, Jennings, Carboni and Johnson2014). To date, no studies have investigated the neural mechanisms underlying these relationships. This study sought to determine whether L+ Z are related to neural efficiency during a verbal learning and memory task.
Initial whole brain analyses revealed diffuse activation in regions commonly associated with verbal learning and retrieval, replicating previous findings (e.g., Bookheimer et al., Reference Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta and Small2000; Cabeza et al., Reference Cabeza, Grady, Nyberg, McIntosh, Tulving, Kapur and Craik1997; Dickerson & Sperling, Reference Dickerson and Sperling2008). As expected, lower levels of both MPOD and serum L+Z concentrations were associated with increased activation (i.e., neural inefficiency) in several of these regions, though results were not identical for the two measures. Overall, serum concentrations predicted activity in regions commonly involved in somatosensory functions (e.g., postcentral gyrus, parietal and central operculum, superior parietal lobule; Eickhoff et al., Reference Eickhoff, Jbabdi, Caspers, Laird, Fox, Zilles and Behrens2010; Molholm et al., Reference Molholm, Sehatpour, Mehta, Shpaner, Gomez-Ramirez, Ortigue and Foxe2006), whereas MPOD was associated with activity in regions more involved in language processing (e.g., inferior frontal gyrus, middle temporal gyrus, supramarginal gyrus, planum polare; Cabeza & Nyberg, Reference Cabeza and Nyberg2000; Costafreda et al., Reference Costafreda, Fu, Lee, Everitt, Brammer and David2006; Friederici, Meyer, & von Cramon, Reference Friederici, Meyer and von Cramon2000). This pattern suggests that acute intake of L+Z, as represented by serum concentrations, may boost performance by aiding in somatosensory processing. Carotenoid consumption over longer periods of time, as represented by MPOD, may increase performance via more efficient higher-order language skills.
Regardless of the specific cognitive functions implicated, pathological aging and cognitive decline in late life have been associated with dysfunction in many of the brain regions that demonstrated a functional relationship to L+Z levels, including left inferior frontal gyrus (Bookheimer et al., Reference Bookheimer, Strojwas, Cohen, Saunders, Pericak-Vance, Mazziotta and Small2000; Clément & Bellville, Reference Clément and Belleville2009), insula (Xie et al., Reference Xie, Bai, Yu, Shi, Yuan, Chen and Li2012), middle temporal gyrus (Convit et al., Reference Convit, de Asis, de Leon, Tarshish, De Santi and Rusinek2000), middle frontal gyrus (Rajah, Languay, & Grady, Reference Rajah, Languay and Grady2011), and central and parietal operculum (Trachtenberg et al., Reference Trachtenberg, Filippini, Ebmeier, Smith, Karpe and Mackay2012). The present results are, therefore, consistent with past studies concluding that a diet rich in L+Z may buffer pathobiological processes in old age by enhancing neural efficiency in structures at risk for deterioration.
Both MPOD and serum L+Z levels was associated with neural efficiency in primary visual areas, suggesting that these carotenoids more generally improve visual processing (as shown behaviorally; Renzi & Hammond, Reference Renzi and Hammond2010) beyond the level of the retina. This finding holds particular relevance among older adults, who exhibit a high prevalence of visual difficulties due to conditions such as macular degeneration, which in turn have been shown to impact functional properties at a neural level (e.g., Baker, Peli, Knouf, & Kanwisher, Reference Baker, Peli, Knouf and Kanwisher2005).
Contrary to expectation, (serum or MP) L and Z did not predict behavioral performance on the verbal memory task. In many respects, however, this observation is consistent with the STAC in the sense that individuals with lower L and Z levels were required to recruit additional neural resources to maintain a similar level of cognitive performance as peers with higher L and Z levels (Park & Reuter-Lorenz, Reference Park and Reuter-Lorenz2009). It is also possible that there was a ceiling effect given the generally high level of cognitive functioning apparent within the present sample and that a more sensitive cognitive measure with greater variability in scores would reveal a relationship. Despite significant correlations with actual cued recall post-scan, the reliance on self-reported cognitive performance within the scanner limited conclusions regarding task accuracy and may have influenced the observed results.
Another important limitation of the present study is its cross-sectional design, which prevents conclusions regarding the directionality of the observed relationship between L+Z and neural efficiency. Although longitudinal studies have indicated that carotenoid consumption preserves neurocognitive health (Min & Min, Reference Min and Min2014), other dietary factors (e.g., antioxidant-rich fruits) left uncontrolled in the present analyses have been shown to impact brain function as measured with fMRI (Bookheimer et al., Reference Bookheimer, Renner, Ekstrom, Li, Henning, Brown and Small2013) and the results should be interpreted with this in mind. For example, it is possible that L and Z levels serve as proxies for other features of a healthful diet that relate to brain health, such as omega-3 fatty acid intake (Witte et al., Reference Witte, Kerti, Hermannstädter, Fiebach, Schreiber, Schuchardt and Flöel2014), and randomized controlled trials will be required to better address issues of causality while isolating the effects of the xanthophylls on neural activity.
Additionally, our sample was entirely Caucasian, highly educated, and, generally, very cognitively healthy. Thus, the generalizability of our findings to individuals characterized by greater diversity in socioeconomic status, racial background, and cognitive function may be limited and represents a critical avenue for future research. Finally, despite the strong chemical and structural similarities between L and Z, future neuroimaging studies may benefit from considering their unique effects in analyses rather than combining them as we have in the present investigation.
To our knowledge, this is the first study to investigate the association of L and Z to cognition using fMRI. Results indicate that L and Z concentrations, measured both acutely (serum) and acquired (retinal), enhance neural efficiency during verbal learning and memory in older adults. Our findings also offer a possible neural mechanism underlying previous findings showing a positive relation between these carotenoids and performance on cognitive tasks (e.g., Feeney et al., Reference Feeney, Finucane, Savva, Cronin, Beatty, Nolan and Kenny2013; Johnson, Reference Johnson2014; Renzi et al., Reference Renzi, Dengler, Puente, Miller and Hammond2014; Vishwanathan, Iannaccone, et al., Reference Vishwanathan, Iannaccone, Scott, Kritchevsky, Jennings, Carboni and Johnson2014). More broadly, the present study adds to the paucity of research investigating the critical relationship between diet and brain health, while identifying a modifiable lifestyle factor that may serve to promote neurocognitive functioning in the rapidly expanding older adult population.
ACKNOWLEDGMENTS
This research project was funded in part by Abbott Nutritional Products (Columbus, OH; research grant to B.R.H., L.M.R., L.S.M.) and the University of Georgia’s Bio-Imaging Research Center (administrative support, L.S.M.). DSM Nutritional Products (Switzerland) provided the supplements and placebos for the larger intervention study from which the data for the present study were derived. Additionally, L.M.R. was an employee of Abbott Nutrition during a portion of the grant period while holding a joint appointment at the University of Georgia. B.R.H. has consulted for Abbott Nutrition. No other potential conflicts of interest exist for any of the study authors, including C.A.L., C.M.M., and J.M.C. All statistical analyses were completed independently of supporting agencies.








