Type 1 diabetes (T1D), an autoimmune disease which requires lifelong insulin administration, affects about 20 to 35 million people worldwide and has a peak incidence in childhood and adolescence( Reference Thrower and Bingley 1 ). The overall incidence of T1D is increasing rapidly but the exact nature of the factors contributing to and triggering the development of T1D is still extensively discussed and not yet known( Reference Thrower and Bingley 1 ), especially for the disease manifestation during puberty( Reference Ziegler, Meier-Stiegen and Winkler 2 ). Already accepted is the interaction of genetic and environmental factors and the polygenic character of T1D susceptibility which triggers autoimmunity in genetically predisposed subjects( Reference Atkinson and Eisenbarth 3 ).
The environmental factors possibly involved in the disease are viral infections, diet, vaccinations and psychosocial factors( Reference Thrower and Bingley 1 , Reference Atkinson and Eisenbarth 3 ). There is evidence from animal and human research which indicates an association between the intake of certain foods as well as nutrients and the development of T1D( Reference Thorsdottir and Ramel 4 ). Three prospective birth cohort studies revealed that early introduction of gluten, other cereals, fruit and berry juices as well as cow's milk increased the risk of β-cell autoimmunity( Reference Norris, Barriga and Klingensmith 5 – Reference Ziegler, Schmid and Huber 7 ). However, there has been still no dietary component identified as a clear risk factor( Reference Virtanen, Nevalainen and Kronberg-Kippila 6 , Reference Knip, Virtanen and Akerblom 8 ). As many nutritional factors probably work in an age-dependent manner, the findings for early infancy might not be transferable to later childhood or adulthood( Reference Thorsdottir and Ramel 4 , Reference Virtanen, Nevalainen and Kronberg-Kippila 6 ).
Data for the correlation of diet and incidence of autoimmunity and T1D for children and adolescents are sparse and often based only on single studies. A high intake of fruits, vegetables( Reference Thorsdottir and Ramel 4 ), cow's milk( Reference Thorsdottir and Ramel 4 , Reference Virtanen and Knip 9 ), meat, dairy products( Reference Muntoni, Cocco and Aru 10 ), coffee and tea( Reference Virtanen and Knip 9 ), as well as total fat( Reference Thorsdottir and Ramel 4 ), protein and carbohydrates( Reference Dahlquist, Blom and Persson 11 ), was hypothesized to increase the risk of T1D in children and adolescents. Additionally, an increased risk of T1D was associated with a higher energy intake and a higher consumption of bread and carbohydrates, especially in the form of disaccharides and sucrose, one year before the manifestation of T1D( Reference Pundziute-Lycka, Persson and Cedermark 12 ). A high consumption of cereals( Reference Muntoni, Cocco and Aru 10 ) as well as a high intake of vitamin D, vitamin E and Zn( Reference Virtanen and Knip 9 ) was found to be protective. However, there is a great necessity to confirm these correlations in large prospective cohort studies. Furthermore, an evaluation of the dietary intake of adolescents at increased risk of T1D in terms of adherence to current recommendations is missing.
The TEENDIAB study, a cohort study in children with a familial risk of T1D, was set up to increase the knowledge on genetic and environmental factors influencing the development of islet autoimmunity and T1D during puberty, as they are hypothesized to differ from those during infancy( Reference Ziegler, Meier-Stiegen and Winkler 2 ). In the present study we assessed the dietary intake of the TEENDIAB children, and measured it against the German Dietary Reference Intakes on the nutrient level( 13 ) and against the Optimized Mixed Diet recommendations developed by the Research Institute of Child Nutrition in Dortmund( Reference Kersting, Alexy and Clausen 14 , Reference Kersting and Alexy 15 ) on the food level. Furthermore, we compared the intake data of the TEENDIAB children with those of a representative sample of children aged 12 years from the EsKiMo study, the nutrition module of KiGGS, the German Health Interview and Examination Survey of Children and Adolescents( Reference Mensink, Bauch and Vohmann 16 ). With these analyses we aimed to characterize the diet of children at increased risk of T1D, to investigate compliance with current reference data and recommendations, and to examine whether there are differences in dietary behaviour between at-risk and non-risk children.
Methods
Study population
Dietary intake was assessed in children participating in the TEENDIAB study. The TEENDIAB study is a prospective, observational cohort study. Subjects are recruited through a network of paediatricians which was generated for recruitment into former studies. Additionally, direct communication to affected families is facilitated by publications within a nationwide diabetes information network. Children can be included in the TEENDIAB study if they are between 8 and 12 years old, if they are resident in Germany and if they have at least one first-degree relative diagnosed with T1D. The subjects are followed up to the age of 18 years to investigate the period of puberty and adolescence in the natural course of T1D development, as previously described in detail( Reference Ziegler, Meier-Stiegen and Winkler 2 ).
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethical committee of the Technical University Munich (No. 2149/08) and Medizinische Hochschule Hannover (No. 5644). Written informed consent was obtained from all subjects.
For the current analysis, the diet of all children enrolled in the TEENDIAB study between February 2009 and January 2012 was assessed. In total, there were nutritional data of 270 participants aged 8–12 years available. The data of two children were incomplete and had to be excluded, which resulted in 268 participants remaining for examination.
Anthropometric and sociodemographic data
A physical examination was performed at study entry to measure weight and height of each child by a trained staff member. Weight, height and BMI were adjusted for gender and age at examination and were expressed as a percentile against national reference data. Children below the 10th percentile were classified as underweight, children between the 10th and 90th percentiles as normal weight and children equal to or above the 90th percentile as overweight( Reference Kronmeyer-Hauschild, Wabitsch and Kunze 17 ).
Sociodemographic data were assessed using a demographic questionnaire at study entry. Based on the school education of the parents and the net income of the family, each parent was assigned to an educational and a net income score. Both scores ranged from 1 to 6 points with higher points for higher educational level and net income. These two scores were summed up individually for each parent. The higher one of both scores was assigned to the child. Based on this, the children were classified into one of three socio-economic classes. Children with 2–5 points belonged to the lower class, those with 10–12 points to the upper class and the ones in between to the middle class.
Dietary evaluation using the diet history interview DISHES Junior
In the TEENDIAB study, diet was assessed using the modified computer-assisted Diet Interview Software for Health Examination Studies Junior (DISHES Junior; Robert Koch Institute, Berlin, Germany). This interview and the software had been updated and adapted for dietary assessment in children and adolescents for the EsKiMo study( Reference Mensink, Bauch and Vohmann 16 , Reference Kurth, Kamtsiuris and Holling 18 , Reference Stahl, Vohmann and Richter 19 ). In the TEENDIAB study, the standardized computerized questionnaire was performed in an interview style on site and in person by trained study nurses. It retrospectively assessed the consumed frequency, type and quantity of foods and beverages of the last 4 weeks( Reference Mensink, Haftenberger and Thamm 20 ). The portion size estimation was assisted by household measures consisting of cups, spoons, plates and bowls, and by a picture book which comprised colour photographs of foods in different portion sizes( Reference Mensink, Haftenberger and Thamm 20 , Reference van Kappel, Amoyel and Slimani 21 ). The interview software of DISHES Junior is linked to the German Nutrient Database, Version II·3 (Bundeslebensmittelschlüssel, BLS; Max Rubner Institut, Karlsruhe, Germany), which allows analysis of the average daily intakes of energy, macronutrients and micronutrients( Reference Ziegler, Meier-Stiegen and Winkler 2 , Reference Mensink, Haftenberger and Thamm 20 ).
The nutritional data of the TEENDIAB children were compared with the German Dietary Reference Intakes on the nutrient level and with the Optimized Mixed Diet recommendations on the food level. The German Dietary Reference Intakes were elaborated by the German Nutrition Society, the Austrian Nutrition Society, the Swiss Society for Nutritional Research and the Swiss Food Association. They provide nutrient-based reference values for the nutrient requirements of defined groups of the healthy population( 13 ). The Optimized Mixed Diet recommendations were established by the Research Institute of Child Nutrition in Dortmund. It provides food-based recommendations for a child-specific diet by implementing the German Dietary Reference Intakes and by additionally applying the current recommendations for the prevention of diet-related diseases. The foods and beverages are grouped into eleven food groups( Reference Kersting and Alexy 15 , Reference Alexy, Clausen and Kersting 22 ) and furthermore classified into three categories: (i) ‘plant foods and beverages’, which should be consumed amply; (ii) ‘animal foods’, with a moderate consumption; and (iii) ‘high-fat, high-sugar foods’, which are recommended only sparingly( Reference Kersting, Alexy and Clausen 14 , Reference Alexy, Clausen and Kersting 22 ). For the current analysis the separation between the two food groups ‘potatoes’ and ‘bread, grains, cereals’ was not completely possible. Therefore, both groups were combined into the new group ‘carbohydrate-rich foods’. Furthermore, we compared the nutrient intakes of the TEENDIAB children with those of a representative sample of children from the EsKiMo study. The EsKiMo study includes a sub-sample of 2506 participants of the KiGGS study and assessed the diet of children aged 6–11 years using a 3 d estimated food record as well as the diet of the children aged 12–17 years using DISHES Junior( Reference Mensink, Bauch and Vohmann 16 ). In order to ensure a comparison of intake data assessed with the same method in both cohorts, we used a sub-sample of children aged 12 years from the EsKiMo study.
For evaluation of the energy and nutrient intakes of the TEENDIAB children, the intake data were expressed as percentage values of the German Dietary Reference Intakes and as percentage values of the EsKiMo intake data. These percentage values were calculated on an individual level for boys, girls and age groups. On the food level, the amounts consumed in each food group were compared with the Optimized Mixed Diet recommendations, which are given as proportions of the food groups by weight (%) as the absolute amounts of intake vary with age, sex and energy requirement( Reference Kersting, Alexy and Clausen 14 ). Therefore, we recalculated the absolute intake data of the children as percentages of the total weight consumed.
Statistical analysis
All statistical analyses were performed using the statistical software package IBM SPSS Statistics 20 for Windows 7. The nutrient and food intake data were not normally distributed, thus are expressed as median and interquartile range (IQR; 25th percentile (P25)–75th percentile (P75)). The Wilcoxon signed-rank test was used to test differences between the TEENDIAB nutrient and food intake data and the respective references values, recommendations as well as the intake data of the EsKiMo cohort. We used Bonferroni's correction method to adjust for multiple comparisons. We considered only P values <0·05/26 = 0·0019 as significant in the analysis regarding comparison with the German Dietary Reference Intakes, P values <0·05/34 = 0·0015 as significant in the analysis regarding comparison with the EsKiMo data, and P values <0·05/10 = 0·005 were considered to be significant in the analysis regarding the Optimized Mixed Diet recommendations.
Results
Characterization of the study population
All 268 children, of whom 53 % were male, were of Caucasian ethnicity with a median age of 10·6 years (IQR: 9·4–11·6 years). Only 3 % of the children were not of German nationality. The percentage of overweight and obese children was with 11 % somewhat lower within the TEENDIAB cohort compared with the representative sample of German children and adolescents from the KiGGS study with about 15–17 % of overweight and obese children( Reference Kurth and Schaffrath Rosario 23 ). The median monthly family net income of the TEENDIAB families was 3000 € (IQR: 2000–4000 €; data available only for 230 children). When grouped into socio-economic classes only 3 % of all participants were ranked into the lower class. The majority of all children, 73 %, had a parent affected with T1D, 23 % had a sibling with T1D and 4 % of the children had more than one first-degree relative with T1D (Table 1).
Table 1 Characteristics of the TEENDIAB children included in the present analysis, Germany, February 2009–January 2012
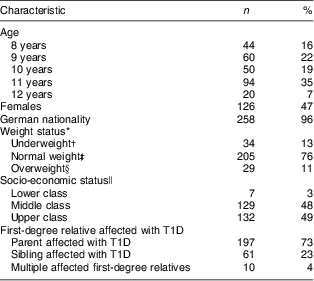
T1D, type 1 diabetes.
n 268 for all except German nationality (n 266).
*BMI was adjusted for gender and age at examination and expressed as a percentile against national reference data( Reference Kronmeyer-Hauschild, Wabitsch and Kunze 17 ).
†Below the 10th age- and gender-specific percentile of the reference population( Reference Korsten-Reck, Widhalm and Berg 31 ).
‡Between the 10th and 90th percentile of the reference population( Reference Korsten-Reck, Widhalm and Berg 31 ).
§Equal to or above the 90th percentile of the reference population( Reference Korsten-Reck, Widhalm and Berg 31 ).
∥Socio-economic classes (lower, middle, upper class) calculated on the basis of the school education of the parents and the monthly net income of the family.
Macronutrient intake data as a percentage of total energy intake
The children consumed 52·0 % of their energy from carbohydrates (23·9 % from mono- and disaccharides and 27·7 % from polysaccharides), 32·6 % from fat (13·9 % from SFA, 11·3 % from MUFA, 4·6 % from PUFA, and the rest from glycerol and lipoids) and 14·3 % from protein. In line with expectations, dietary fibre only made up 1·1 % of the total energy intake (Fig. 1).
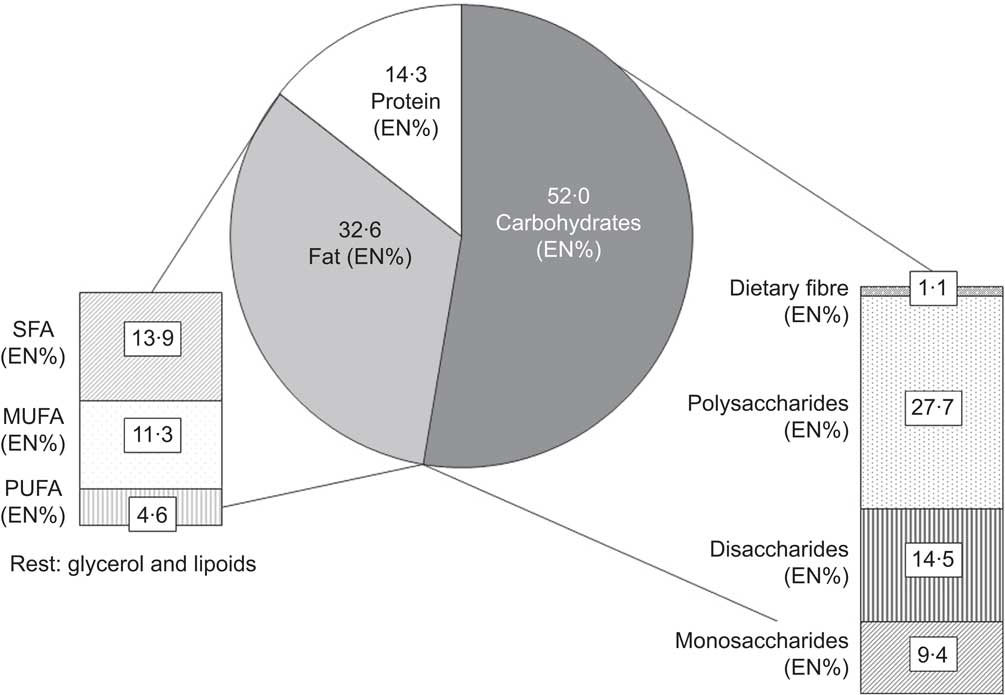
Fig. 1 Macronutrient intakes as a percentage of total energy intake (EN%) among the TEENDIAB children (n 268), Germany, February 2009–January 2012
Dietary intake data measured against the German Dietary Reference Intakes
The TEENDIAB children had a significantly higher energy intake compared with the German Dietary Reference Intakes (103·6 % of the reference (IQR: 87·5–125·0 %; P < 0·001)) and consumed significantly more protein (244·5 % of the reference (IQR: 193·8–309·4 %; P < 0·001)), dietary fibre (111·3 % of the reference (IQR: 93·6–141·7 %; P < 0·001)) and cholesterol (186·2 % of the reference (IQR: 152·4–223·1 %; P < 0·001)). Within the group of micronutrients, the children had intakes above the recommended values for most of the minerals (Ca, P, Fe, Zn, K, Mg) and vitamins (A, C and B-complex). Their intake was highest for vitamin K at 803·4 % of the reference (IQR: 633·4–1080·4 %; P < 0·001) as well as for Na at 546·4 % of the reference value (IQR: 449·2–659·3 %; P < 0·001), and lowest for vitamin D at 8·9 % of the reference (IQR: 5·8–13·2 %; P < 0·001), iodine at 58·1 % of the reference value (IQR: 46·1–73·3 %; P < 0·001) and folate at 61·3 % of the reference (IQR: 47·9–77·5 %; P < 0·001). Of all micronutrients, only vitamin E at 103·9 % of the reference (IQR: 78·2–133·7 %) and pantothenic acid at 97·3 % of the reference (IQR: 79·9–123·5 %) were not significantly different from the recommended values (Table 2).
Table 2 Daily energy and nutrient intakes, percentage of the German Dietary Reference Intakes and comparison against the EsKiMo intake data: TEENDIAB children, Germany, February 2009–January 2012
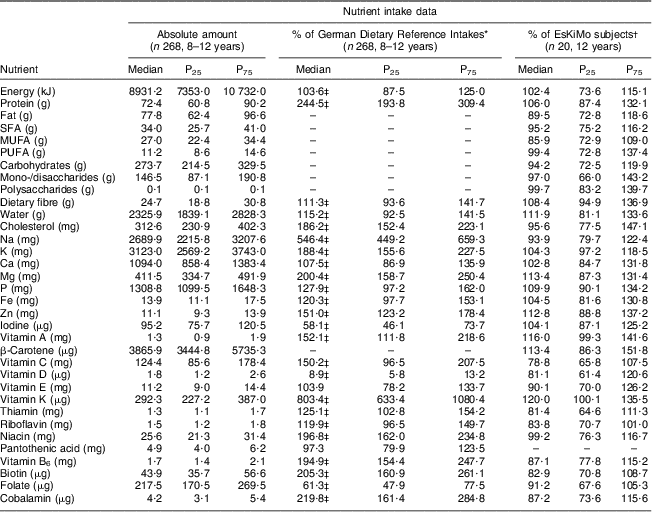
P25, 25th percentile; P75, 75th percentile.
*From reference 13.
†From reference 25.
‡Wilcoxon signed-rank test, reported intakes v. German Dietary Reference Intakes, P value <0·0019.
Dietary intake data of the TEENDIAB children compared with a representative sample
The comparison of the sub-sample of the TEENDIAB study consisting of twenty children aged 12 years with that of the EsKiMo cohort assessed with the same method showed very similar energy and nutrient intakes. There was no significant difference for the intake of any of the analysed nutrients between both cohorts (Table 2).
Food consumption data measured against the Optimized Mixed Diet recommendations
The food intake data of the TEENDIAB children were additionally compared with the Optimized Mixed Diet recommendations. The amounts of beverages, meat and meat products as well as sweets, snacks and sweetened beverages consumed by the TEENDIAB children exceeded the recommended proportions of the Optimized Mixed Diet recommendations (43·9 % v. 40 %, P = 0·145 for beverages; 5·4 % v. 2 %, P < 0·001 for meat and meat products; 10·2 % v. 3 %, P < 0·001 for sweets, snacks and sweetened beverages). In contrast, the consumed amounts of vegetables, fruits, carbohydrate-rich foods, milk and dairy products as well as oil, margarine and butter were below the recommended proportions (7·0 % v. 10 %, P < 0·001 for vegetables; 5·9 % v. 10 %, P < 0·001 for fruits; 15·0 % v. 16 %, P < 0·001 for carbohydrate-rich foods; 10·6 % v. 18 %, P < 0·001 for milk and dairy products; 0·8 % v. 1 %, P < 0·001 for oil, margarine and butter). Only the consumed amounts of fish and eggs were within the recommended food group proportions of less than 1 % of the total food intake (Table 3).
Table 3 Daily food consumption in food groups according to the Optimized Mixed Diet recommendations, expressed as weight consumed and percentage of the total weight: TEENDIAB children, Germany, February 2009–January 2012
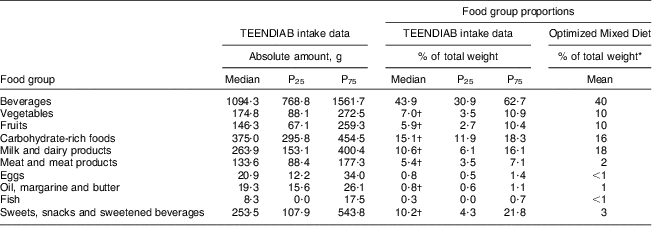
*From reference 14.
†Wilcoxon signed-rank test, reported intakes v. Optimized Mixed Diet recommendations, P value <0·005.
Discussion
Dietary intake and behaviour is discussed as a potential factor influencing the development of islet autoimmunity and T1D( Reference Thrower and Bingley 1 , Reference Atkinson and Eisenbarth 3 ). Little is known about dietary intake and behaviour in children at increased risk of T1D. Here we compared the dietary intake of children participating in the TEENDIAB study with the German Dietary Reference Intakes and intake data of the EsKiMo subjects on the nutrient level and with the Optimized Mixed Diet recommendations on the food level with the following main findings. The TEENDIAB children had intakes significantly below the German Dietary Reference Intakes only for three micronutrients, which were folate, iodine and vitamin D. They highly exceeded the reference intake values for vitamin K, Na and protein. Comparing a sub-sample of the TEENDIAB study with the EsKiMo cohort assessed with the same dietary assessment method revealed similar intakes. Compared with the Optimized Mixed Diet recommendations, most critically it has to be considered that the TEENDIAB children exceeded the recommendations for meat and meat products as well as for sweets, snacks and sweetened beverages and that they were below the recommendations for fruits, vegetables and carbohydrate-rich foods.
To our knowledge, the present study is the first one which assessed the dietary intake in adolescents at increased risk of T1D and therefore opens up the opportunity to evaluate the adherence of this cohort to current recommendations. Using a diet history interview provided detailed information on the dietary intake and behaviour of the children with the possibility to calculate food and nutrient intakes.
The macronutrient intake of the TEENDIAB children expressed as a percentage of the total energy intake was 52·0 % for carbohydrates, 32·6 % for fat and 14·3 % for protein, and was therefore within the range of the German Dietary Reference Intakes. The recommended macronutrient distribution ranges as a percentage of total energy are >50 % for carbohydrates, 30–35 % for fat and 10–15 % for protein( 13 ). However, the fatty acid distribution was not desirable for the cohort. Assuming the reference values for adults as being valid also for children, SFA and non-SFA (MUFA and PUFA) are supposed to be in the ratio 1:2 with SFA accounting for a maximum of 10 % of total energy and with PUFA accounting for 7–10 % of total energy( 13 , Reference Stahl 24 ). The intake of 13·9 % of energy from SFA of the TEENDIAB children exceeded the reference value, whereas the intake of 4·6 % of energy from PUFA was below the reference of the German Dietary Reference Intakes( Reference Kersting and Alexy 15 ). These dietary patterns were found very similarly within the EsKiMo cohort( Reference Stahl 24 , Reference Mensink, Heseker and Richter 25 ).
Although the contribution of protein of total energy intake was within the range of the reference values, the absolute protein intake of the TEENDIAB children exceeded the German Dietary Reference Intake about 1·5-fold. This observation might be reflected by the fact that the consumption of meat and meat products was also highly above the recommendations. According to present knowledge, there is no direct evidence of a protein intake above the recommendations being harmful for healthy individuals. Nevertheless, the intake of animal protein is generally connected with a concurrent intake of fat and cholesterol( 13 ). The cholesterol intake of the TEENDIAB children actually also exceeded the recommendation by 86 %. Furthermore, a high protein intake is discussed to be a risk factor for developing T1D, especially in early childhood diet( Reference Thrower and Bingley 1 , Reference Knip, Virtanen and Akerblom 8 ) and maybe also during early puberty( Reference Dahlquist, Blom and Persson 11 ).
The Na intake of the TEENDIAB children reached a median value of 546 % of the German Dietary Reference Intakes. As Na consumption through extra salting could not be recorded with DISHES Junior( Reference Mensink, Heseker and Richter 25 ), the actual median Na intake of the children might be even higher. The urinary Na excretion increases with an increased Na intake, but additionally triggers an increased urinary Ca excretion which might have serious consequences as an adequate Ca supply is especially essential during periods of bone growth( 13 ). Vitamin K exceeded the recommendations by about seven times. However, there is no upper intake level for vitamin K assigned by the European Food Safety Authority( 13 ).
The intake of vitamin D within the TEENDIAB study was considerably below the reference value, which is in line with the finding for the EsKiMo children( Reference Mensink, Heseker and Richter 25 ). However, the TEENDIAB children had a median absolute daily intake of 1·8 μg vitamin D, which is within the range of a feasible dietary intake from common foods of 1–2 μg/d( 13 ). Since the TEENDIAB study does not assess nutritional supplements, there was no possibility to evaluate the implementation of the German Dietary Reference Intakes to complement the dietary intake of vitamin D by supplementation if the exposure to sunlight is not sufficient for endogenous vitamin D synthesis. The role of vitamin D in the development of T1D is conversely discussed, but there is no clear evidence that vitamin D intake is associated with T1D risk( Reference Thrower and Bingley 1 , Reference Knip, Virtanen and Akerblom 8 ). The result for iodine within the TEENDIAB cohort is again consistent with the observation from the EsKiMo study, since these children also have a median intake of only 50 % of the respective reference value( Reference Stahl 24 , Reference Mensink, Heseker and Richter 25 ). As the intake of this mineral is assumed to be actually above the recorded amount due to difficulties in capturing consumption in the form of iodized table salt used in the food industry and in the household setting, the low intake probably does not have to be seen as alarming( Reference Mensink, Heseker and Richter 25 ). The third micronutrient for which the TEENDIAB children had an intake below the German Dietary Reference Intakes was folate. The assessment of the folate intake within the EsKiMo study revealed that these children were also undersupplied in respect to the reference value( Reference Stahl 24 ).
The assessment of the TEENDIAB intake data on the food level showed that the food intake of the TEENDIAB children was not well balanced under general disease preventive aspects. According to the recommendations of the Optimized Mixed Diet, the consumption of meat and meat products as well as of sweets, snacks and sweetened beverages needs to be decreased in both cohorts, whereas the consumption of plant-based foods needs to be increased( Reference Stahl 24 ). In contrast to the EsKiMo subjects, the TEENDIAB children additionally showed an intake of milk and dairy products which was below the recommendations of the Optimized Mixed Diet. The finding of an intake below the recommendations for vegetables and fruits and above for sweets, snacks and sweetened beverages could also be seen within the DONALD (Dortmund Nutritional and Anthropometric Longitudinally Designed) Study, conducted by the authors of the Optimized Mixed Diet recommendations( Reference Kersting, Alexy and Kroke 26 , Reference Burrows, Martin and Collins 27 ). Under T1D-related aspects, a lower intake of carbohydrate-rich foods, fruits, vegetables and dairy products may be positive as high intakes of these foods were hypothesized to increase the risk of T1D in children and adolescents( Reference Thorsdottir and Ramel 4 , Reference Virtanen and Knip 9 , Reference Dahlquist, Blom and Persson 11 ). A high intake of carbohydrates, especially in the form of disaccharides and sucrose( Reference Pundziute-Lycka, Persson and Cedermark 12 ), as well as of meat and meat products( Reference Muntoni, Cocco and Aru 10 ) was associated with the development of T1D.
There are some limitations of the current analysis. First of all, it is an interim analysis of the dietary behaviour of the TEENDIAB participants and therefore only included the data of 268 children at baseline. In total, a number of 1500 children are planned for the study, providing the opportunity to confirm these results after completion of the whole study. Furthermore, as the TEENDIAB study aims to investigate the natural course of the development of islet autoimmunity and T1D during puberty( Reference Ziegler, Meier-Stiegen and Winkler 2 ), it does not provide healthy controls. However, comparing the intake data of the children at increased risk of T1D with those of non-risk children was important to evaluate the dietary behaviour more precisely. Therefore, we evaluated the diet of the TEENDIAB children against the data of the EsKiMo subjects. As there was a methodological difference in the assessment of the dietary intake for the children aged 8–11 years between both cohorts, we conducted this comparison only in a small sub-sample of the TEENDIAB participants. Nevertheless, we considered DISHES Junior as being most suitable for assessing the diet of children aged 8–14 years with one harmonized method, since there is no indirect dietary assessment method which is able to report the true intake of a person( Reference Burrows, Martin and Collins 27 , Reference Livingstone and Robson 28 ). The retrospective documentation of dietary intake used within DISHES Junior relies heavily on children's self-reports. The children need very good memory and the ability to correctly estimate consumed portion sizes( Reference Livingstone and Robson 28 ). Therefore, parents were asked to serve as proxy respondents, especially for younger children. Another issue which is important to note is that the preliminary TEENDIAB cohort included in the current analysis revealed a selection bias towards a higher socio-economic background of the children. This might be due to higher engagement and health interest with an increasing socio-economic status. This bias needs to be considered when evaluating the diet of the children since several studies have shown that dietary quality follows a social gradient, i.e. higher social status is associated with healthier diet( Reference Eagle, Sheetz and Gurm 29 , Reference Drewnowski 30 ). Furthermore, as the TEENDIAB children all have at least one first-degree relative with T1D, the diet within the family and of the participants could be different from that of children from a representative sample of the EsKiMo study. However, the comparison against the intake data of the EsKiMo children did not provide much evidence for this assumption.
Conclusion
Children participating in the TEENDIAB study were sufficiently provided with macronutrients and micronutrients, with the exception of vitamin D, iodine and folate for which their intakes were considerably below the German Dietary Reference Intakes. However, their vitamin K, Na and protein intakes were considerably above. Compared with a control cohort of healthy children and adolescents from the EsKiMo study, the nutrient intakes of the TEENDIAB children were very similar. Furthermore, the TEENDIAB children consumed too much meat and meat products as well as sweets, snacks and sweetened beverages, which could be also observed within two other cohorts of German children and adolescents. Thus, being at increased risk of T1D seems not to improve or worsen the main dietary patterns of the TEENDIAB children. In future, the TEENDIAB study aims to investigate the impact of the dietary intake and behaviour as well as their interactions with other exogenous and endogenous factors on the development of islet autoimmunity and T1D during puberty.
Acknowledgements
Sources of funding: The TEENDIAB study is supported by Kompetenznetz Diabetes mellitus (Competence Network for Diabetes mellitus) funded by the Federal Ministry of Education and Research (FKZ 01GI0805). Kompetenznetz Diabetes mellitus had no role in the design, analysis or writing of this article. Conflicts of interest: The authors declare that they have no conflict of interest. Authorship: K.S.W. researched data, performed statistical analysis, interpreted the results and wrote the manuscript. F.H., J.R., B.A., A.W. and C.R. acquired data and reviewed/edited the manuscript. O.K. is the Co-Investigator of the TEENDIAB study and reviewed/edited the manuscript. A.-G.Z. is the Principal Investigator of the TEENDIAB study, designed the study, reviewed the data, contributed to the statistical analysis, was involved in the interpretation of the results and wrote the manuscript. C.W. researched data, contributed to the statistical analysis, was involved in the interpretation of the results and wrote the manuscript. Each author has seen and approved the contents of the submitted manuscript. Acknowledgements: The authors thank Simone Schneider, Kerstin Remus, Sarah Bläsig, Evelin Sadeghian and Anika Bokelmann for data collection and expert technical assistance. They wish to thank all families participating in the TEENDIAB study and also all paediatricians, diabetologists and family doctors in Germany for recruitment and continuous support.






