Introduction
Humankind has been interested in space throughout the ages and studies of the universe and our own solar system have been ongoing since the first observations of celestial bodies. In the current era space exploration has provided in situ data for the different bodies in our solar system. As well as images, a wide range of atmospheric and geoscientific data, including geological features, mineralogical composition, interior composition, presence of surface and subsurface water, and atmospheric composition and chemistry, have been returned by both orbiting and landing missions. To fully comprehend the underlying processes occurring in these bodies, missions and telescope observations are, however, not sufficient and additional modelling studies, both numerical and analogue, are necessary.
A large number of analogue simulation facilities capable of mimicking planetary surface and astrobiological conditions exists worldwide (ten Kate & Motamedi, Reference ten Kate, Motamedi, Beysens and van Loon2015). It is practically impossible to simulate all conditions on a solar system body in one facility, therefore different facilities focus on different aspects. In this paper we focus on a facility designed to study experimentally organic compounds on rocky bodies in our solar system. Organic compounds are nearly all molecules containing carbon, apart from a few exceptions, for example the atmospheric gases CO and CO2, and have been detected throughout the universe (Ehrenfreund et al., Reference Ehrenfreund, Spaans and Holm2011). Although the name suggests otherwise not all organic compounds are produced by living organisms. Life uses four major types of organic compounds: carbohydrates, lipids, proteins and nucleic acids (DNA and RNA). These types are themselves built up from smaller organic molecules, such as sugars, polysaccharides, fatty acids, amino acids and nucleobases. Some of these organic molecules are thought to have played a role in the origin of life and are therefore referred to as prebiotic molecules. A wide variety of organic compounds are formed and have been observed in space. Large organic compounds, such as polycyclic aromatic hydrocarbons (PAHs), are readily formed in the interstellar medium (Frenklach & Feigelson, Reference Frenklach and Feigelson1989; Parker et al., Reference Parker, Zhang, Kim, Kaiser, Landera, Kislov, Mebel and Tielens2012). PAHs also been have been linked to processes playing a role in the origin of life (Groen et al., Reference Groen, Deamer, Kros and Ehrenfreund2012). Smaller, more complex organic compounds are formed during the evolution of protoplanetary disks (Ciesla & Sandford, Reference Ciesla and Sandford2012). Mechanisms include irradiation of interstellar ices (Bernstein et al., Reference Bernstein, Dworkin, Sandford, Cooper and Allamandola2002), aqueous alteration of a protoplanet or meteorite (Zolensky & McSween, Reference Zolensky, McSween, Kerridge and Matthews1988; Shock, Reference Shock1990), Fischer–Tropsch-type syntheses during, for example, collisions or shock waves (Hayatsu & Anders., Reference Hayatsu and Anders1981), or thermal decomposition of certain minerals (McCollom, Reference McCollom2003). Even the simplest sugar has recently been detected in the interstellar medium (Beltrán et al., Reference Beltrán, Codella, Viti, Neri and Cesaroni2009). These space-based organics can be incorporated and further processed in planetary bodies, comets, meteorites and interplanetary dust particles (IDPs) during the formation of a planetary system. Nucleobases and PAHs have been detected in comets (Kissel & Krueger, Reference Kissel and Krueger1987), PAHs, carboxylic acids (CAs), nucleobases and amino acids have been detected in meteorites (Sephton, Reference Sephton2002; Pizzarello et al., Reference Pizzarello, Cooper and Flynn2006; Martins et al., Reference Martins, Botta, Fogel, Sephton, Glavin, Watson, Dworkin, Schwartz and Ehrenfreund2008), and interplanetary dust particles also contain a wide range of organics (Flynn et al., Reference Flynn, Keller, Wirick, Jacobsen, Kwok and Sandford2008). Planets in our solar system have been constantly bombarded with large impactors, such as comets and asteroids, even as recently as 1.7 Gyr ago (Bottke et al., Reference Bottke, Vokrouhlický, Minton, Nesvorný, Morbidelli, Brasser, Simonson and Levison2012), and with an ongoing flux of smaller impactors, such as meteorites and IDPs. However, the link between the origin of life and extraplanetary organics remains unknown. Whereas many hypotheses discuss whether these organics contribute to the origin of life, the fate of extraplanetary organics after delivery onto (early) planetary bodies is essentially unknown. A range of factors can alter the state and composition of the delivered organics (e.g. ten Kate, Reference ten Kate2010). Here we present a new facility specifically designed to simulate planetary (sub)surface conditions that was recently commissioned at Utrecht University.
The Planetary Analogues Laboratory for Light, Atmosphere, and Surface Simulations
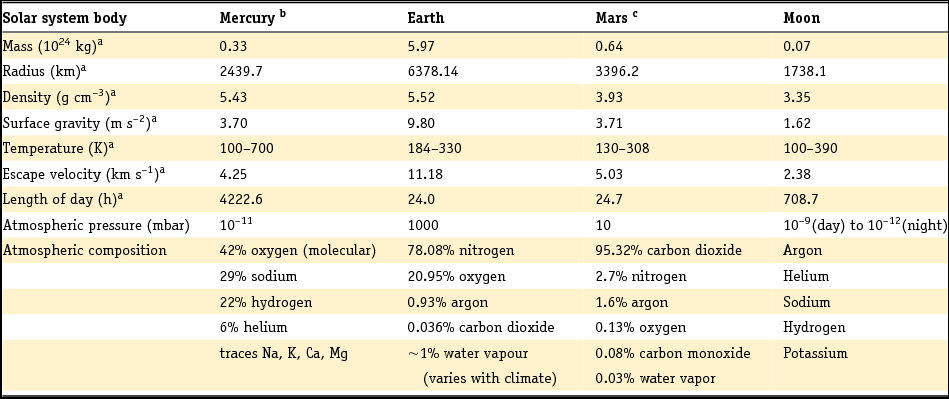
To study organic processes in a planetary surface scenario while recreating several of the conditions discussed in the previous section, we designed and built the Planetary Analogues Laboratory for Light, Atmosphere, and Surface Simulations (PALLAS). PALLAS is not only suitable for studies on organic compounds, but also for studying microorganisms exposed to different planetary conditions as well as other processes requiring non-terrestrial conditions, such as evaporation studies of lakes on Mars. Below we give an overview of PALLAS's specifications and show preliminary calibration data to give a feel for the capabilities of this facility. Table 1 gives an overview of selected surface and atmospheric parameters of solar system bodies whose surfaces can be simulated within PALLAS. Additionally, specific temperature, pressure and atmosphere conditions can be selected that can be extrapolated to asteroid or comet surfaces and interiors.
Table 1. Selected surface and atmospheric parameters of selected solar system bodies.

b Broadfoot et al. (Reference Broadfoot, Kumar, Belton and McElroy1974), Shemansky & Broadfoot (Reference Shemansky and Broadfoot1977), Potter & Morgan (Reference Potter and Morgan1985, Reference Potter and Morgan1986), Vasavada et al. (Reference Vasavada, Paige and Wood1999), Bida et al. (Reference Bida, Killen and Morgan2000), McClintock et al. (Reference McClintock, Suffix, Bradley, Killen, Mouawad, Sprague, Burger, Solomon and Izenberg2009).
c Catling (Reference Catling and Gornitz2009).
Chamber description
PALLAS (Fig. 1) is a 50 × 50 × 50 cm stainless steel vacuum chamber (Pfeiffer Vacuum) equipped with various ports and windows, and a large door for sample access. A differentially pumped sampling volume, the atmospheric sample chamber (ASC), is mounted onto the main chamber and connected via both a gate valve and a needle valve. The ASC is equipped with a turbo pump (Pfeiffer Vacuum Turbo HiPace 80) attached to a diaphragm pump (Pfeiffer Vacuum MVP 070–3), a mass spectrometer (Pfeiffer Vacuum QMG 220 M1, PrismaPlus Compact) and a pressure gauge (Pfeiffer Vacuum PKR251, 1011–1100 mbar). The entire system (chamber + ACS) can be pumped down to pressures around 10–8 mbar through the gate valve. A xenon arc discharge lamp (LOT-Oriel, 450 W UV enhanced Xe, 180–900 nm) is available to create the desired solar spectrum. A deuterium light source (Hamamatsu S2D2 VUV) can additionally be mounted inside the chamber to enhance the ultraviolet (UV) spectrum. The xenon lamp stands on top of the chamber and irradiates the samples through a UV transparent fused-silica window (99.5% transmission at 193 nm, transparent down to EUV (10 nm)). An airtight tube is mounted between the lamp housing and the fused-silica window and can be filled with N2 to minimise UV loss and ozone formation. Samples are placed on temperature-controlled tables and can be variably irradiated in the beam spot of the UV source. The intensity of the beam is measured using an Ocean Optics Maya2000PRO spectrometer, optimised for the 150–400 nm wavelength range. The temperature of the sample tables is controlled using a JULABO FP89-AL ultra-low refrigerated heating circulator. Three gas inlet valves are connected to the chamber to insert atmospheric gases. One inlet is connected to a N2 line, which is used to vent the chamber while preventing atmospheric water from entering. Gases can be either premixed or mixed inside the chamber to obtain the desired atmospheric conditions. Atmospheric pressures inside the chamber are monitored with a pressure gauge (Pfeiffer Vacuum CMR361, 0.1-1100 mbar).

Fig. 1. PALLAS. A. A schematic drawing showing the chamber, with four side ports, two top windows, the right with a borosilicate window and the left with a UV transparent fused-silica window, and top port to mount the deuterium UV source, the main door with a borosilicate window, and mounted on the left the mass spectrometer. B. A picture showing the actual setup in the laboratory, with the atmospheric sample chamber and its mass spectrometer and turbopump, gate valve and needle valve. The solar simulator placed on top of the chamber and controlled by the supply on the right. The computer is used to monitor and log mass spectra, pressures and UV spectra. Both the lamp and the diaphragm pump are connected to the main laboratory venting system with adjustable hoods to remove ozone and gases that are pumped out of the chamber.
Brief experimental protocol
Samples are placed in the desired configuration on the sample tables, and then the chamber is closed and carefully pumped down through either the gate valve or the needle valve. When the pressure inside the chamber has reached the desired value (at least in the order of 10–7 mbar) a background mass spectrum is recorded. If both background and pressure requirements are met, both the gate valve and the needle valve are closed and the chamber is filled with the desired atmosphere. The chamber pressure can be monitored on the chamber pressure gauge. The ASC is continuously pumped. To carry out atmospheric analyses a little gas is let into the ACS up to pressures of around 10–6 mbar. Higher pressures may damage the mass spectrometer. Atmospheric analyses can be recorded continuously or in intervals. The sample temperature can be regulated between –90 and +100°C as required for the simulated scenario.
Example experiment
PALLAS is designed to be versatile to allow a wide range of experiments. An organic-mineral interaction example experiment is described here. In this experiment selected minerals are spiked with selected organics through mechanical mixing, vapour deposition or dissolution–evaporation. The samples are analysed before being subjected to the conditions in the chamber, using non-destructive techniques including infrared and Raman spectroscopy. Samples are prepared in batches of at least three, one to be placed in the chamber under the UV beam, a second in the chamber in the dark and a third as a dark control outside the chamber. After the experiments the samples are analysed again using infrared and Raman spectroscopy, followed by extraction of the organics for further analysis using mass spectrometry and liquid chromatography. The mineral residue is analysed using scanning and transmission electron microscopy. Additional analysis can be carried out with secondary ion mass spectrometry (nanoSIMS) and nuclear magnetic resonance.
UV radiation
UV radiation has a very strong photodegradation effect on a wide range of organic compounds and could therefore have a sterilising effect on planetary surfaces, such as Mars (e.g. Oro & Holzer, Reference Oro and Holzer1978; ten Kate et al., Reference ten Kate, Garry, Peeters, Quinn, Foing and Ehrenfreund2005, Reference ten Kate, Garry, Peeters, Foing and Ehrenfreund2006; Stalport et al., Reference Stalport, Coll, Szopa, Cottin and Raulin2009; Moores & Schuerger, Reference Moores and Schuerger2012). This UV photodegradation is predominantly a surface process, since UV does not penetrate a rocky surface deeper than a few 100 nanometres, depending on composition (180 nm has been used as a reference value (Jeong et al., Reference Jeong, Kim and Im2003; Schuerger et al., Reference Schuerger, Clausen and Britt2011). Additionally, at wavelengths that are not directly damaging to organic molecules UV can have a photocatalytical effect on metal oxides (Shkrob et al., Reference Shkrob, Marin, Adhikary and Sevilla2011), causing, for example, photo-oxidation of these organic molecules (Shkrob et al., Reference Shkrob, Chemerisov and Marin2010). Furthermore, photodissociation of H2O by UV leads to the formation of highly reactive OH radicals, which in turn can also react with organic compounds.
The Sun put out more UV in the early stages of the solar system (Cnossen et al., Reference Cnossen, Sanz-Forcada, Favata, Witasse, Zegers and Arnold2007; Claire et al., Reference Claire, Sheets, Cohen, Ribas, Meadows and Catling2012), a factor that needs to be taken into account when simulating conditions representing the early solar system. As result of their differing atmospheric compositions, the terrestrial planets have very different ultraviolet histories (Cockell, Reference Cockell2000). For example, CO2 absorbs wavelengths shorter than 190 nm, but everything longer than that will reach the surface, as is the case on Mars (Patel et al., Reference Patel, Zarnecki and Catling2002). Present-day Earth is protected from most of the damaging radiation (<300 nm) through its ozone layer, but early Earth's atmosphere did not contain any ozone and therefore more UV reached the early Earth's surface. Fig. 2A shows the UV spectrum of the Sun, the Archean Earth, present-day Earth with and without ozone layer and present-day Mars, in arbitrary units. Additionally, the spectrum of the xenon lamp is plotted to show its relation to the aforementioned spectra. Fig. 2B shows the spectrum of the lamp measured directly and through the fused-silica window with and without N2 in the air-closed connection tube. The lamp has a warm-up time of about 20 min; a cool lamp versus a warm lamp has a difference of ~15% in intensity.

Fig. 2. The UV spectrum as received by samples in PALLAS. A. Surface scenarios: the UV spectrum at the sample location, compared to selected scenarios: the Sun, the UV flux on the Archean Earth's surface, the current Earth's surface with and without the effect of ozone and Mars' surface. Note that the spectra are plotted in arbitrary units and that the Mars UV spectrum has been scaled, to highlight the difference in the current day Mars and Earth UV scenarios. B. Effect of fused-silica window: the difference in UV intensity on the samples without the fused-silica window, with the window, and with the window and the N2 filled cylinder between the window and the lamp.
Atmospheric composition
PALLAS can be used to simulate a range of atmospheric conditions. In the case of experiments involving microorganisms the atmospheric composition in the facility is particularly important. There are two constraints: the pressure (PALLAS is a low-pressure chamber, so to keep the chamber isolated from the laboratory environment the pressure inside the chamber needs to be slightly lower than ambient pressure in the laboratory (~1000 mbar)) and the presence of corrosive gases (even though PALLAS and most of its parts are made out of stainless steel, compounds such as Cl, SO2 and H2SO4 can have corrosive effects when applied in large amounts, and these gases are relatively difficult to remove only by pumping, so precautions have to be taken when simulating conditions involving these species). The vacuum inside the chamber is shown to be very stable: when filled to 10 mbar and left closed without pumping for 18 months the pressure rose 20% to 12 mbar. To monitor the atmospheric composition a small amount of gas is leaked into the ASC. Fig. 3 shows the linear drop in atmospheric pressure in the main chamber when continuously sampling gases to monitor. Here we measured a drop in pressure of about 18% over 30 days. Table 1 gives an overview of the current atmospheric composition on the Earth and Mars, whereas Fig. 4 shows a schematic evolution of the terrestrial and Martian atmospheres. This evolution is important when simulating early Earth and Mars conditions.

Fig. 3. Chamber pressure change during continuous sampling. During continuous sampling a tiny leak is created between the main chamber and the ASC. This leak leads to an internal pressure in the order of 10–6 mbar in the ASC and enables continuous scanning of the atmosphere in the chamber.

Fig. 4. Approximate evolution of the Earth's atmosphere and Martian CO2. Showing the evolution of the main terrestrial atmospheric gases as well as the main Martian atmospheric gas, CO2, as function of time. The era of heavy impacts and the window in which life on Earth originated are shown because both had a large influence on the atmospheric evolution. (Based on Ahrens, Reference Ahrens1993; Zahnle et al., Reference Zahnle, Schaefer, Fegley, Deamer and Szostak2010; Canfield, Reference Canfield2005; Catling, Reference Catling and Gornitz2009; Farquhar, Reference Farquhar and Gornitz2009).
Temperature
The temperature on the terrestrial planets and other rocky bodies in our solar system ranges from 100 to 700 K (see Table 1). Not only does temperature have a large effect on the processes occurring in atmospheres, it also has a great effect on chemical reactions on the surface by, for example, enabling liquid water to exist. Simulating the full 100–700 K temperature range in a facility the size of PALLAS is very difficult. Within the scope of research that PALLAS is designed for, temperature variations between 183 and 373 K, directly enabling Moon, Mars and early Earth simulations, are sufficient. Within this temperature range more generic conditions can be simulated that can be further extrapolated to, for example, comet and asteroid conditions using dedicated numerical models.
Surface composition
Mineral–organic interactions are important for a variety of modern geochemical phenomena and were potentially also important on extraterrestrial bodies and for the origin of life of Earth (see Cleaves et al., Reference Cleaves, Michalkova Scott, Hill, Leszczynski, Sahai and Hazen2012 and references therein).
Mineral surfaces are hypothesised to play a role in protecting, selecting, concentrating, templating and catalysing reactions of prebiotic organic molecules. Well-studied minerals include clay minerals (e.g. Cairns-Smith & Hartman, Reference Cairns-Smith and Hartman1986), various transition metals (e.g. Fe, Ni, Co and Cu), sulphide minerals, metaloxides, carbonates and olivine (Cleaves et al., Reference Cleaves, Michalkova Scott, Hill, Leszczynski, Sahai and Hazen2012).
Specific processes that can be studied in PALLAS include photocatalysis, the substrate-mediated redox reactions of organics with UV and visible light by iron-rich minerals (e.g. Jia et al., Reference Jia, Zhao, Fan, Dilimulati and Wang2012) and metal-oxides (e.g. Fox & Dulay, Reference Fox and Dulay1993; Shkrob et al., Reference Shkrob, Chemerisov and Marin2010, Reference Shkrob, Marin, Adhikary and Sevilla2011), and aqueous alterations of minerals and mineral–organic aggregates, processes that, for example, play a role in meteorites (Shock & Schulte, Reference Shock and Schulte1990).
Conclusion
Planetary environment simulation facilities are used worldwide to better understand the chemical physical processes occurring in the bodies in our solar system. PALLAS is the newest member of this family and allows us to recreate temperature, atmospheric and ultraviolet conditions on planetary surfaces. PALLAS is designed to host a wide range of research projects, including studies on organic–mineral interactions and microorganisms under extraterrestrial conditions.