Schizophrenia is associated with a significant risk of suicide (Reference Harris and BarracloughHarris & Barraclough, 1997; Reference Inskip, Harris and BarracloughInskip et al, 1998). Risk factors for suicide in schizophrenia are similar to those in the general population. There are, however, other risk factors that are specific to the disorder (Reference SirisSiris, 2001). Prediction of risk of suicide in general is difficult, owing to the low base rate of suicide and the relative imprecision of risk factors (Reference Goldney, Hawton and Van HeeringenGoldney, 2000; Reference Powell, Geddes and HawtonPowell et al, 2000). As with other disorders, however, careful identification of risk factors is important to assist clinicians caring for patients with schizophrenia, as the former often have to make crucial decisions based on risk assessment. Risk factors have been investigated in several studies. Several reviews summarising the studies of risk factors in schizophrenia are available, but these are largely descriptive and have usually not taken account of the designs of the investigations. Systematic review procedures offer the best means of aggregating and summarising findings from individual studies. We conducted a systematic review of the international literature on studies of risk factors for suicide in schizophrenia, focusing entirely on studies most likely to provide valid estimates of risk factors (cohort and case-control studies).
METHOD
Study eligibility
Studies were selected for inclusion in this review if they met the following criteria:
-
(a) patient diagnosis of schizophrenia (including its subtypes), paraphrenia, delusional psychoses, paranoid psychosis, psychosis not otherwise specified, schizophreniform disorder, schizotypal disorder or schizoaffective disorder;
-
(b) at least 90% of the participants aged 16 years or over;
-
(c) cohort studies, with a minimum follow-up period of 1 year, and case-control studies;
-
(d) specific risk factors for suicide were investigated.
Search strategy
A broad search strategy for potential articles was used in order to include all relevant studies. Electronic searches of Medline (1966 to June 2004), EMBASE (1980 to June 2004), PsycINFO (1872 to June 2004) and Biological Abstracts (1985 to June 2004) were made with subject headings including SCHIZOPHRENIA, SCHIZOAFFECTIVE PSYCHOSIS, SUICIDE, with COHORT ANALYSIS, CASE CONTROL STUDIES, COHORT STUDIES, RISK FACTORS, FOLLOW UP STUDIES; and text words including SCHIZOPHREN*, SUICID* with RISK*, FOLLOW UP STUD*, CASE CONTROL STUD*, COHORT STUD* and COHORT ANALYSIS. No language restrictions were applied to the search. We hand-searched the journal Schizophrenia Research (1991, 1993, 1995-1999, 2001). A total of 1329 articles were identified from searching the electronic databases. Identified studies were screened for suitability independently by two investigators. Where a study was reported in more than one article, data were extracted from the most recent report. Bibliographies of eligible papers were checked for possible relevant studies. We consulted international experts in the field to check whether there were any omissions from our identified studies. Where there were uncertainties about the data in studies we approached authors for clarification.
Design of studies
The identified studies were categorised using the following order to reflect strength of study design (Reference Sackett, Haynes, Guyatt, Sackett, Haynes and GuyattSackett et al, 1991): 1, prospective cohort study; 2, retrospective cohort study; 3, nested case-control study; 4, case-control study, with similar patient groups; 5, case-control study in which the status of the controls was unclear or different.
Data extraction
Data were extracted from the reports independently by two members of the research team using a structured pro forma. Data were extracted on the following variables:
-
(a) socio-demographic: gender, ethnicity, religion, civil status, children, employment, social class;
-
(b) family history: psychiatric disorder, depression, alcohol misuse, suicide;
-
(c) personal history: childhood broken home/parental loss, education, IQ, living circumstances, recent loss and life events;
-
(d) clinical history: positive symptoms of schizophrenia, delusions, hallucinations (command or other), paranoia, suspiciousness, negative symptoms of schizophrenia, flat affect, social withdrawal, agitation/motor restlessness, worthlessness/low self-esteem, hopelessness, sleep disturbance, insight, fear of mental disintegration, medication, adherence to treatment, compulsory admission, attempted suicide, suicide threats or ideation, depression (past and recent), alcohol misuse/dependence, drug misuse/dependence, substance misuse/dependence (drugs and/or alcohol), violence, impulsivity, hostility, suspiciousness, anxiety, social relationships and physical illness.
Two approaches to the extraction of study results were used. Where numbers of suicides and non-suicides were known for patients with and without the risk factor, a 2 × 2 table was created from these numbers and used in the meta-analysis. Otherwise, if an estimate of the odds ratio for an association with a risk factor was stated, together with a measure of its precision (e.g. a standard error, confidence interval or P value), these numbers were used in the analysis. If there were insufficient data to use either of these approaches the study was excluded from the review for that risk factor.
We only present meta-analyses on variables for which data were available from more than one study, where it is possible to ascertain results are repeatable. A full list of the variables examined only in single studies is available from the authors upon request. Meta-analyses are also only presented for variables for which there are dichotomous results. Where findings are based on continuous measures we provide details where these add further information to the results from dichotomous analyses.
Statistical analysis
Study results were combined using the DerSimmonian and Laird random effects method of meta-analysis (Reference Deeks, Altman, Bradburn, Egger, Davey Smith and AltmanDeeks et al, 2001). Risk factors were expressed as odds ratios because of the inclusion of case-control studies in the analysis. Between-study heterogeneity was tested using Cochran's Q. A sensitivity analysis was performed including only the strongest designs, to determine whether the magnitude and significance of risk factors was dependent on including results from studies of less robust design.
RESULTS
We identified 29 studies that met the review criteria (Fig. 1; Table 1). The main reasons for excluding studies identified in the original search were: risk factors not reported; case-control or cohort study design not used; or no extractable data provided. In some of the included studies the diagnoses had been updated to modern criteria by the original authors. The numbers of studies in each design category were as follows:
-
(a) prospective cohort studies: n 3 (Reference Cohen, Test and BrownCohen et al, 1990; Reference Lim and TsoiLim & Tsoi, 1991; Casadebaig & Philippe, Reference Casadebaig and Philippe1999a ,Reference Casadebaig and Philippe b );
-
(b) retrospective cohort studies: n=2 (Reference Dingman and McGlashanDingman & McGlashan, 1986; Reference Fenton, McGlashan and VictorFenton et al, 1997; Reference Stephens, Richard and McHughStephens et al, 1999; Reference FentonFenton, 2000);
-
(c) nested case-control studies: n=3 (Reference Allebeck, Varia and KristjanssonAllebeck et al, 1987; De Hert & Peuskens, Reference De Hert and Peuskens1995, Reference De Hert and Peuskens1997; Reference Peuskens, De Hert and CosynsPeuskens et al, 1997; Reference Rossau and MortensenRossau & Mortensen, 1997; De Hert et al, Reference De Hert, Gelan and Peuskens1999, Reference De Hert, McKenzie and Peuskens2001);
-
(d) case-control studies with similar controls: n=14 (Reference Cohen, Leonard and FarberowCohen et al, 1964; Reference Shaffer, Perlin and SchmidtShaffer et al, 1974; Reference RoyRoy, 1982; Reference Drake, Gates and CottonDrake et al, 1984; Reference Drake and CottonDrake & Cotton, 1986; Reference Law and KhooLaw, 1986; Reference Wolfersdorf, Barth, Steiner, Platt and KreitmanWolfersdorf et al, 1989; Reference Cheng, Leung and LoCheng et al, 1990; Reference Hu, Sun and LeeHu et al, 1991; Reference Modestin, Zarro and WaldvogelModestin et al, 1992; Reference Havaki-Kontaxaki, Kontaxakis and ProtopappaHavaki-Kontaxaki et al, 1994; Reference Taiminen and KujariTaiminen & Kujari, 1994; Reference Steblaj, Tavcar and DernovsekSteblaj et al, 1999; Reference Taiminen, Huttunen and HeiläTaiminen et al, 2001; Reference Wolfersdorf and NeherWolfersdorf & Neher, 2003);
-
(e) case-control studies with different or unclear controls: n=7 (Reference WarnesWarnes, 1968; Reference Wilkinson and BaconWilkinson & Bacon, 1984; Reference Breier and AstrachanBreier & Astrachan, 1984; Reference Roos, Boraine and BodemerRoos et al, 1992; Reference Roy and DraperRoy & Draper, 1995; Reference Shah and GanesvaranShah & Ganesvaran, 1999; Reference Funahashi, Ibuki and DomonFunahashi et al, 2000).

Fig. 1 Results of the search for relevant papers.
Table 1 Studies included in the review
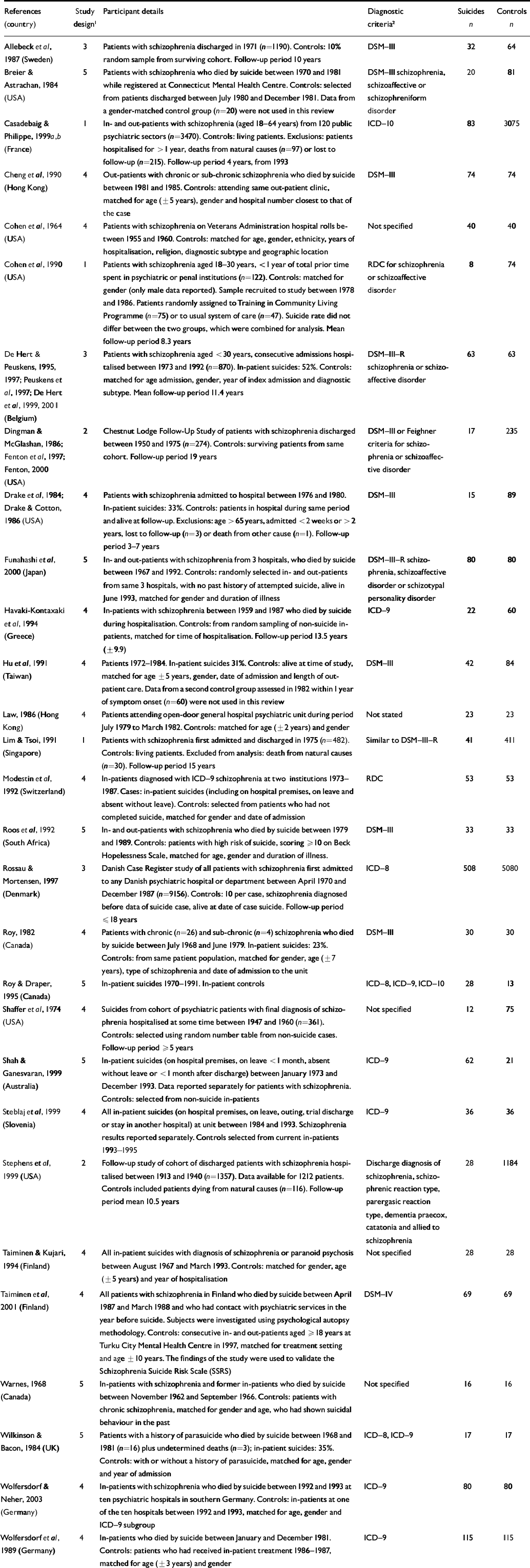
| References (country) | Study design 1 | Participant details | Diagnostic criteria 2 | Suicides n | Controls n |
|---|---|---|---|---|---|
| Allebeck et al, 1987 (Sweden) | 3 | Patients with schizophrenia discharged in 1971 (n=1190). Controls: 10% random sample from surviving cohort. Follow-up period 10 years | DSM-III | 32 | 64 |
| Breier & Astrachan, 1984 (USA) | 5 | Patients with schizophrenia who died by suicide between 1970 and 1981 while registered at Connecticut Mental Health Centre. Controls: selected from patients discharged between July 1980 and December 1981. Data from a gender-matched control group (n=20) were not used in this review | DSM-III schizophrenia, schizoaffective or schizophreniform disorder | 20 | 81 |
| Casadebaig & Philippe, 1999a,b (France) | 1 | In- and out-patients with schizophrenia (aged 18-64 years) from 120 public psychiatric sectors (n=3470). Controls: living patients. Exclusions: patients hospitalised for > 1 year, deaths from natural causes (n=97) or lost to follow-up (n=215). Follow-up period 4 years, from 1993 | ICD-10 | 83 | 3075 |
| Cheng et al, 1990 (Hong Kong) | 4 | Out-patients with chronic or sub-chronic schizophrenia who died by suicide between 1981 and 1985. Controls: attending same out-patient clinic, matched for age (±5 years), gender and hospital number closest to that of the case | DSM-III | 74 | 74 |
| Cohen et al, 1964 (USA) | 4 | Patients with schizophrenia on Veterans Administration hospital rolls between 1955 and 1960. Controls: matched for age, gender, ethnicity, years of hospitalisation, religion, diagnostic subtype and geographic location | Not specified | 40 | 40 |
| Cohen et al, 1990 (USA) | 1 | Patients with schizophrenia aged 18-30 years, <1 year of total prior time spent in psychiatric or penal institutions (n=122). Controls: matched for gender (only male data reported). Sample recruited to study between 1978 and 1986. Patients randomly assigned to Training in Community Living Programme (n=75) or to usual system of care (n=47). Suicide rate did not differ between the two groups, which were combined for analysis. Mean follow-up period 8.3 years | RDC for schizophrenia or schizoaffective disorder | 8 | 74 |
| De Hert & Peuskens, 1995, 1997; Peuskens et al, 1997; De Hert et al, 1999, 2001 (Belgium) | 3 | Patients with schizophrenia aged <30 years, consecutive admissions hospitalised between 1973 and 1992 (n=870). In-patient suicides: 52%. Controls: matched for age admission, gender, year of index admission and diagnostic subtype. Mean follow-up period 11.4 years | DSM-III-R schizophrenia or schizo-affective disorder | 63 | 63 |
| Dingman & McGlashan, 1986; Fenton et al, 1997; Fenton, 2000 (USA) | 2 | Chestnut Lodge Follow-Up Study of patients with schizophrenia discharged between 1950 and 1975 (n=274). Controls: surviving patients from same cohort. Follow-up period 19 years | DSM-III or Feighner criteria for schizophrenia or schizoaffective disorder | 17 | 235 |
| Drake et al, 1984; Drake & Cotton, 1986 (USA) | 4 | Patients with schizophrenia admitted to hospital between 1976 and 1980. In-patient suicides: 33%. Controls: patients in hospital during same period and alive at follow-up. Exclusions: age >65 years, admitted <2 weeks or > 2 years, lost to follow-up (n=3) or death from other cause (n=1). Follow-up period 3-7 years | DSM-III | 15 | 89 |
| Funahashi et al, 2000 (Japan) | 5 | In- and out-patients with schizophrenia from 3 hospitals, who died by suicide between 1967 and 1992. Controls: randomly selected in- and out-patients from same 3 hospitals, with no past history of attempted suicide, alive in June 1993, matched for gender and duration of illness | DSM-III-R schizophrenia, schizoaffective disorder or schizotypal personality disorder | 80 | 80 |
| Havaki-Kontaxaki et al, 1994 (Greece) | 4 | In-patients with schizophrenia between 1959 and 1987 who died by suicide during hospitalisation. Controls: from random sampling of non-suicide in-patients, matched for time of hospitalisation. Follow-up period 13.5 years (±9.9) | ICD-9 | 22 | 60 |
| Hu et al, 1991 (Taiwan) | 4 | Patients 1972-1984. In-patient suicides 31%. Controls: alive at time of study, matched for age ±5 years, gender, date of admission and length of out-patient care. Data from a second control group assessed in 1982 within 1 year of symptom onset (n=60) were not used in this review | DSM-III | 42 | 84 |
| Law, 1986 (Hong Kong) | 4 | Patients attending open-door general hospital psychiatric unit during period July 1979 to March 1982. Controls: matched for age (±2 years) and gender | Not stated | 23 | 23 |
| Lim & Tsoi, 1991 (Singapore) | 1 | Patients with schizophrenia first admitted and discharged in 1975 (n=482). Controls: living patients. Excluded from analysis: death from natural causes (n=30). Follow-up period 15 years | Similar to DSM-III-R | 41 | 411 |
| Modestin et al, 1992 (Switzerland) | 4 | In-patients diagnosed with ICD-9 schizophrenia at two institutions 1973-1987. Cases: in-patient suicides (including on hospital premises, on leave and absent without leave). Controls: selected from patients who had not completed suicide, matched for gender and date of admission | RDC | 53 | 53 |
| Roos et al, 1992 (South Africa) | 5 | In- and out-patients with schizophrenia who died by suicide between 1979 and 1989. Controls: patients with high risk of suicide, scoring ≥ 10 on Beck Hopelessness Scale, matched for age, gender and duration of illness. | DSM-III | 33 | 33 |
| Rossau & Mortensen, 1997 (Denmark) | 3 | Danish Case Register study of all patients with schizophrenia first admitted to any Danish psychiatric hospital or department between April 1970 and December 1987 (n=9156). Controls: 10 per case, schizophrenia diagnosed before data of suicide case, alive at date of case suicide. Follow-up period ≤18 years | ICD-8 | 508 | 5080 |
| Roy, 1982 (Canada) | 4 | Patients with chronic (n=26) and sub-chronic (n=4) schizophrenia who died by suicide between July 1968 and June 1979. In-patient suicides: 23%. Controls: from same patient population, matched for gender, age (±7 years), type of schizophrenia and date of admission to the unit | DSM-III | 30 | 30 |
| Roy & Draper, 1995 (Canada) | 5 | In-patient suicides 1970-1991. In-patient controls | ICD-8, ICD-9, ICD-10 | 28 | 13 |
| Shaffer et al, 1974 (USA) | 4 | Suicides from cohort of psychiatric patients with final diagnosis of schizophrenia hospitalised at some time between 1947 and 1960 (n=361). Controls: selected using random number table from non-suicide cases. Follow-up period ≥5 years | Not specified | 12 | 75 |
| Shah & Ganesvaran, 1999 (Australia) | 5 | In-patient suicides (on hospital premises, on leave <1 month, absent without leave or <1 month after discharge) between January 1973 and December 1993. Data reported separately for patients with schizophrenia. Controls: selected from non-suicide in-patients | ICD-9 | 62 | 21 |
| Steblaj et al, 1999 (Slovenia) | 4 | All in-patient suicides (on hospital premises, on leave, outing, trial discharge or stay in another hospital) at unit between 1984 and 1993. Schizophrenia results reported separately. Controls selected from current in-patients 1993-1995 | ICD-9 | 36 | 36 |
| Stephens et al, 1999 (USA) | 2 | Follow-up study of cohort of discharged patients with schizophrenia hospitalised between 1913 and 1940 (n=1357). Data available for 1212 patients. Controls included patients dying from natural causes (n=116). Follow-up period mean 10.5 years | Discharge diagnosis of schizophrenia, schizophrenic reaction type, parergasic reaction type, dementia praecox, catatonia and allied to schizophrenia | 28 | 1184 |
| Taiminen & Kujari, 1994 (Finland) | 4 | All in-patient suicides with diagnosis of schizophrenia or paranoid psychosis between August 1967 and March 1993. Controls: matched for gender, age (±5 years) and year of hospitalisation | Not specified | 28 | 28 |
| Taiminen et al, 2001 (Finland) | 4 | All patients with schizophrenia in Finland who died by suicide between April 1987 and March 1988 and who had contact with psychiatric services in the year before suicide. Subjects were investigated using psychological autopsy methodology. Controls: consecutive in- and out-patients aged ≥ 18 years at Turku City Mental Health Centre in 1997, matched for treatment setting and age ± 10 years. The findings of the study were used to validate the Schizophrenia Suicide Risk Scale (SSRS) | DSM-IV | 69 | 69 |
| Warnes, 1968 (Canada) | 5 | In-patients with schizophrenia and former in-patients who died by suicide between November 1962 and September 1966. Controls: patients with chronic schizophrenia, matched for gender and age, who had shown suicidal behaviour in the past | Not specified | 16 | 16 |
| Wilkinson & Bacon, 1984 (UK) | 5 | Patients with a history of parasuicide who died by suicide between 1968 and 1981 (n=16) plus undetermined deaths (n=3); in-patient suicides: 35%. Controls: with or without a history of parasuicide, matched for age, gender and year of admission | ICD-8, ICD-9 | 17 | 17 |
| Wolfersdorf & Neher, 2003 (Germany) | 4 | In-patients with schizophrenia who died by suicide between 1992 and 1993 at ten psychiatric hospitals in southern Germany. Controls: in-patients at one of the ten hospitals between 1992 and 1993, matched for age, gender and ICD-9 subgroup | ICD-9 | 80 | 80 |
| Wolfersdorf et al, 1989 (Germany) | 4 | In-patients who died by suicide between January and December 1981. Controls: patients who had received in-patient treatment 1986-1987, matched for age (± 3 years) and gender | ICD-9 | 115 | 115 |
RDC, Research Diagnostic Criteria.
1. Study design: 1, prospective cohort; 2, retrospective cohort; 3, nested case-control; 4, case-control: controls equivalent (patient status, timing, etc.); 5, case-control: controls unclear or different.
2. Schizophrenia only unless stated.
Socio-demographic factors
Suicide risk was associated with male gender (Fig. 2). White people were more at risk than non-White people, but this finding was based on only three studies; when the study in design category 5 (Reference Breier and AstrachanBreier & Astrachan, 1984) was omitted, the association was not significant (OR=2.18, 95% CI 0.16-30.39; heterogeneity P=0.22). No association was found with religious denomination (data not shown). Those who were married or cohabiting were at somewhat lower risk, although this finding, based on 15 studies, was not statistically significant. Omitting the four studies in design category 5 did not affect the result (OR=0.68, 95% CI 0.45-1.04; heterogeneity P=0.26). Single marital status was not a risk factor. This appears to be a robust finding, having been investigated in 16 studies. Being divorced did not appear to influence suicide risk, even when the study in design category 5 (Reference Wilkinson and BaconWilkinson & Bacon, 1984) was omitted (OR=1.97, 95% CI 0.88-4.43, heterogeneity P=0.36). Similarly, the impact of having children was examined in only two studies, although there was a trend toward a protective effect. Being employed had no impact on risk. Unemployment was not associated with risk. Difference in categorisation of social class precluded meta-analysis of the findings of four studies in which it was examined (Reference Shaffer, Perlin and SchmidtShaffer et al, 1974; Reference Wilkinson and BaconWilkinson & Bacon, 1984; Reference Hu, Sun and LeeHu et al, 1991; Reference Modestin, Zarro and WaldvogelModestin et al, 1992).

Fig. 2 Demographic characteristics. Studies identified by first-named author and year only. Study design: 1, prospective cohort; 2, retrospective cohort; 3, nested case-control; 4, case-control: controls equivalent (patient status, timing, etc.); 5, case-control: controls unclear or different. *Estimates based on reported incidence rate ratio (counts of cases and controls not available).
Personal, social and family history
Suicide risk was not related to coming from a broken home or having lost a parent (Fig. 3). Limited education was unrelated to risk, but there was a non-significant trend for risk to be greater in those with higher education. When the study in design category 5 (Reference Shah and GanesvaranShah & Ganesvaran, 1999) was omitted there was a significant association of higher education with risk (OR=5.66, 95% CI 1.91-16.8; heterogeneity P=0.6), but this was based on just two studies. Two studies were identified that investigated the impact of IQ on suicide risk (Reference FentonFenton, 2000; Reference De Hert, McKenzie and PeuskensDe Hert et al, 2001) but dichotomous data from these studies could not be extracted for our meta-analyses. Both, however, showed a significant association of risk with higher IQ.

Fig. 3 Personal, social and family history characteristics. Studies identified by first-named author and year only. Study design: 1, prospective cohort; 2, retrospective cohort; 3, nested case-control; 4, case-control: controls equivalent (patient status, timing, etc.); 5, case-control: controls unclear or different.
Participants living alone or not living with their families were at greater risk of suicide. Although the converse - living with family - was not significantly associated with reduced risk of suicide in the full analysis, it became so when the two studies in design category 5 (Brier & Astrachan, 1984; Reference Wilkinson and BaconWilkinson & Bacon, 1984) were omitted (OR=0.52, 95% CI 0.31-0.88; heterogeneity P=0.58). In the single study that examined it, number of friends was not associated with suicide risk (Reference Cohen, Test and BrownCohen et al, 1990).
Suicide was associated with recent loss events. A family history of depression was correlated with suicide risk, although family history of any psychiatric disorder was not. There were insufficient data on family history of alcohol misuse for analysis. This also applied to family history of suicide, although a positive association was found in the largest and methodologically robust study included in this analysis (Reference De Hert, McKenzie and PeuskensDe Hert et al, 2001; OR=7.39, 95% CI 2.04-26.8).
Characteristics of the disorder
Positive symptoms of schizophrenia
The results of the studies of positive symptoms of schizophrenia (Fig. 4) were conflicting (heterogeneity P<0.001): two studies reported a statistically significant positive association and two reported a significant negative association. In a further study, which recorded symptoms on a continuous scale, there was an association of total number of positive symptoms and risk (Reference FentonFenton, 2000). Delusions and hallucinations were also investigated separately. Delusions were not associated with suicide risk, although again there was significant heterogeneity (P=0.02). When the study in design category 5 (Reference Roos, Boraine and BodemerRoos et al, 1992) was omitted, delusions appeared to be associated with lower risk (OR=0.48, 95% CI 0.24-0.94; heterogeneity P=0.04). In a single study a scaled measure of paranoid ideation was associated with suicide risk (Reference Cohen, Test and BrownCohen et al, 1990) and in another similar study a measure of suspiciousness was also associated with risk (Reference FentonFenton, 2000). Hallucinations were associated with a lower risk of suicide. The finding for the three studies of command hallucinations showed significant heterogeneity (P=0.006). Although there was no overall association with suicide risk, this was based on relatively few data, and two of the studies were in design category 5.

Fig. 4 Characteristics of the disorder. Studies identified by first-named author and year only. Study design: 1, prospective cohort; 2, retrospective cohort; 3, nested case-control; 4, case-control: controls equivalent (patient status, timing, etc.) 5, case-control: controls unclear or different.
Negative symptoms of schizophrenia
There were conflicting data on negative symptoms in general (heterogeneity P=0.003), with no overall association with suicide risk (Fig. 4). A protective association was found in a single study using a negative symptom scale, which also found a protective association for flat affect (Reference FentonFenton, 2000). There were limited data on social withdrawal, but the result of the meta-analysis did not show an association with suicide.
Affective symptoms
Agitation (or motor restlessness) was associated with suicide (Fig. 4). The same was true for both a sense of worthlessness (or low self-esteem) and hopelessness. There was a trend towards an association with sleep disturbance, but the data were very limited. No study examined anxiety as a dichotomous variable; however, no association with suicide was found in a study using a continuous measure of anxiety (Reference Cohen, Test and BrownCohen et al, 1990).
Reaction to illness and treatment
Insight into the nature of the illness was not associated with suicide, but there was considerable heterogeneity in the result (Fig. 4). This finding did not change when the study in design category 5 (Reference WarnesWarnes, 1968) was omitted from the analysis (OR=1.70, CI 0.33-8.75; heterogeneity P<0.001). Fear of mental disintegration was associated with risk, but again there was considerable heterogeneity in this finding. This result remained positive when the two studies in design category 5 were omitted from the analysis, but the confidence intervals were very wide (OR=81, CI 13.8-481). Suicide risk was considerably increased in participants with poor adherence to treatment (defined as failure to take medication as prescribed or to attend follow-up). Patients who had been compulsorily admitted to hospital were not at greater risk of suicide, although there was significant heterogeneity (P=0.03).
Suicidal phenomena
Previous suicidal phenomena were assessed in a variety of ways in the studies, all but one of which were significantly associated with suicide in the meta-analyses (Fig. 5). On the basis of the results of 22 studies, a history of attempted suicide strongly increased the risk of suicide, a finding that was largely unaffected when the studies in design category 5 were omitted from the analysis (OR=4.44, 95% CI 3.06-6.45). Suicide risk was also associated with both attempted suicide being a reason for the last admission (OR=2.87, 95% CI 1.66-4.95) and an attempt during that admission (OR=8.91, 95% CI 3.40-23.4) (data not shown in Fig. 5). The findings for suicide threats were contradictory; this may be due to one study selecting controls from among patients with high scores (≥10) on the Beck Hopelessness Scale (Reference Roos, Boraine and BodemerRoos et al, 1992), whereas the other study, which involved a more robust design (Reference De Hert, McKenzie and PeuskensDe Hert et al, 2001), showed a strong association. Suicide was linked to both past and recent suicidal ideation.

Fig. 5 Suicidal phenomena. Studies identified by first-named author and year only. Study design: 1, prospective cohort; 2, retrospective cohort; 3, nested case-control; 4, case-control: controls equivalent (patient status, timing, etc.); 5, case-control: controls unclear or different.
Comorbid disorders and behaviour
Depression
Both a history of depression and recent depression were associated with suicide (Fig. 6). The different result for recent depression in one study may be explained by the selection of high-risk controls (Reference Roos, Boraine and BodemerRoos et al, 1992). Omitting this study from the analysis resulted in an even stronger association (OR=12.7, 95% CI 6.72-24.1), with little heterogeneity (P=0.43).

Fig. 6 Comorbid disorders and behaviour. Studies identified by first-named author and year only. Study design: 1, prospective cohort; 2, retrospective cohort; 3, nested case-control; 4, case-control: controls equivalent (patient status, timing, etc.); 5, case-control: controls unclear or different. *Estimates based on reported incidence rate ratio (counts of cases and controls not available).
Alcohol and drug misuse
Suicide risk was not associated with alcohol misuse or dependence (Fig. 6), a finding that was unaffected by omission of the studies in design category 5 (Reference Roos, Boraine and BodemerRoos et al, 1992; Reference Shah and GanesvaranShah & Ganesvaran, 1999) (OR=1.17, 95% CI 0.69-1.99; heterogeneity P=0.81). On the other hand, suicide risk was considerably increased in the presence of drug misuse or dependence, a finding again unaffected by omitting the two studies in design category 5 (Reference Roos, Boraine and BodemerRoos et al, 1992; Reference Shah and GanesvaranShah & Ganesvaran, 1999) (OR=3.51, 95% CI 2.06-5.97; heterogeneity P=0.88). Where authors did not define the substance of misuse there was no association with suicide risk, although this result showed considerable heterogeneity and may reflect the fact that the majority of patients in this category could have been alcohol misusers.
Violence, impulsivity and physical illness
There was considerable variation in the findings for violence between individual studies, although the overall result did not indicate an association (Fig. 6). Omitting the study in design category 5 (Reference WarnesWarnes, 1968) did not alter this finding (OR=1.66, 95% CI 0.67-4.14; heterogeneity P=0.015). Impulsivity was associated with increased risk, although this finding was based on the results of only two studies. Suicide was not associated with physical illness, a finding unaffected by omitting the study in design category 5 (Reference Shah and GanesvaranShah & Ganesvaran, 1999) (OR=1.22, 95% CI 0.54-2.72; heterogeneity P=0.16).
DISCUSSION
We adopted a thorough and systematic approach to searching the world literature for studies of risk factors for suicide in schizophrenia, including searching for studies in any language. Some authors reanalysed their original data for us, or supplied us with additional data. This is therefore the most comprehensive review of risk factors for suicide in schizophrenia that has been conducted to date. Its findings indicate that suicide risk in patients with schizophrenia is related less to the core psychotic symptoms of the disorder and more to affective symptoms, agitation or motor restlessness, and to awareness that the illness is affecting mental functioning. Previous suicidal behaviour is a strong risk factor. Drug misuse and loss events also appear to increase risk. Treatment compliance is important. Hallucinations are associated with decreased risk.
Limitations of the study
As with all systematic reviews based on published studies, the findings of our review are subject to publication bias. This bias is increased by the tendency among authors to provide little or no data when investigation of potential risk factors produces non-significant associations, since this results in their exclusion from the meta-analysis. Reviews of this type are also subject to potential bias resulting from the fact that some investigations - especially cohort studies - examine relatively few potential risk factors, whereas others - notably case-control studies - include more. Also, some potential risk factors have been examined in a fairly large number of studies, whereas others have received less attention. However, the approach we have used in this review provides the best synthesis of the evidence that is available from currently published information.
We only included investigations that met the criteria of being either cohort or case-control studies. The patients could have any of the diagnoses within the broad spectrum of schizophrenia. We also included studies in which some of the patients had schizoaffective disorder. The psychopathology of schizoaffective disorder overlaps with that of schizophrenia and this disorder also has a high suicide risk (Reference Fenton, McGlashan and VictorFenton et al, 1997). It was not possible to analyse risk according to specific diagnoses because the numbers of cases of schizoaffective disorder were either not supplied or were small.
One of the main drawbacks of a meta-analytical study of this kind is that there is considerable variation between investigators in the definition of individual risk factors. This variability necessitates compromise on the specificity of definitions in order to allow inclusion of the largest possible number of studies.
Specific criteria were used to group the studies according to research design. Cohort studies are likely to yield the most robust findings, followed by nested case-control studies, and then case-control studies with similar patient groups (Reference Sackett, Haynes, Guyatt, Sackett, Haynes and GuyattSackett et al, 1991). Relatively few of the studies were in the former categories. However, their findings did not differ markedly from those of other categories of study for most variables. We have re-examined all the findings excluding studies with the least robust design (case-control studies with controls that differed from those of the cases or where their status was unclear). This resulted in changes to some of the findings.
The advantage of meta-analysis of summary data is that it not only allows the findings of a range of studies to be synthesised, but also greatly reduces the danger of findings from individual studies leading to spurious conclusions. The degree of heterogeneity in the analyses of some factors is testimony to how much findings can vary between studies and how misleading single studies can be, especially when based on small numbers of participants and/or weaker research designs. A disadvantage of this approach is that it is not possible to adjust estimates of risk factors for effects of confounding factors, since this would require access to individual patient data.
Factors associated with risk of suicide
Although this meta-analysis has shown that some of the risk factors for suicide in schizophrenia are similar to those for suicide in the general population, it has highlighted certain risk factors that are clearly specific to schizophrenia and its consequences. The odds ratio for suicide in men compared with women of 1.57 is somewhat less than the ratio observed in the general populations of most countries (Reference Cantor, Hawton and Van HeeringenCantor, 2000). The excess risk in White patients is in keeping with the situation in the general populations of multiracial countries at the time the studies examining this factor were conducted. It was, however, a weak finding, which was no longer positive when the sensitivity analysis was applied. We could not examine age as a risk factor because it was used as a matching factor in some of the case-control studies, and in other studies for which age data were supplied there were differences in manner of reporting. Married or cohabiting patients did not appear to be at markedly lower risk. This is perhaps surprising, as being married might reflect less severe illness or later-onset disorders, which tend to be less damaging (Reference Eaton, Mortensen and HerrmanEaton et al, 1992). In contrast to the risk in the general population, being single or divorced was not associated with greater risk. The living circumstances of patients appeared to be important, in that those living alone or not living with their families were at increased risk; again, this might reflect severity of the disorder. Life events in the form of recent losses appear to be associated with suicide risk, in keeping with their role in suicide risk in general.
The most robust findings were of risk of suicide being strongly associated with comorbid affective disorders, specific affective symptoms (agitation, sense of worthlessness and hopelessness) and a history of suicidal thinking, threats and (especially) non-fatal suicidal acts. It was not possible to distinguish between depressive symptoms that were part of the schizophrenic illness, occurred after an episode of illness or represented a separate disorder. Further support for the importance of depression as a risk factor came from the positive association of risk with a family history of affective disorders. Although family history of suicide did not emerge from the meta-analysis as a factor, perhaps because it is a relatively rare phenomenon, it was a risk factor in the largest study that examined this factor (Reference De Hert, McKenzie and PeuskensDe Hert et al, 2001).
With regard to the characteristics of schizophrenia, we could not examine age at onset or duration of the disorder as potential risk factors because of considerable variation in the way this was recorded in different studies, and because of matching for this factor in some studies. Using a different study design to address this problem has shown that the majority of suicides in cases of schizophrenia occur early in the course of the illness (Reference Palmer, Pankratz and BostwickPalmer et al, 2005). Active psychotic symptoms were not associated with increased risk; indeed, hallucinations were associated with a reduced risk of suicide, as were delusions when the studies of more robust design were examined. Also, command hallucinations were not associated with increased risk, although some authors have cited command hallucinations as causing patients with schizophrenia to complete suicide (Reference Planansky and JohnstonPlanansky & Johnston, 1973; Reference Barraclough, Bunch and NelsonBarraclough et al, 1974). In separate single studies, paranoid ideation (Reference Cohen, Test and BrownCohen et al, 1990) and suspiciousness (Reference FentonFenton, 2000) were associated with risk. Suicide risk was not associated with negative symptoms, although there was significant heterogeneity in the result. Findings based on a scale of negative symptoms (Reference FentonFenton, 2000) suggest that risk is probably inversely related to such symptoms.
Developing schizophrenia after having achieved academically has been claimed to be associated with particular risk of suicide (Reference Drake, Gates and CottonDrake et al, 1984). Meta-analysis provides some support for this. The results of two studies also indicated increased risk associated with higher IQ. Fear of mental disintegration was significantly associated with suicide risk, although there was considerable variation between studies regarding the possible role of insight into the nature of the illness. Surprisingly, given the significance of alcohol misuse as a major risk factor for suicide in the general population (Reference Murphy, Hawton and Van HeeringenMurphy, 2000), it does not appear to be a risk factor in schizophrenia. On the other hand, drug misuse or dependence was strongly associated with suicide risk. Drug misuse is twice as common in people with schizophrenia as in the general population (Reference Bühler, Hambrecht and LöfflerBühler et al, 2002).
We were unable to examine treatments in this review, partly because it is difficult to compare these across studies and partly because medication was often referred to in general terms, such as ‘antipsychotics' or ‘antidepressants'. However, our review has shown that suicide risk is considerably increased in patients who adhere poorly to treatment. Although akathisia is often cited by clinicians as a risk factor for suicide, the association is based on case reports only (Reference Shear, Frances and WeidenShear et al, 1983; Reference Drake and EhrlichDrake & Ehrlich, 1985). No study in this review provided data on akathisia as a possible risk factor and so the association was not confirmed.
Limitations in predicting risk
A further methodological issue, which needs to be borne in mind when considering the findings of this review, is that evaluation of potential risk factors (e.g. symptoms) often took place a long time before death occurred, and these factors might have changed in the intervening period. Another issue is that suicide is a relatively uncommon event, even in a disorder such as schizophrenia, which is characterised by relatively high risk. The prediction of suicide both in the general population (Reference Goldney, Hawton and Van HeeringenGoldney, 2000) and in psychiatric patients (Reference Powell, Geddes and HawtonPowell et al, 2000), using risk factors that are by their nature somewhat crude and are often present in a sizeable proportion of the patient population, is always going to be difficult.
Clinical implications
The main factors to be taken into account when assessing risk of suicide in patients with schizophrenia are affective symptoms or syndromes, suicidal thoughts, threats or behaviour, poor adherence to treatment, fears of the impact of the illness on mental functioning, and drug misuse. The nature of the schizophrenic disorder seems to be less important and, in the case of positive symptoms, may be misleading. Prevention of suicide is thus likely to result from active treatment of affective symptoms and syndromes, improving adherence to treatment, use of medication that may have special anti-suicidal effects, and maintaining special vigilance in patients with risk factors, especially when faced with significant loss events.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Risk of suicide in people with schizophrenia is strongly associated with depression, previous suicide attempts, drug misuse, agitation or motor restlessness, fear of mental disintegration, poor adherence to treatment and recent loss.
-
▪ Active psychotic features have less predictive value.
-
▪ Prevention of suicide in schizophrenia may be best addressed through treatment of affective symptoms, improving adherence to treatment and maintaining special vigilance in patients with risk factors, especially after loss events.
LIMITATIONS
-
▪ The findings may be subject to the influences of publication bias and differential attention to risk factors between the studies.
-
▪ Relatively few of the included studies were of robust cohort design.
-
▪ It was not possible to adjust the findings for the potential influence of confounding factors.
Acknowledgements
This study was supported by a grant from the National Health Service Executive South East Region Research Committee. Keith Hawton is sup supported by Oxfordshire Mental Healthcare Trust, Camilla Haw by St Andrew's Hospital, Northampton, and Julia Sinclair by the Medical Research Council. We thank Dr A. Shah, Professor J. Modestin, Professor T. Taiminen and Dr R. Tavcar for supplying us with additional data from their studies, and Professor T. Barnes, Professor T. Crow, Dr M. De Hert, Dr E. Fuller Torrey, Dr H. Heila, Professor E. Johnstone and Professor P. B. Mortensen for checking our list of studies.










eLetters
No eLetters have been published for this article.