Hwabyung (HB; known as an anger syndrome) is characterized by chronic suppression of anger, continued feelings of unfairness and resentment, subjective anger, external anger, somatic complaints, heat sensation, chest tightness, sighing, fatigue, insomnia, dysphoric affect, and indigestion (American Psychiatric Association, 1994; Min, Reference Min and Koh2013). HB was listed as culture-bound syndrome in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994). However, it was deleted from the category of cultural syndromes in the DSM-V (American Psychiatric Association, 2013). The prevalence of HB syndrome has been estimated to be between 4.2% and 13.3% in the Korean general population and Korean immigrants in Western countries, and the prevalence is known to be higher in females than in males and is lower in higher social classes (Kim & Park, Reference Kim and Park2004; Lee & Lee, Reference Lee and Lee2008; Min et al., Reference Min, Namkoong and Lee1990; Rhi, Reference Rhi2004).
Prior studies have shown that HB is frequently comorbid with many psychiatric disorders, including major depressive disorder, generalized anxiety disorder, panic disorder, somatization disorder, dysthymia, and post-traumatic embitterment disorder (PTED), a subtype of post-traumatic stress disorder (PTSD; Joe et al., Reference Joe, Lee, Kim, Won, Lim and Ha2017; Kim et al., Reference Kim, Chung, Suh, Jung, Lee, Kim and Kim2010; Lin et al., Reference Lin, Lau, Yamamoto, Zheng, Kim, Cho and Nakasaki1992; Min & Suh, Reference Min and Suh2010). As with many other psychiatric illnesses, the onset of HB has been associated with exposures to stressful life events. For example, Chung et al. (Reference Chung, Song and Kim2013) found in a sample of South Koreans that major stressful life events that preceded the onset of HB were spousal infidelity, serious financial problems, and family conflicts. Chung et al. (Reference Chung, Song and Kim2013) further showed that the risk for developing HB increased with lower social support, higher spousal criticisms, and higher interpersonal relationship problems.
Several researchers suggested that the collectivism cultures in East Asian countries play a strong etiologic role in HB. In collectivistic cultures, people are socialized to internalize stress and repress negative emotions such as anger, hostility, and psychological distress to maintain harmonious social relationships (Chiao, Reference Chiao2015). If aversive emotion is not relieved properly and accumulated over a long period, then the negative emotion reaches a threshold limit and often explodes in rage, which can lead to the development of HB (Pang, Reference Pang1990). After a review of large medical records of the patients with HB, Min (Reference Min2008) concluded that one of the most common precipitating factors of HB in South Korean women was ‘unfair treatment’. Many South Korean female patients with HB felt that they were victims of unfair treatment, especially the chronic verbal and physical violence of husbands and in-law families. The patients typically felt that they were supposed to suppress their anger to conform to the Korean traditional patriarchal system and collectivism cultures where housewives are obliged to obey their husbands and in-law families. Other precipitating factors of HB reported by Min (Reference Min2008) included poverty resulting from unfair social deprivation, experiences of social injustice, discrimination, and unfair juridical decisions.
In a functional magnetic resonance imaging (FMRI) study on neural responses to neutral, sad, and angry facial stimuli, Lee et al. (Reference Lee, Paik, Kang, Chung, Kwon, Khang and Ham2009) found that as compared to healthy controls, HB patients showed increased activations in the lingual gyrus and fusiform gyrus and reduced activation in the thalamus in responses to all three types of facial stimuli. HB patients also showed lower activities in the right anterior cingulate cortex (ACC) in response to the neutral condition than did healthy controls. These findings indicated that the suppression of emotion may lead to aberrant function of the brain regions of the visual pathway and functional impairment in the ACC. However, as the sample size of the Lee et al.’s study is small (n = 24), the findings need to be replicated in a larger sample to draw a firm conclusion.
While several psychosocial and neurological risk factors contributing to the development of HB have been suggested, the genetic etiology of HB remains poorly understood. In an early study, Min et al. (Reference Min, Namkoong and Lee1990) found that the rates of HB and psychiatric illnesses such as depression, anxiety disorders, insomnia, personality disorders, violence, and suicide were higher in the first-degree relatives of the HB patients than those of healthy controls, suggesting that HB may have a genetic basis. The major goal of the present study, therefore, was to explore genetic and environmental influences on the HB symptoms in South Korean adolescent and young adult twins. We also examined age and sex differences in genetic and environmental influences on HB during adolescence and young adulthood.
Methods
Sample
The sample included 1,601 twins consisting of 143 pairs of monozygotic male (MZM), 67 pairs of dizygotic male (DZM), 295 pairs of monozygotic female (MZF), 114 pairs of dizygotic female (DZF), 117 pairs of opposite-sex dizygotic (OSDZ) twins, and 129 twins with non-participating co-twins drawn from the South Korean Twin Registry (Hur et al., Reference Hur, Jeong, Chung, Shin and Song2013). The mean age of the twins was 19.1 ± 3.1 years (range 12–29 years). Twins under 20 years of age were recruited mostly from schools throughout South Korea, while those of 20 years of age or older were from Facebook, twin clubs on the internet, and colleges throughout South Korea. The present sample has an over-representation of females (62%), which was due in part to the fact that some of the male twins were in the military service at the time of the recruitment of the subjects because it is mandatory for young adult males in South Korea to complete army service. In terms of parental educational achievement, 49% of fathers and 41% of mothers of twins completed university education or more.
Zygosity of the twins was assessed using a three-item zygosity questionnaire. As compared to DNA analysis, this questionnaire method has been shown to achieve over 90% accuracy (Ooki et al., Reference Ooki, Yamada and Asaka1993). The number of monozygotic (MZ) twins was much greater than that of dizygotic (DZ) twins in the present sample, which likely reflected the low DZ twin birth rates in the South Korean population for the birth cohorts in the present study rather than sampling bias (Hur & Kwon, Reference Hur and Kwon2005).
Measures
To measure HB, we used the HB symptom scale (Kwon et al., Reference Kwon, Kim, Park, Lee, Min and Kwon2008), which consists of 15 self-report items concerning typical symptoms of HB. Psychometric properties of the HB symptom scale have been intensively studied, and reliabilities and validity of the scale were found to be acceptable (Kwon et al., Reference Kwon, Kim, Park, Lee, Min and Kwon2008). A telephone interview was conducted to assess twins. In the interview, twins were instructed to rate themselves on a 5-point Likert scale from not true (0) to certainly true (4) for each of the 15 items. The ratings were summed to obtain a total score so that higher scores represent more severe symptoms. As with many other psychopathology measures, the total score of the HB symptom scale was somewhat positively skewed, with a skewness index of 0.65. We performed square-root transformation of the scores of the HB symptoms scale, which resulted in a distribution of skewness of -0.14. Thus, twin correlation and model-fitting analyses below were based on the transformed data. Cronbach's alpha of the 15 items was 0.92 in the present sample, which was close to that in the normative sample (Kwon et al., Reference Kwon, Kim, Park, Lee, Min and Kwon2008).
Statistical Analysis
To estimate genetic and environmental influences on HB, we conducted maximum likelihood twin correlation and structural equation model-fitting analyses. A general sex-limitation model (Neale & Cardon, Reference Neale and Cardon1992) was applied to the raw data for HB. The full model included additive genetic factors (A) (i.e., the sum of the average effect of all alleles that influence a trait), shared environmental factors (C) (i.e., those environmental factors shared between the two members of a twin pair), and individual-specific environmental factors (E) (i.e., those environmental factors unique to each member of a twin pair and measurement error). On the basis of the degree of genetic relatedness, the correlations for A were set at 1.0 for MZ, and 0.50 for the same-sex DZ twins for both sexes. The correlation for C was set at 1.0 for both types of twins for both sexes. The correlation for E was set at 0.0 because by definition, this factor was uncorrelated between the two members of a twin pair. To determine sex differences in genetic and environmental influences on HB, we allowed the magnitudes of A, C, and E to differ across sexes in the full model. In addition, to test sex-specific genetic effects, the genetic correlation for opposite-sex DZ twins was set to be different from 0.5. Age was treated as a covariate in the model.
We used the maximum likelihood raw data option in Mx (Neale et al., Reference Neale, Boker, Xie and Maes2003), which calculates twice the negative log-likelihood (-2LL) of the data. To determine the best-fitting, most parsimonious model, parameters in the full model are constrained sequentially, and the resulting changes in -2LL were evaluated. As the difference in -2LL is chi-square distributed, when models were nested to each other, the likelihood ratio test (LRT) was used to compare alternative models. A significant change in chi-square would indicate that the reduction of the parameters in the nested model causes a significant decrease in model fit, whereas a non-significant change would suggest that constraining parameters in the nested model is acceptable. When alternative models were not nested to each other, Akaike's information criterion (AIC = -2LL - 2df) for alternative models were compared to evaluate superiority among competing models. Models having lower AIC are considered more parsimonious and are thus preferred (Akaike, Reference Akaike1987).
Results
Descriptive Statistics and Twin Correlations
Table 1 presents means, standard deviations, and maximum likelihood twin correlations for HB by zygosity. Although there was no significant sex difference in the variance of HB, the mean was significantly higher in females than in males (t = 4.7, p < .001). The correlation between age and HB was significant but small (r = 0.07).
TABLE 1 Means, Standard Deviations, and Maximum Likelihood Twin Correlations and Their 95% CIs for Hwabyung Syndrome by Zygosity

Note: 95% CIs are in parentheses. MZM = monozygotic male twins; DZM = dizygotic male twins; MZF = monozygotic female twins; DZF = dizygotic female twins; OSDZ = opposite-sex dizygotic twins. Twin correlations were based on the square-root transformed data.
As a preliminary step, we computed maximum likelihood twin correlations across zygosity groups to predict the results from general sex-limitation model-fitting analysis. As can be seen in Table 1, MZ twin correlations were greater than DZ twin correlations in both sexes, suggesting the presence of genetic effects on HB. The sizes of DZ twin correlations were only slightly greater than half the MZ correlations in both sexes, suggesting that shared environmental influences may be very small. The size of OSDZ twin correlation was not significantly lower than same-sex DZ twins, which suggested that genes for HB may not be sex-specific. MZ correlations were much less than 1.0 in both sexes, indicating that individual-specific environmental factors may be important in HB. These observations of the twin correlations were formally tested using general sex-limitation model-fitting analysis.
Model-Fitting Analysis
Table 2 presents the results of model-fitting analysis. When we set the genetic correlation to be the same across OSDZ and same-sex twins, the resulting chi-square change was negligible (Model 2). When we removed age covariate from the full model (Model 3), the resulting chi-square change was significant, suggesting that the age covariate should be retained in the model. We next equated the magnitudes of additive genetic, shared, and individual-specific environmental effects across two sexes, which yielded no significant change in chi-square (Model 4). Taken together, these result suggested that sex-specific genes might not be present in HB, and that the magnitudes of genetic and shared and individual-specific environmental influences on HB might be the same across sexes.
TABLE 2 Model-Fitting Results for the Hwabyung Symptoms Scale
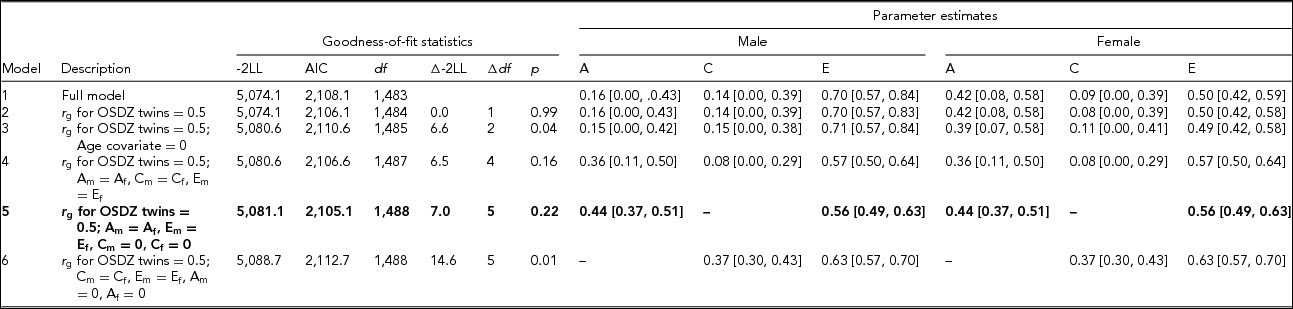
Note: Model-fitting analyses were based on the square-root transformed data. LL = log likelihood. The best-fitting model is indicated in bold type. A = additive genetic variance, C = shared environmental variance, E = individual-specific environmental variance plus measurement error. r g = genetic correlation. OSDZ = opposite-sex dizygotic twin. Subscripts m and f represent male and female, respectively.
Models 5 and 6 removed shared environmental effects and additive genetic effects, respectively, from Model 2. The resulting chi-square change was significant in Model 6 but not in Model 5, indicating that shared environmental effects are negligible, while genetic influences are significant. From these model comparisons, we concluded that Model 5 was the best fit, where additive genetic and individual-specific environmental effects including measurement error was 44% (95% CI [37%, 51%]) and 56% (95% CI [49%, 63%]), respectively.
Discussion
The present study was the first to report heritability of HB. Model-fitting analysis revealed that 44% of the variation in HB symptoms was due to genetic effects, with the remaining variance being attributable to individual-specific environmental influences and measurement error. The genetic contribution to HB found in the present study points to a need to conduct molecular genetic studies in the future for a better understanding of the pathophysiology of HB. In an association study of the oxytocin receptor gene (OXTR rs53576), Kim et al. (Reference Kim, Sherman, Mojaverian, Sasaki, Park, Suh and Taylor2011) found that as compared to those who carry the A allele, those with the G allele exhibited more culturally congruent patterns of emotion regulation. While suppression of emotion is a normative behavior in Korean collectivistic cultures, expression of emotion is a normative behavior in Western individualistic cultures. Consistent with cultural norms, the American G allele carriers showed less habitual use of emotional suppression than did the American A allele carriers, whereas the Korean G allele carriers showed greater habitual use of emotional suppression than did the Korean A allele carriers. These results suggest a possibility that expression of genes related to HB may be modified by cultural norms. Although emotion regulation is likely to be an endophenotype of HB, the Kim et al. study employed a candidate gene approach that has been criticized for the high rate of false positive findings (Marian, Reference Marian2012). Thus, it is necessary in the future to carry out genome-wide association studies of HB to identify genes involved in HB symptoms.
The magnitude of genetic influence found in HB in the present sample was broadly similar to those found in affective disorders and stress-related psychopathology such as depression, anxiety disorders, somatization, and PTSD (Duncan et al., Reference Duncan, Ratanatharathorn, Aiello, Almli, Amstadter, Ashley-Koch and Koenen2017; Shimada-Sugimoto et al., Reference Shimada-Sugimoto, Otowa and Hettema2015). Given the frequent comorbidity of HB with these disorders, multivariate genetic studies may need to be performed in the future to elucidate shared genetic basis of HB and related disorders.
In the present sample, we found no significant sex differences in genetic and environmental influences on HB. Similar results were reported in twin studies of anxiety disorders (Shimada-Sugimoto et al., Reference Shimada-Sugimoto, Otowa and Hettema2015) but not in those of depression or PTSD (Bangasser & Valentino, Reference Bangasser and Valentino2014; Perry et al., Reference Perry, Goldstein-Piekarski and Williams2017). Given the higher incidence of stress-related psychiatric disorders in females (Marcus et al., Reference Marcus, Young, Kerber, Kornstein, Farabaugh, Mitchell and Rush2005) and sex differences in biological pathways to psychiatric disorders reported in the literature (e.g., Perry et al., Reference Perry, Goldstein-Piekarski and Williams2017), it is possible that our sample size was not sufficiently large to detect sex differences in genetic and environmental influences on HB. Future twin studies should, therefore, increase sample size to test sex differences.
Our twin model assumes that there is no gene environment (GE) correlation or gene by environment interaction (G × E). It has been argued that omission of GE correlation and G × E in a twin model may lead to an overestimation of genetic and/or environmental variance components (Eaves et al., Reference Eaves, Silberg and Erkanli2003). Genetically vulnerable individuals may create a stressful environment that can precipitate anger, which can contribute to the development of HB (GE correlation). Genetically influenced risk factors for HB include hasty, impatient, and convulsive personality characteristics (Moon et al., Reference Moon, Kim and Whang1988), poor social skills and stress coping strategies (Kim & Kim, Reference Kim and Kim2013), and low self-esteem (Park & Chae, Reference Park and Chae2001). Longitudinal twin studies may be necessary to delineate the process of GE correlation in the development of HB.
Genetic factors in HB may be expressed differently across different environmental settings (G × E). Parental education is an example of shared environment. Given the findings that lower parental education is associated with a higher risk factor for HB (Kim & Park, Reference Kim and Park2004; Lee & Lee, Reference Lee and Lee2008; Min et al., Reference Min, Namkoong and Lee1990; Rhi, Reference Rhi2004), we conducted a G × E model-fitting analysis including the parental educational level as a moderator. However, genetic and environmental influences on HB in our sample did not differ significantly across the levels of parental education (data available upon request), suggesting that our heritability estimate may not be inflated due to the effect of gene by parental education interaction.
Family conflicts have been consistently suggested to be risk factors for the development of HB (Kim & Park, Reference Kim and Park2004). However, our study showed that shared environmental effects were negligible and that environmental effects on HB were mainly individual-specific, suggesting that family conflicts relevant to HB may be specific to individuals within the family. If family conflicts are subsumed under shared environmental influences in the development of HB, then our heritability estimate may encompass the gene by family conflict interaction component. If, however, family conflicts are subsumed under individual-specific environment, then our estimate of individual-specific environment may include the gene by family conflict interaction component.
Unfortunately, we did not measure family conflicts in our study, and we were not able to examine how family conflicts act and interact with genetic effects on HB.
There were a few limitations in this study that deserve mention. First, we collected the HB data through telephone interviews. Although some studies suggest that telephone interview is comparable to the face-to-face interviews in assessing psychiatric phenotypes (e.g., Sobin et al., Reference Sobin, Weissman, Goldstein, Adams, Wickramaratne, Warner and Lish1993), others suggest that responses elicited by telephone are susceptible to social desirability bias (e.g., Sibbald et al., Reference Sibbald, Addington-Hall, Brenneman and Freeling1994). Therefore, it is necessary to replicate our findings with the face-to-face interview method. Second, our sample only included adolescents and young adults, and therefore heritability of HB found in our sample may not be generalized in other age groups. Third, HB is known to be more prevalent in middle-aged adults than in adolescent populations (Min, Reference Min2008). Thus, one could argue that patients with HB may not be present and only mild cases may have been available in our sample. However, on the basis of the diagnostic criteria of the HB symptom scale (Kwon et al., Reference Kwon, Kim, Park, Lee, Min and Kwon2008), approximately 10% of the present sample could be considered at risk for the development of HB, which is within the range of the prevalence rate of HB estimated for the general population in South Korea (Kim & Park, Reference Kim and Park2004; Lee & Lee, Reference Lee and Lee2008; Min et al., Reference Min, Namkoong and Lee1990; Rhi, Reference Rhi2004). Finally, we employed a symptom scale to measure the phenotype of HB. However, heritability estimate may be different if a clinical diagnosis of HB is employed.
Acknowledgments
This work was supported by the ‘Development of Health Prediction Technology based on Big Data’ (K18092) funded by the Ministry of Science and ICT (MSIT) of South Korea, given to the Korea Institute of Oriental Medicine (KIOM).




