Oxidative stress is involved in the development of numerous diseases, including CVD and cancer(Reference Ceconi, Boraso and Cargnoni1–Reference Klaunig and Kamendulis3). It has been suggested that antioxidants may attenuate oxidative stress and thereby inhibit the development of oxidative stress-related diseases. The majority of epidemiological cohort and case–control studies studying associations between dietary antioxidants, or the serum concentration of antioxidants, and the incidence of oxidative stress-related diseases have been supportive of this hypothesis(Reference Hak, Ma and Powell4–Reference Mahdavi, Faramarzi and Seyedrezazadeh8). Thus, the second expert report from the World Cancer Research Fund and the American Institute of Cancer Research concluded that intake of foods containing specific antioxidant compounds such as carotenoids, vitamin C and vitamin E probably reduces the risk of several types of cancer(9). In contrast to these observations, most randomised controlled trials (RCT) using high doses of antioxidant compounds in supplements (β-carotene, vitamin C and vitamin E) have not been supportive. Although some of these studies have shown beneficial effects, several studies have demonstrated no or even harmful effects, especially in smokers(10, Reference Omenn, Goodman and Thornquist11). A recent meta-analysis investigating all-cause mortality in RCT using antioxidant supplements concluded that supplementation was dose-dependently associated with an increased risk of death(Reference Bjelakovic, Nikolova and Gluud12). Thus, although fruits and vegetables containing a large number of various antioxidants seem to be beneficial, supplements containing one or a few antioxidant compounds in pharmacological doses seem to have no or even harmful effects.
To study the possible preventive role of dietary antioxidants in oxidative stress-related diseases, we measured the total antioxidant content of more than 3500 foods(Reference Carlsen, Halvorsen and Holte13–Reference Halvorsen, Carlsen and Phillips15). In the present RCT, we used a comprehensive combination of antioxidant-rich foods (such as berries, nuts, spices, fruits and vegetables) providing dietary antioxidants at levels that are similar to that only previously tested using supplements(Reference Bjelakovic, Nikolova and Gluud16). In the second intervention group, kiwi fruits, which are a rich source of vitamin C, were provided.
The primary aim of the present RCT was to test the compliance and tolerability of two food-based antioxidant interventions. Furthermore, as one of the interventions provided dietary antioxidants at levels similar to those previously administered in RCT using supplements, in which adverse effects among smokers have been indicated, the safety of these diets was evaluated. To our knowledge, no human intervention study has previously investigated the effects of similar amounts of total antioxidants provided by food sources alone.
Subjects and methods
Subjects and sample size
The participants were recruited via advertisement in a local newspaper. The inclusion criteria were men between 45 and 75 years, smoking at least five cigarettes/d and a stable weight range ( ± 4 kg in the previous 3 months) and BMI < 35 kg/m2 (for the last 6 months). The exclusion criteria were any history of symptomatic CVD (myocardial infarction, angina pectoris, coronary artery bypass graft surgery, percutaneous coronary intervention, congestive heart failure and heart failure classified according to the New York Heart Association grades III and IV), diabetes mellitus (type 1 or type 2) or other significant clinical disorders, including cancer and gastrointestinal diseases. Individuals following a vegetarian or near-vegetarian diet or having allergy to experimental foods were also excluded. Moreover, subjects with a history of serious or unstable medical or psychiatric disorder, current use of, or in need of, lipid-lowering drug treatment, aspirin or non-steroidal anti-inflammatory drugs, nutritional supplements or herbs for weight loss within the 4 weeks before inclusion, or participating in a drug trial during the last 30 d were not included. Sample size was determined using literature values and results from previous studies on expected effects(Reference Brevik, Andersen and Karlsen17, Reference Erlund, Freese and Marniemi18). In the absence of background data on other endpoints, plasma antioxidant variables were used as primary endpoints for sample size calculations.
Study design and intervention
The study followed a randomised, parallel design with an 8-week intervention period. A 4-week run-in period was included before randomisation. During the run-in and intervention periods, the participants were instructed to avoid any single vitamins or antioxidant supplementation and pain or cold remedies containing aspirin or aspirin-like drugs. As coffee is the major contributor of intake of dietary antioxidants in Norway, intake was limited to three cups/d. Furthermore, all participants were instructed to limit their use of berries, nuts, pomegranates, tomatoes, kiwi fruit and tea during the run-in and intervention periods.
After the run-in period, subjects were randomly assigned to one of the three groups: antioxidant-rich diet group, kiwi fruit group or control group. Randomisation was performed in blocks of twelve, with group allocation provided in pre-sealed, numbered envelopes.
Subjects were provided with relevant food items at weekly follow-ups. For the antioxidant-rich diet group, the weekly amounts and the respective antioxidant content of the different food items are presented in Table 1. In total, the antioxidant-rich foods provided 300 mmol antioxidants/week, as estimated by the ferric-reducing/antioxidant power (FRAP) assay (Table 1)(Reference Carlsen, Halvorsen and Holte13–Reference Halvorsen, Carlsen and Phillips15), in which the amounts of total antioxidants are estimated based on the substance's ability to reduce Fe3+. The antioxidant-rich diet included green tea, juice of rose hips, orange, apple and carrot, juice of cranberries, raspberries and grapes, juice of black chokeberry, bilberries, grapes and cherries, bilberry juice, unsweetened bilberry jam, bilberries, blackberries, strawberries, raspberries, pomegranates, dark blue table grapes, Brussel sprouts, broccoli, red cabbage, kale, blue potatoes, tomatoes, dark chocolate (70 % cocoa), pecan nuts, sunflower seeds and walnuts. Extra virgin olive oil, rosemary, thyme and oregano were provided once at the start of the intervention period. The weekly amounts that were administered were, as far as possible, based on practical issues, i.e. size of container, suggested portion sizes (i.e. 100 g for fruit and vegetables) and taste (for nuts and seeds baked in bread). If not otherwise specified, all berries, fruits and vegetables were purchased from Odd Langdalen Frukt & Grønnsaker Engros AS (Oslo, Norway). To increase the palatability of the diet, the sunflower seeds and walnuts were used as ingredients in bread.
Table 1 Antioxidant-rich food items provided to the antioxidant-rich diet group during the intervention period

* Food items were provided by the Norwegian University of Life Sciences (Ås, Norway).
† Food items were provided as ingredients in bread.
‡ Food items were provided once at the start of the intervention period.
Participants in the kiwi fruit group consumed three kiwi fruits/d (Actinidia deliciosa; Odd Langdalen Frukt & Grønnsaker Engros AS). Weekly amounts were provided at follow-ups. Participants in the control group were advised to follow their habitual diet during the 8-week period, and were monitored at biweekly follow-ups.
Participants were included between March 2004 and March 2005. The Easter holiday, summer vacation and Christmas holiday were avoided to prevent possible confounding by seasonal changes in the participants' diet. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the regional ethics for medical research committee and all participants gave a written, informed consent before inclusion. The study is registered as ‘Oslo antioxidant study’ (NCT00520819) at www.clinicaltrials.gov
Compliance, tolerability and registration of undesirable events and diet
A detailed questionnaire was completed at each weekly follow-up to record compliance, as well as any challenges and difficulties related to the intake of the intervention items.
The questionnaire was designed to record any dietary items that were not consumed during the preceding week, as well as the acceptance of the dietary intervention (i.e. whether the participants found it palatable or not). To ease the completion of the questionnaire, all participants were instructed to bring the remaining food items to the weekly follow-up. Compliance was based on the information obtained from the questionnaires. Individual counselling was given to the participants in the antioxidant-rich diet group by a trained nutritionist to help them consume the provided food items by suggesting recipes and giving practical advice in preparing the food. For those who had problems implementing parts of the intervention diet, food items were exchanged within other antioxidant-rich foods (Table 1).
Undesirable events were recorded at weekly follow-ups by a specific question related to the occurrence of any adverse events. If any events were reported, questions about specific symptoms were asked and recorded in the questionnaire.
Tolerability was evaluated based on the data related to the acceptance of the dietary intervention, as well as reported undesirable events.
Habitual dietary intake, as well as dietary intake during the intervention period, was recorded using a 7 d food record with a picture book. The 7 d food record with a picture book used in the present study has been validated using a weighed food record as a reference method, demonstrating that the reported energy and nutrient intake are valid on a group level(Reference Lillegaard and Andersen19). The food records were completed in the last week of the run-in period (i.e. the week preceding randomisation) and during the last week of the intervention period. Average daily intake of energy and nutrients was computed using the food database AE-07 and KBS software system (KBS, 2008; version 4.9; Oslo, Norway) developed at the Department of Nutrition, University of Oslo, Norway. The food database AE-07 is based on the 2006 edition of the Norwegian food composition table (http://www.norwegianfoodcomp.no). The antioxidant values in AE-07 are based on the comprehensive antioxidant food database developed at the Department of Nutrition, University of Oslo(Reference Carlsen, Halvorsen and Holte13, Reference Halvorsen, Holte and Myhrstad14). A total of four participants failed to return their dietary records; thus, data on dietary intake were available for thirty-two participants in each group.
Laboratory analysis
Overnight, fasting blood samples were collected between 08.00 and 10.00 hours before and after the intervention period. Plasma and serum were prepared from blood samples within 1 h after sampling, and stored at − 70°C until time of analysis unless immediately analysed.
Analysis of antioxidants in plasma
To preserve vitamin C in the plasma, heparin plasma was immediately acidified using an equal volume of 10 % meta-phosphoric acid and stored at − 70°C until analysis within 3 months. The vitamin C isoform ascorbic acid was determined by HPLC(Reference Karlsen, Blomhoff and Gundersen20, Reference Karlsen, Blomhoff and Gundersen21). Plasma calibrators quantified against the National Institute of Standards and Technology 970 standardised reference material were used as standards.
For the determination of tocopherols by HPLC, proteins were precipitated by the addition of three volumes of isopropanol followed by centrifugation at 3000 g at 4°C for 15 min. The internal standard tocol was added with the isopropanol. A total of 5 μl of the clear supernatant was used for the analysis by HPLC(Reference Richheimer, Kent and Bernart22). Standards prepared in 1 % bovine serum albumin in PBS were used for quantification.
The carotenoids lutein, zeaxanthin, β-kryptoxanthin, α-carotene, β-carotene and lycopene were determined in plasma by HPLC. Proteins were precipitated and removed by the addition of a 4·5 volume of isopropanol followed by centrifugation at 3000 g at 4°C for 15 min. The internal standard astaxanthin was added with isopropanol. A total of 25 μl of the clear supernatant was used for the analysis. The mobile phases consisted of A (20 % water and 24 % acetone in ethanol) and B (acetone). The gradient conditions were as follows: from 2 to 100 % mobile phase B within 20 min, followed by 100 % mobile phase B for 15 min. Detection was performed at 453 nm using a variable wavelength detector. Plasma calibrators quantified against the National Institute of Standards and Technology 968c standardised reference material were used as standards.
Quercetin was analysed by HPLC and electrochemical detection after enzymatic hydrolysis(Reference Erlund, Alfthan and Siren23). Phenolic acids and enterolactone were analysed by GC–MS after enzymatic hydrolysis(Reference Kilkkinen, Erlund and Virtanen24).
Analysis of total antioxidant capacity in plasma
FRAP was determined in plasma and in the extracts of plasma free of uric acid and proteins (modified FRAP)(Reference Benzie and Strain25). For the preparation of extracts free of uric acid and proteins, 10 μl uricase (0·1 units/10 μl) in Tris buffer (pH 8·5, 400 mmol/l) were added to 60 μl plasma. After incubation for 5 min at room temperature, 120 μl ethanol were added to precipitate the proteins. Samples were placed at 4°C for 5 min before centrifugation at 13 000 g at 4°C for 5 min. The uric acid- and protein-free supernatants were used for FRAP analysis.
Total peroxyl radical-trapping activity was measured in plasma by a modified version of an assay described elsewhere(Reference Serafini, Bugianesi and Salucci26). Briefly, plasma was added to the reaction mixture made up to a 200 μl final volume before incubation. The oxidation reaction was initiated by the addition of 2,2′-azobis(2-amidinopropane). The decay of fluorescence was monitored every minute for 60 min (at 495 nm (excitation) and 570 nm (emission)) on a fluorescence microplate reader (GENios Standard TECAN Italia, Milano Due, Segrate, Italy). Results are expressed as μm-trolox equivalents.
Plasma samples for the oxygen radical absorbance capacity (ORAC) assay were thawed and kept on ice. To isolate the protein-free fraction, plasma proteins were precipitated by the addition of 0·5 m-perchloric acid (PCA) to an equal volume of plasma before centrifugation at 13 000 g at 4°C for 5 min. Plasma (total) or the protein-free supernatant (PCA precipitated) was used for the ORAC assay, with the oxidation reaction initiated with 2,2′-azobis (2-amidinopropane) dihydrochloride(Reference Davalos, Gomez-Cordoves and Bartolome27). Fluorescence was recorded every 3 min (485 nm (excitation) and 520 nm (emission)) with the use of a FLUOstar OPTIMA (BMG LABTECH, Offenburg, Germany). ORAC values were calculated using the differences of areas under the fluorescence decay curves between the blank (phosphate buffer) and a sample (net area). Results are expressed as μmol trolox equivalents/l plasma.
Total phenolics were analysed by a modified version of a previously described method. Briefly, 50 μl plasma were mixed with 150 μl ethanol for 2 min, before centrifugation at 3000 g at 4°C for 15 min. A total of 50 μl of the clear supernatant was used for the determination of total phenolics using the Folin–Ciocalteau reagent(Reference Maskarinec, Chan and Meng28). Quercetin prepared in ethanol served as standard solutions, and the results are reported as μm-quercetin equivalents.
Clinical parameters associated with tissue damage
Creatinine kinase, alanine aminotranferase, aspartate aminotransferase, albumin, lactate dehydrogenate and creatinine kinase-isoenzyme MB (CK-MB) band were analysed at the Department of Clinical Chemistry at Oslo University Hospital Ullevål (Oslo, Norway) using a Cobas Integra 400/800 analyser (Roche Diagnostics GmbH, Mannheim, Germany).
Biomarkers associated with endogenous antioxidant defence and oxidative damage
Glutathione was determined according to the ‘homocysteine by HPLC’ kit provided by Biorad Laboratories GmbH (Munchen, Germany). Standard solutions prepared in PBS served as calibrators. Erythrocyte antioxidant enzymes were measured in erythrocytes isolated from whole blood using citrate cell preparation tubes. Lysates of erythrocytes were prepared by the addition of an equal amount of water. These were used for the determination of the antioxidant enzymes such as glutathione reductase, glutathione peroxidase, catalase and superoxide dismutase(Reference Young, Dragstedt and Haraldsdottir29). Glutathione S-transferase was determined on a Cobas Mira analyser using a modified version of a previously reported assay(Reference Habig, Pabst and Jakoby30). Briefly, the erythrocyte lysates were diluted to 1:3 in 150 mm-KCl containing 2 mm-EDTA and 0·1 % Triton X-100 (pH 7·0). A total of 6 μl lysate was transferred to a reaction mixture containing 484 μl of 1 mm-glutathione in 100 mm-phosphate buffer and 10 μl of 1-chloro-2,4-dinitrobenzene in dimethylsulfoxide. Enzyme activities were calculated relative to the amount of Hb, which was measured by a standard kit and Drabkins reagent (Randox Laboratories Limited, Crumlin, Antrim, UK).
Measurement of DNA damage (strand breaks) by the comet assay was performed in lymphocytes as described previously(Reference Singh, McCoy and Tice31). In addition to DNA strand breaks, oxidised bases were measured; they were converted to breaks by digesting nucleoids in the gel using endonuclease III (which recognises oxidised pyrimidines) and formamidopyrimidine-DNA glycosylase (specific for oxidised purines).
The diacron-reactive oxygen metabolites test was performed according to the information from the manufacturer (Diacron International, Grossetto, Italy).
Liquid chromatography–MS/MS (electrospray ionisation, negative mode) was used for the determination of 8-epi PGF2α (isoprostanes) in EDTA plasma. Plasma was hydrolysed and acidified, before isolated isoprostanes were washed and concentrated using a C-18 solid-phase extraction column. The eluate was dried, and the residue was dissolved in water–methanol (1:1). A total of 50 μl was used for chromatographic analysis. Separation was achieved with the use of two SUPELCOSIL™ ABZ+Plus columns connected in series at 22°C. The mobile phases were (A) acetonitrile–formic acid (100:0·1) and (B) water–formic acid (100:0·1) at a flow rate of 0·35 ml/min. The gradient conditions were as follows: mobile phase A from 35 to 55 % for 18 min followed by 55 % A for 2 min.
Statistical analysis
Kruskal–Wallis one-way ANOVA was used to evaluate whether baseline values or changes during the intervention period differed between the groups. Where significant results were obtained, Student's t test or the Mann–Whitney test was performed to compare the mean and median values, respectively, between the groups. Data for the participants who did not complete the intervention period were excluded from the statistical analysis.
In the antioxidant-rich diet group, changes during the intervention period were significantly different from changes in the control group for a large number of variables. To assess whether all the tests in combination indicate an intervention effect, we have used the non-parametric combination methodology as described by Pesarin(Reference Pesarin32). This method describes how to combine the P value of individual permutation tests into a single test, comparing the antioxidant-rich diet and the control group using Fisher's combination method.
All statistical analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). A P value of 0·050 or below was considered statistically significant.
Results
Of the 137 individuals responding to the advertisement in a local newspaper, 118 were found to be potentially eligible and screened. A total of 102 participants were included and of these, 100 completed the study (thirty-three in the antioxidant-rich diet group, thirty-three in the kiwi fruit group and thirty-four in the control group). The remaining two participants did not complete the intervention, one was excluded in the kiwi fruit group due to a non-fatal cardiovascular event and the other participant was excluded in the antioxidant-rich diet group due to lack of compliance. All baseline characteristics (Table 2) were similar for the three groups.
Table 2 Baseline descriptives of the study participants
(Mean values and ranges)
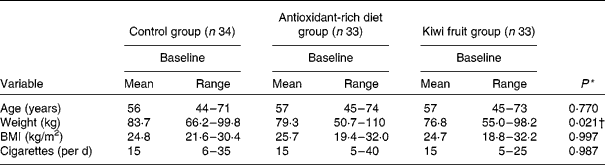
* P values are obtained by comparing baseline means between the groups using the Kruskal–Wallis test.
† Baseline means are significantly different between the kiwi fruit and the control groups (P < 0·05).
Compliance to the experimental diets
With minor exceptions, the participants in the antioxidant-rich diet group consumed all food items that were administered. Of the two excluded participants, one dropped out due to lack of acceptance of the intervention items. Acceptance rates of green tea, red cabbage, kale and blueberry jam were approximately 70 %, as some of the participants exchanged these food items with juice, blueberries, dark blue grapes and dark chocolate. Compliance for most other foods was approximately 100 %.
In the kiwi fruit group, all participants reported to consume the provided kiwi fruits, with only minor deviation.
Tolerability of the experimental diets
Only mild undesirable effects were reported during the intervention period. In the antioxidant-rich diet group, four participants reported mild gastrointestinal problems presumably related to the intervention, i.e. mild abdominal pains and mild occasional diarrhoea. Of these, three participants reported that these symptoms were experienced only during the first 2 weeks of the intervention period, whereas one participant experienced continued mild gastrointestinal problems during the entire intervention period. In the kiwi fruit group, four participants reported mild gastrointestinal problems, two of these only for the initial 2 weeks of intervention. However, these symptoms were perceived as manageable and all participants completed the intervention.
During the intervention period, four participants in the antioxidant-rich diet group, four participants in the kiwi fruit group and two participants in the control group reported upper respiratory tract symptoms related to the common cold.
Reported intake of energy and other nutrients
For all three groups, reported habitual dietary intakes at baseline and changes during the intervention period are listed in Table 3. Intakes of total energy increased significantly during the intervention period in both intervention groups when compared with the control group. In the antioxidant-rich diet group, an increase of 19 % in total energy intake was observed. However, no changes in body weight were observed (0·14 (sd 1·73), − 0·20 (sd 3·16) and − 0·30 (sd 1·64) kg) in the control, antioxidant-rich diet and kiwi fruit groups, respectively. Furthermore, intake of fibre increased significantly in both intervention groups. While the increase in the kiwi group corresponded to a 16 % increase from baseline, a more substantial increase of 76 % was observed in the antioxidant-rich diet group (Table 3).
Table 3 Daily intake of energy, micronutrients and antioxidants*, at baseline and changes during the intervention period
(Mean values, standard deviations, ranges, medians† and 95 % confidence intervals)

E %, percentage of energy.
* Calculations are based on the 7 d food record.
† Variables are non-normally distributed.
‡ P values are obtained by comparing the changes during the intervention period between the groups using the Kruskal–Wallis test.
§ Changes are significantly different compared with changes in the control group.
∥ Including supplements.
¶ Total antioxidants are estimated from ferric-reducing/antioxidant power values.
Minor, but significant, changes in the intakes of carbohydrates and proteins were observed in both intervention groups. Intakes of SFA and PUFA were only slightly altered in the kiwi fruit groups, whereas the intake of SFA decreased by 18 % and the intake of PUFA increased by 46 % in the antioxidant-rich diet group.
Reported intake of dietary antioxidants
Baseline reported intakes of total antioxidants were about 30 mmol/d in all groups (Fig. 1 and Table 3). In the antioxidant-rich diet group, the median increase in the intake of dietary antioxidants was 31·4 mmol/d, whereas only minor changes were observed in the kiwi fruit and control groups.

Fig. 1 Total intake of antioxidants in the antioxidant-rich diet group and the control group before and during the intervention. The food group ‘grain products’ includes bread, breakfast cereals and cakes. The food group ‘other foods’ includes spices, oils, meat, fish, eggs and dairy products. Intakes of antioxidants are determined using ferric-reducing/antioxidant potential values. ![]() , Grains;
, Grains; ![]() , other foods;
, other foods; ![]() , chocolate;
, chocolate; ![]() , vegetables;
, vegetables; ![]() , wine;
, wine; ![]() , tea;
, tea; ![]() , fruits and berries;
, fruits and berries; ![]() , nuts and seeds;
, nuts and seeds; ![]() , juice;
, juice; ![]() , coffee.
, coffee.
Both the antioxidant-rich diet and kiwi fruit groups significantly increased their intake of vitamin C compared with the control group. Furthermore, substantial and significant increases in the intakes of β-carotene and tocopherol were observed in the antioxidant-rich diet group (Table 3).
The contribution from different foods and food groups to the intake of total antioxidants in the antioxidant-rich diet group as compared with the control group is illustrated in Fig. 1. In both groups, coffee contributed more than 70 % of total antioxidant intake at baseline. In the antioxidant-rich diet group, fruits and berries, coffee and nuts and seeds contributed about 25, 20 and 7·0 mmol/d, respectively, whereas food groups such as tea, wine, chocolate and grain products together contributed about 10 mmol/d during the experimental period.
Safety of the experimental diets
Safety of the experimental diets was investigated using clinical parameters, as well as biomarkers of oxidative stress. As a measure of potential harmful effects of the diets, several common clinical parameters for tissue damage were measured, including creatinine kinase, creatinine kinase-MB, alanine aminotranferase, aspartate aminotransferase, albumin and lactate dehydrogenate. Baseline values and changes during the intervention are given in Table 4. As a measure of oxidative stress, biomarkers associated with endogenous antioxidant defence and oxidative damage were investigated. Baseline values and changes during the intervention in glutathione, antioxidant enzymes and biomarkers of oxidative damage are listed in Table 5. No effects were observed in biomarkers associated with endogenous antioxidant defence, or in any of the investigated biomarkers of oxidative damage in the antioxidant-rich diet group as compared with the control group. In the kiwi fruit group, the change in γ-glutamyltransferase was significantly different as compared with the control group. However, the absolute change in the kiwi fruit group was minor, whereas a substantial decrease was observed in the control group.
Table 4 Baseline values and changes during the intervention in clinical parameters related to tissue damage
(Medians*, ranges and 95 % confidence intervals)

CK, creatinine kinase; CK-MB, creatinine kinase-MB; ALAT, alanine aminotranferase; ASAT, aspartate aminotransferase; LDH, lactate dehydrogenate.
* Variables are non-normally distributed.
† P values are obtained by comparing the changes during the intervention period between the groups using the Kruskal–Wallis test.
Table 5 Baseline values and changes during the intervention in biomarkers associated with the endogenous antioxidant defence and oxidative damage
(Mean values, standard deviations, ranges, medians* and 95 % confidence intervals)

GR, glutathione reductase; GPx, glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase; GST, glutathione S-transferase; AU, arbitrary units; γ-GT, γ-glutamyltransferase; D-ROM, diacron-reactive oxygen metabolite.
* Variables are non-normally distributed.
† P values are obtained by comparing the changes during the intervention period between the groups using the Kruskal–Wallis test.
‡ Changes are significantly different compared with changes in the control group (P < 0·05).
Plasma concentrations of dietary antioxidants
At baseline, there were no significant differences between the groups in dietary antioxidants in plasma (Table 6).
Table 6 Baseline values and changes during the intervention in dietary antioxidant compounds measured in plasma
(Mean values, standard deviations, ranges, medians* and 95 % confidence intervals)

DOPAC, 3,4-dihydroxyphenyl acetic acid; FRAP, ferric-reducing/antioxidant power; ORAC, oxygen radical absorbance capacity; TE, trolox equivalents; TRAP, total peroxyl radical-trapping activity; QE, quercetin equivalents.
* Variables are non-normally distributed.
† P values are obtained by comparing the changes during the intervention period between the groups using the Kruskal–Wallis test.
‡ Changes are significantly different when compared with changes in the control group (P < 0·05).
∥ P values are from the Mann–Whitney U test.
¶ Phenolic compounds were not analysed in the kiwi fruit group.
Following the antioxidant-rich diet, substantial increases in the median plasma concentrations of lutein, α-carotene, β-carotene, quercetin, vanillic acid, protocatechuic acid, homovanillic acid, 3,4-dihydroxyphenyl acetic acid, gallic acid and p-coumaric acid were observed (15–161 % increase from baseline). Additionally, an increase of 8 % in protein-free ORAC, a measure of total antioxidant activity, was observed. These changes were significantly different when compared with the control group (Table 6). Moreover, a trend towards an increase was observed for the phenolic acid 3-hydroxyphenylacetic acid (P = 0·053).
In the kiwi fruit group, increases that were significantly different from the control group were observed for lutein and modified total peroxyl radical-trapping activity, measurements of total antioxidant capacity. Furthermore, a decrease in γ-tocopherol was observed in the kiwi fruit group.
Combination statistics
Because several biomarkers of antioxidant status were altered in the antioxidant-rich diet group, combination statistics, where all individual tests are combined into one global test of the intervention effect, were performed(Reference Pesarin32). Combination of the individual parameters to one single test shows that there is an intervention effect that is significantly different between the antioxidant-rich diet and control groups. The variables that contributed significantly to the intervention effect, results of the permutation tests and the combined statistics are summarised in Table 7.
Table 7 Plasma variables significantly contributing to the intervention effect in the antioxidant-rich diet group listed in order of declining P values (from permutation tests)

DOPAC, 3,4-dihydroxyphenyl acetic acid.
* The combined P value is associated with the combination test of an intervention effect between the antioxidant-rich diet group and the control group.
Discussion
We have demonstrated that male smokers are able to comply with and tolerate a comprehensive diet rich in antioxidants, as well as a simpler dietary change involving kiwi fruit. No potential harmful or pro-oxidative effects were observed, demonstrating the safety of the dietary intervention strategies.
The reported compliance was very good for both intervention strategies, and the majority of the participants were able to comply with the comprehensive antioxidant-rich diet and to the kiwi fruit diet during the 8-week intervention period. In the antioxidant-rich diet group, a few food items were unfamiliar to the participants, such as green tea, red cabbage, kale and blueberry jam. Some of these items were replaced with other foods such as broccoli, blue grapes, blueberries and dark chocolate. Only one participant was excluded due to lack of compliance. Although the participants in the antioxidant-rich diet group increased their intake of fruit and vegetables from 400 to 1550 g/d (data not shown), only a few experienced minor undesirable events during the 8-week intervention period. Similarly, very few experienced problems in the kiwi fruit group. A total of eight participants equally divided between the intervention groups experienced mild gastrointestinal problems related to the intervention diet. Of these, three participants in the antioxidant-rich diet group and two participants in the kiwi fruit group reported just temporary discomfort. Those with continuous problems perceived these as manageable and were able to complete the intervention. Due to the low number of participants experiencing adverse events, it is not possible to establish which dietary constituents may have been responsible. The kiwi fruit group was provided with three kiwi fruits/d. Kiwi fruits were selected due to their high antioxidant content, as well as their high content of vitamin C. Furthermore, a few studies have indicated beneficial effects of kiwi fruit consumption(Reference Collins, Horska and Hotten33–Reference Chang and Liu35). The amount was chosen based on the previous experience of the tolerability of kiwi fruit consumption over time(Reference Duttaroy and Jorgensen34).
We have previously assessed the content of total antioxidants in several thousand dietary plants including fruits, berries, vegetables, cereals, nuts and herbs(Reference Halvorsen, Holte and Myhrstad14, Reference Halvorsen, Carlsen and Phillips15, Reference Dragland, Senoo and Wake36). These data, as well as previously published data on potential bioactivity(Reference Battino, Beekwilder and Denoyes-Rothan37–Reference Willcox, Catignani and Lazarus44), were used to select plant-based dietary items with a high content of antioxidants for the antioxidant-rich diet (Table 1). Different dietary sources, including juices, berries, fruits, vegetables, nuts, oil, chocolate and spices, were selected to provide a broad range of dietary antioxidants.
The antioxidant-rich foods that were administered provided approximately 300 mmol antioxidants/week, as determined by the FRAP assay. In RCT using supplements, daily dosages of up to 2 g vitamin C (11·4 mmol) or 5 g vitamin E (11·6 mmol) have been administered(Reference Bjelakovic, Nikolova and Gluud16). Both of these correspond to about 23 mmol each in the FRAP assay. Administration of vitamins C and E at these dosages would therefore equal 300 mmol/week, which is similar to the amounts of dietary antioxidants that are provided by the antioxidant-rich foods. At baseline, the daily intake of dietary antioxidants was approximately 30 mmol/d, and coffee contributed to nearly 70 % of the total antioxidant intake (Fig. 1). Following the antioxidant-rich diet, a mean increase of 31 mmol antioxidants/d was observed, corresponding to an increase of 220 mmol/week. The main contributors were coffee (34 %), juices (29 %), nuts and seeds (11 %), and fruits and berries (10 %) (Fig. 1). The goal of increasing the intake of dietary antioxidants by 300 mmol/week was not fully achieved by all participants. This observation may be explained by the participants who exchanged parts of their habitual diet with intervention items. Although the total intake of antioxidants increased substantially, the contribution from the habitual intake may have been reduced during the intervention period.
The food items provided in the antioxidant-rich diet provided about 3070 kJ/d. The participants reduced their reported energy intake from other food sources by only 1120 kJ/d, causing an average increase of 19 % in energy intake during the intervention period. However, no increase in body weight was observed following the intervention (Table 2), although such an increase in energy intake for 8 weeks theoretically should promote an increase in body weight of about 3 kg. As the present study was not designed to accurately assess total energy intake or expenditure, this apparent effect on body weight needs to be addressed in future studies.
Alternatively, the antioxidant-rich diet provided a number of foods rich in polyphenols, as well as fibre. Data from epidemiological and experimental studies suggest that polyphenol-rich food items may exert beneficial effects on body weight. For instance, it has been proposed that green tea may reduce lipolysis of long-chained TAG, interfere with fat emulsification and inhibit fatty acid synthase activity(Reference Cabrera, Artacho and Gimenez45). Moreover, results from epidemiological and clinical studies have suggested that consumption of nuts, independent of their high energy content, may promote weight loss or at least limit weight gain(Reference Coates and Howe46, Reference Sabate47). Furthermore, the antioxidant-rich diets were rich in fibre, a dietary component that potentially may contribute beneficially in body-weight management and limit weight gain by prolonging postprandial satiety(Reference Anderson, Baird and Davis48, Reference Astrup, Kristensen and Gregersen49). Taken together, our data may suggest that a diet rich in antioxidant foods might be beneficial in relation to body-weight regulation. This deserves further investigations.
An important aim of the present study was to investigate the safety of a diet designed to provide dietary antioxidants at levels similar to what previously only have been tested in supplementation studies. As a measure of safety, commonly applied clinical parameters associated with tissue damage, in addition to biomarkers associated with endogenous antioxidant defence and oxidative damage to lipids and DNA, were investigated. No potential harmful or pro-oxidative effects were observed following any of the intervention diets.
In the α-Tocopherol, β-Carotene Cancer Prevention study as well as in the Carotene and Retinol Efficacy Trial, where large doses of β-carotene were administered to middle-aged male smokers, the authors reported increased incidence of cancer in the lung(10, Reference Omenn, Goodman and Thornquist50). Furthermore, it has been suggested that the increased mortality or morbidity observed in some antioxidant supplementation studies may be related to pro-oxidative effects(Reference Bjelakovic, Nikolova and Gluud12). However, few of the large RCT using antioxidant supplementation have reported data related to specific biomarkers of oxidative damage. A recent review has investigated the relationship between oxidative damage to DNA and antioxidants from dietary sources and supplements(Reference Moller and Loft51). The authors conclude that intervention with antioxidant-rich foods appears to be more effective than supplementation in lowering the excretion of oxidatively damaged DNA. This conclusion is supported by other intervention studies where intake of dietary antioxidants has mediated potential beneficial effects by decreases in certain measures of oxidative damage(Reference Bub, Watzl and Abrahamse52–Reference Weisel, Baum and Eisenbrand58) or induction of endogenous antioxidant defence by increases in plasma glutathione or induction of glutathione peroxidase activity(Reference Weisel, Baum and Eisenbrand58–Reference Young, Nielsen and Haraldsdottir60). To our knowledge, such effects have not been investigated in studies where large doses of antioxidant supplements have been administered.
RCT using supplementation are usually conducted over long periods of time (i.e. years), whereas food intervention studies are conducted over short- to medium-term periods (i.e. weeks to months). Due to the varying time aspects, it may not appear easy to compare the effects on endpoints between such studies. However, pro-oxidative effects may be regarded as an acute condition, which over time may be responsible for disease development by accumulation of potentially harmful products. Thus, pro-oxidative effects measured by intermediate biomarkers of oxidative damage may be compared between acute and chronic studies as a measure of potential health effects and safety of the dietary regimens. Taken together, it may be plausible to suggest that a diet rich in antioxidants does not mediate harmful or pro-oxidative effects, although the intake of total antioxidants may be similar to that used in RCT using supplements.
In the antioxidant-rich diet group, several antioxidants increased in fasting plasma during the intervention (Table 6). It has been proposed that antioxidant compounds with differing chemical properties may constitute an antioxidant network in the plasma and other compartments(Reference Packer, Weber and Rimbach61, Reference Yeum, Russell and Krinsky62). It is hypothesised that these may play an important role in endogenous protection against oxidative insults. A number of the measured antioxidant compounds (i.e. polyphenols) have a rapid metabolism and elimination(Reference Manach, Scalbert and Morand63). Although it was not expected to observe large changes in these compounds using overnight, fasting blood samples, we observed several significant increases. Polyphenolic compounds have been demonstrated to be efficient antioxidants in vitro, and in the circulation, these may interact with other compounds of both lipophilic and hydrophobic nature(Reference Yeum, Russell and Krinsky62). It has been suggested that their rapid metabolism and elimination may serve as a detoxification pathway for oxidative challenges(Reference Yeum, Russell and Krinsky62).
Increased concentration of certain antioxidant compounds following a fruit and vegetable-based intervention has been reported(Reference Dragsted, Pedersen and Hermetter59, Reference Broekmans, Klopping-Ketelaars and Schuurman64–Reference Roberts, Gordon and Walker68), although, to our knowledge, the present study is the first to report increases in such a large number of antioxidant compounds with differing chemical properties following intervention with foods rich in antioxidants. The use of combination statistics confirms that a true intervention effect is observed mainly on these variables.
Although participants in the kiwi fruit group significantly increased their intake of vitamin C, no increase in plasma levels was observed, which is in conflict with data from similar studies(Reference Duttaroy and Jorgensen69). However, the lack of effects in our study may be explained by the choice of the study population. It is well established that smokers have a higher turnover of vitamin C(Reference Murata70), and it is possible that absorbed vitamin C is rapidly degraded.
We have demonstrated that intake of dietary antioxidants at doses similar to those previously tested in supplementation studies mediates a substantial improvement in plasma antioxidants without promoting harmful or pro-oxidative effects, in a population at an increased risk of CVD. In addition, we present evidence that both diets modulated a number of genes and gene pathways related to stress and defence responses(Reference Bohn, Myhrstad and Thoresen71).
In conclusion, male smokers can comply with a diet abundant in antioxidants and to a kiwi fruit diet for 8 weeks. Only minor and temporary side effects were experienced. We have demonstrated that the antioxidant-rich diet was particularly effective in terms of increasing plasma antioxidants. Although the level of consumed antioxidants in this diet may be compared with that previously tested in RCT using supplements, no potentially harmful or pro-oxidative effects were observed. Future studies are needed to explore the impact of such dietary intervention strategies on the risk of chronic diseases related to oxidative stress.
Acknowledgements
The following authors declare no potential conflict of interest: A. K., M. Sv., I. S., M.-A. S., N. B., J. S., A. B., I. E., M. Se., S. F. R., G. I. B., M. H. C., S. K. B., M. C. M., L. O. D., A. K. D., K. H., P. L., H. A., A. C., S. T. Conflict of interests: R. B. and C. A. D. had an interest in Bioindex AS and Vitas AS. Bioindex was established by Birkeland Innovation, the Technology transfer office at the University of Oslo, while Vitas was established by Oslo Innovation Centre. The present study was supported by grants from the Throne Holst's Foundation, the Research Council of Norway and the Norwegian Cancer Society. The participants recruited via advertisements and from the Department of Preventive Cardiology, Oslo University Hospital Ulleval are greatly appreciated. Substantial contribution to the study coordination is appreciated by the enthusiastic support of Lisa Flakk at the Department of Preventive Cardiology at Oslo University Hospital Ulleval. The laboratory staff at the Centre for Clinical Heart Research, Department of Cardiology, Oslo University Hospital Ulleval provided invaluable help during the study. Kari Holte, Anne Randi Enget, Magnhild Kverneland, Aud Jørgensen and Vibeke Kegel at the Department of Nutrition and Department of Human Nutrition provided valuable contribution to the sample analysis. We also thank Petter Gjeitanger at the Central Kitchen at the Oslo University Hospital Ulleval and the bakers at Åpent Bakeri in Oslo for their contribution to the preparation of the dietary items that were provided during the intervention. R. B. formulated the present hypothesis. A. K., M. Sv., I. S., S. F. R., S. K. B., M. C. M., A. K. D., K. H., P. L., C. A. D., H. A., A. C., S. T. and R. B. contributed to the clinical study design, intervention, sample handling and data collection. A. K., J. S. and P. L. were responsible for the statistical analysis. M.-A. S. and M. Sv. were responsible for the participants and their follow-up, as well as administration of the intervention items during the study. A. K., N. B. and A. B. were responsible for the analysis of parameters related to antioxidant status and oxidative damage. I. E. was responsible for the analysis of polyphenols in the plasma. M. Se. was responsible for the analysis of plasma total peroxyl radical-trapping activity, whereas G. I. B. performed the analysis of plasma ORAC. S. F. R. and K. H. prepared and provided several food items (as specified in the Subjects and methods section). L. O. D. was responsible for the analysis of antioxidant enzymes in erythrocytes. M.-A. S. and C. A. D. were responsible for handling of the data associated with the registration of dietary intake. M. H. C. was responsible for computing intake of the dietary antioxidants. A. K., P. L. and R. B. participated in the interpretation of the data. A. K. was responsible for drafting the manuscript, which was revised and approved by all authors.










