Despite an increase in the number of available and effective antidepressants, many patients with depression respond poorly to drug treatment. At least a third of patients make an inadequate response to their first antidepressant monotherapy. Responses to subsequent courses are also limited. Optimising antidepressant use by attempting to ensure that patients take an adequate dose for an adequate length of time with measures to improve concordance is the first strategy recommended for managing incomplete response. If that is unsuccessful, further strategies include the use of higher doses, switching to another antidepressant of the same or different class, augmenting the antidepressant with either psychotherapy or a medication which is not an antidepressant (such as lithium or antipsychotics), or combining with another recognised antidepressant.
There is very little evidence, however, to guide practice in treatment-refractory patients. This is reflected in the limited number of options that the National Institute for Health and Clinical Excellence (NICE) recommends for consideration in the treatment of these patients. The STAR*D (Sequenced Treatment Alternatives to Relieve Depression) study has been a welcome addition and provides evidence for a variety of options for up to four failed treatment trials (Reference McGrath, Stewart and FavaMcGrath 2006). It reported on antidepressant combinations, but did not show any single combination to be superior. Despite the risks of an increased burden of side-effects or drug–drug interactions with antidepressant combinations, such combinations are common in clinical practice.
This article is based on searching the literature indexed in MEDLINE and published in English since 1950. The search was conducted using keywords ‘antidepressants’, ‘combination’, ‘depression’, ‘refractory’ and the names of individual antidepressant drugs, to identify randomised controlled trials (RCTs), open-label trials, case series and case reports on efficacy and toxicity from combining antidepressants currently available in the UK. Bupropion has been excluded as it is not licensed as an antidepressant in the UK. We examine the combinations by class of antidepressant (detailed reviews of individual studies can be found elsewhere, e.g.: Reference Dodd, Horgan and MalhiDodd 2005; Reference Rojo, Ros and AgüeraRojo 2005) and we review the nature and extent of the side-effect burden and potential risks of these combinations. Defining treatment resistance is a difficult issue, with many definitions available in the literature, and is beyond the scope of this article. Where available, we have reported the populations studied with particular combinations. Only key references are cited in this article. A full reference list is available from the authors on request.
Selective serotonin reuptake inhibitor (SSRI) combinations
SSRI with SSRI
Selective serotonin reuptake inhibitors (SSRIs) are widely used antidepressants. They differ to some extent in their receptor profile and exhibit significantly different pharmacokinetics.
The SSRI–SSRI combination has only been tried in two open-label studies (Reference Dodd, Horgan and MalhiDodd 2005). In both instances either fluvoxamine (50–100 mg; n= 7) or fluoxetine (20 mg; n= 6) was combined with citalopram, with apparent good clinical improvement in patients who did not respond to SSRI monotherapy. It has been proposed that addition of another SSRI increases the active S-enantiomer of citalopram compared with its R-enantiomer, leading to greater reuptake inhibition (Reference Bondolfi, Lissner and KoselBondolfi 2000). It is also possible that any clinical effect may be due to an increase in the total SSRI dose. There is some evidence for the latter (Reference Baker, Tweedie and DuvalBaker 2003), but most results suggest a flat dose–response relationship for SSRIs when used as monotherapy (Reference Adli, Baethge and HeinzAdli 2005).
Nausea and tremor are common with the citalopram–fluvoxamine combination but no serious side-effects were noted from either reported series. Serotonin syndrome is a potential serious adverse reaction with this combination (Box 1).
BOX 1 Serotonin syndrome
Definition
A predictable consequence of excessive serotonergic agonism in the central nervous system
Mechanism
Although no single receptor appears to be responsible, it is likely to be mediated through 5-HT2A receptor agonism. Noradrenergic hyperactivity may play an important role
Differential diagnosis
Anticholinergic poisoning, malignant hyperthermia, neuroleptic malignant syndrome
Treatment
Mild: withdraw the offending agent, supportive care (correction of vital signs), benzodiazepines
Moderate: as above; 5-HT2A antagonists (cyproheptadine, atypical antipsychotics, chlorpromazine)
Severe: as above; sedation, neuromuscular paralysis, intubation
(Adapted from Reference Boyer and ShannonBoyer 2005)
SSRI with tricylic antidepressant (TCA)
This is a popular combination at least in some parts of the world (Reference Rojo, Ros and AgüeraRojo 2005). The rationale of combining an SSRI with a tricylic antidepressant (TCA) arises from two hypotheses.
The first is that the noradrenergic and serotonergic effects of these agents can be effectively utilised in combination (Reference GillmanGillman 2007). It has been suggested that the serotonin–noradrenaline reuptake inhibitors (SNRIs) venlafaxine and duloxetine have a fixed ratio of serotonergic and noradrenergic effects that may limit their therapeutic efficacy in some patients. The combination of a predominantly noradrenergic TCA such as nortriptyline and an SSRI may overcome this ceiling effect and produce a different sodium:5-HT reuptake blockade ratio. However, there is no evidence that this ratio is related in any way to clinical effectiveness.
The second hypothesis is that cytochrome P450 (CYP450) inhibition of SSRIs can increase plasma levels of TCAs, giving rise to better clinical effect. Reference Levitt, Joffe and KamilLevitt et al (1999) suggested that the efficacy of the combination is largely due to increased TCA levels in patients who failed monotherapy with either an SSRI or a TCA. This is supported by Reference Weilburg, Rosenbaum and BiedermanWeilburg et al (1989), who showed that fluoxetine alone could not sustain remission in a significant number of patients who initially responded to the combination of a TCA and fluoxetine. Hepatic metabolism of TCAs can be inhibited by the effect of SSRIs on the CYP450 system; however, the extent of this inhibition varies between SSRIs used (Table 1). For example, the paroxetine–imipramine combination preferentially increases the desipramine metabolite (a potent noradrenergic reuptake inhibitor), whereas sertraline produces more modest elevations in desipramine levels (Reference Lydiard, Anton and CunninghamLydiard 1993). Rapid metabolisers of TCAs may show a good response when combining a TCA with an SSRI that inhibits CYP2D6 (Reference Conus, Bondolfi and EapConus 1996).
We identified three RCTs (total n = 181), four open-label trials (total n = 85) and three case series (total n = 46) that used SSRI–TCA combinations (a full list of references is available on request). Desipramine, a predominantly noradrenergic agent, has been studied in combination with fluoxetine. However, an RCT involving patients had not responded to standard (20 mg/day) fluoxetine monotherapy failed to demonstrate significant benefits for this combination compared with high-dose (40–60 mg/day) fluoxetine monotherapy (Reference Fava, Rosenbaum and McGrathFava 1994). It has been suggested that the desipramine–fluoxetine combination may be more useful for non-responders than for partial responders, although this has not been supported in a larger RCT (Reference Fava, Alpert and NierenbergFava 2002). Not surprisingly, treatment-resistant depression shows poorer response than non-resistant depression with this combination.
Nelson and colleagues suggested that, compared with monotherapy, combination treatment of depression using noradrenaline and serotonin reuptake inhibitors might ameliorate a greater number of symptoms in individual patients and be better at achieving remission (Reference Nelson, Mazure and JatlowNelson 2004). Additional advantages of the SSRI–TCA combination may be a more rapid response compared with using a TCA alone, although this is uncertain owing to the small numbers studied and baseline differences in the reported series. In any event, this speed of onset effect could not be replicated in a later RCT (Reference Nelson, Mazure and JatlowNelson 2004).
Side-effects
Tricyclic toxicity can occur as a result of raised plasma levels. This is a particular risk for the 7% of White people who lack sufficient CYP2D6 to metabolise TCAs (Reference Albers, Reist and HelmesteAlbers 1996). Resultant cardiovascular problems can be life-threatening, especially in the elderly or the predisposed or if there is an overdose of the SSRI–TCA combination. Paroxetine can increase the anticholinergic side-effects of TCAs. Dry mouth and gastrointestinal distress are the most common problems in combining fluoxetine with desipramine (Reference Dodd, Horgan and MalhiDodd 2005). Elimination of TCAs can be prolonged by fluoxetine, but blood levels are not closely correlated with dosage and are unpredictable (Reference WestermeyerWestermeyer 1991).
Fava and colleagues recommend using small doses of TCAs and plasma-level monitoring in such combinations (Reference Fava, Alpert and NierenbergFava 2002). Citalopram, owing to its relative lack of CYP inhibition, may be a safer SSRI to use in combination with a TCA, although no clinical data are available to support this.
SSRI with monoamine oxidase inhibitor (MAOI)
In theory, combining these two drugs could result in enhanced serotonin transmission by an additive effect. However, irreversible MAOIs such as phenelzine and tranylcypromine are dangerous in combination with SSRIs and any benefits are outweighed considerably by the risks. Patients who are inadvertently exposed to this combination show a very high occurrence of the toxic serotonin syndrome. Fatalities have been reported and death can occur even after an SSRI has been stopped before an MAOI is started. When switching from an SSRI to an MAOI, a washout period of at least 5 times the half-life of the SSRI is recommended to prevent serotonin syndrome (Reference Lane and BaldwinLane 1997). Most SSRIs require 2 weeks of washout before starting MAOIs; fluoxetine, however, because of its long half-life, requires a minimum of 5 weeks.
SSRI with moclobemide
Moclobemide selectively and reversibly blocks the monoamine oxidase A enzyme. The SSRI–moclobemide combination has been tried with the same rationale as the SSRI–MAOI combination.
Three small open-label trials (total n= 46) found moclobemide to be effective in combination with SSRIs (Reference Dodd, Horgan and MalhiDodd 2005). Both SSRI and moclobemide were started at lower than usual doses and titrated slowly up.
Side-effects
Nausea and insomnia were common side-effects, and hypomania and akathisia was seen in one patient on the sertraline–moclobemide combination. Despite being a reversible inhibitor of monoamine oxidase A, moclobemide can cause life-threatening serotonin toxicity, especially in the case of an SSRI overdose. The combination seems relatively safe at therapeutic doses, although careful consideration is needed for patients at risk of suicide.
SSRI with mirtazapine or mianserin
This is one of the most popular combinations and has been proposed on various grounds:
-
• rapid onset of effect is possible, owing to the receptor profile of noradrenergic and specific serotonergic antidepressants (NaSSAs) (see below);
-
• side-effects of the SSRI may be nullified by the NaSSA and vice versa;
-
• additive effects are possible because of different mechanisms of action;
-
• SSRIs can increase plasma levels of NaSSAs through CYP450 enzyme inhibition.
Administered acutely, SSRIs initially suppress 5-HT reuptake at somatodendritic (presynaptic) sites facilitating autoreceptor activation and reduced serotonin transmission. In due course, desensitisation of these autoreceptors enhances serotonin neurotransmission. Mirtazapine, being an α2-adrenergic antagonist, reduces autoreceptor (heteroreceptor) feedback at the somatodendritic site directly. This potentially enhances serotonin transmission at a quicker pace. Thus, it could be predicted that a combination of both medications could induce a more rapid and robust antidepressant effect than each medication administered alone.
This combination has positive evidence from three RCTs and an open-label trial (Reference Dodd, Horgan and MalhiDodd 2005) – in two of the RCTs (total n= 135) mianserin was combined with fluoxetine. The combination was better tolerated than the individual agents alone, with a significantly more rapid onset of action than with fluoxetine alone. In the third RCT (n = 60), mirtazapine combined with paroxetine showed good tolerance and significantly better response compared with high doses of either agent alone (Reference Debonnel, Gobbi and TurcotteDebonnel 2000). However, in a fourth RCT (n = 295), the combination of sertraline and mianserin was only as efficacious as 100 mg sertraline alone in patients previously unresponsive to 6 weeks of sertraline alone. An open-label study (n = 20) followed by a small RCT (n = 26) of mirtazapine 15–30 mg in combination with other antidepressants (including SSRIs) at near-maximum doses revealed a significant response and good tolerance (Reference Carpenter, Yasmin and PriceCarpenter 2002). Comparison of SSRI–NaSSA combinations with other antidepressant–NaSSA combinations has not been undertaken to date.
Side-effects
Sedation, weight gain and headache are the most commonly reported side-effects of this combination. Restless legs syndrome has been reported in three patients from an RCT sample receiving fluoxetine 20 mg/day in combination with mirtazapine 15 mg/day (Reference Prospero-Garcia, Torres-Ruiz and Ramirez-BermudezProspero-Garcia 2006). Although mirtazapine monotherapy is a possible treatment for some symptoms of serotonin syndrome (e.g. insomnia and agitation), there are case reports of new-onset serotonin syndrome with the combination (Reference BenazziBenazzi 1998).
SSRI with SNRI
This combination of an SSRI and the SNRI venlafaxine is now being seen in practice, but it does not make for rational polypharmacy as venlafaxine has predominant SSRI activity, particularly at low doses. Reference Gonul, Akdeniz and DonatGonul et al (2003) report on four patients who only partially responded to high-dose venlafaxine but fully responded to SSRI–venlafaxine combination. To reduce the risk of serotonin toxicity, the SSRIs were added to lower than the maximum dose of venlafaxine.
Side-effects
Low doses of venlafaxine combined with fluoxetine can cause urinary retention, constipation, dry mouth and blurred vision. This might be due to adrenergic stimulation mimicking anticholinergic effects. There is a potential risk of serotonin toxicity with this combination.
There are no published data on duloxetine in combination with SSRIs. Duloxetine can inhibit CYP2D6 and this may need to be considered if such a combination is attempted (Table 1).
SSRI with trazodone
Trazodone is a dual 5-HT2A antagonist and serotonin reuptake inhibitor. Its 5-HT2A blockade is believed to reduce the side-effects associated with the stimulation of 5-HT2A, including sexual dysfunction, insomnia and anxiety. Trazodone has been largely used more for its sedative than its antidepressant properties. It may be the most commonly combined antidepressant with SSRIs for this reason. It has been suggested that the mechanism of any additional antidepressant activity may be through SSRI-induced inhibition of the breakdown of both trazodone and its active metabolite m-chlorophenylpiperazine.
Good response to the combination has been demonstrated in a small (n= 26) double-blind RCT involving a treatment-resistant sample defined using Thase & Rush criteria (Reference Maes, Vandoolaeghe and DesnyderMaes 1996). In a case series involving eight consecutive patients taking fluoxetine as monotherapy, three reported reduced insomnia and depression when trazodone 100 mg/day was added (Reference Nierenberg, Cole and GlassNierenberg 1992). This sample was heterogeneous for both severity of depression and response to previous medications.
Side-effects
Despite being 5-HT2 antagonists, trazodone and nefazodone can produce serotonin syndrome in combination with either SSRIs or SNRIs. This may be mediated through increased 5-HT1A transmission. Higher levels of trazodone can produce marked side-effects, including priapism. Citalopram and fluoxetine do not seem to increase trazodone levels significantly, at least in lower doses (Reference Prapotnik, Waschgler and KönigPrapotnik 2004).
SSRI with reboxetine
Reboxetine is a noradrenaline reuptake inhibitor. Its combination with SSRIs can produce pharmacological effects similar to TCAs but with a more favourable side-effect profile due to a lower affinity for other receptors. The SSRI–reboxetine combination is now being increasingly used. It is proposed to have quicker onset of effects, at least experimentally. However, the combination mirrors the pharmacological profile of an SNRI and in the absence of compelling data it seems illogical to use two drugs rather than one.
Various open-label trials have been reported, involving reboxetine in doses of up to 8 mg/day (Reference Rubio, San and López-MuñozRubio 2004). Interestingly, the combination appears to work better for non-psychotic than psychotic depression. Results are less favourable for dysthymia. An open-label series of 141 patients who were partial responders or non-responders to SSRIs showed 50.4% response and 35% remission at 12 weeks when reboxetine was added (Reference López-Muñoz, Alamo and RubioLópez-Mu–oz 2007). In another case series, involving patients who had failed to respond to SSRIs (n = 43), venlafaxine (n = 12) or mirtazapine (n = 6), the addition of reboxetine to the current drug was effective (Reference Rubio, San and López-MuñozRubio 2004).
Side-effects
The combination of an SSRI with reboxetine is generally well tolerated and side-effects are largely related to effects of individual drugs. Nausea, headaches, nervousness with insomnia, urinary retention and periorbital oedema were reported, especially in combination with fluoxetine.
Tricyclic antidepressant combinations
The combination of TCAs with SSRIs has been considered in the previous section.
TCA with MAOI
The amount of serotonin and noradrenaline available in synaptic junctions can increase significantly if they are neither taken back (reuptake) nor destroyed (by a monoamine oxidase enzyme). This provides the basis for combining TCAs with MAOIs.
The combination of TCAs with MAOIs has been reported on in three double-blind controlled trials, two open-label trials, a controlled trial of the combination against electroconvulsive therapy, and many case series. Despite the positive reports of efficacy in case series (Reference White, Razani and SimpsonWhite 1982), the controlled trials are largely negative.
In a double-blind controlled trial of 135 outpatients with mild to moderate depression, most of whom had been previously treated with a TCA, trimipramine alone proved to be superior to the combination of an MAOI (phenelzine or isocarboxazid) with trimipramine or an MAOI alone (Reference Young, Lader and HughesYoung 1979). The combination was, however, found more likely to benefit women with severe depression lacking energy.
Another double-blind controlled trial (n = 79), which excluded treatment-resistant depression, found that the combination of amitriptyline and tranylcypromine had no advantage over either drug alone, although patients on the combination improved more according to their self-ratings after 6 weeks. A substantial proportion did not complete the study (23%) and the combined treatment was less well tolerated than single treatments (Reference O'Brien, McKeon and O'ReganO'Brien 1993).
In the third double-blind controlled trial of patients with depression, the combination of amitriptyline and tranylcypromine was not superior to either drug alone (Reference Razani, White and WhiteRazani 1983). This trial had been preceded by an open-label study by the same team, involving 30 newly admitted randomly assigned patients with depression, who were not necessarily treatment refractory. In this sample, the combination of amitriptyline and tranylcypromine was not superior to either drug alone and was associated with a slight increase in side-effects (Reference White, Razani and SimpsonWhite 1982).
The second open-label trial, of isocarboxazid and amitriptyline (n= 25), involved patients with major depression who had failed to respond to at least four previous antidepressants. Follow-up for 3 years of the 12 who responded to combination drugs showed that treatment efficacy diminished after 2 years (Reference Berlanga and Ortega-SotoBerlanga 1995).
In a controlled trial, electroconvulsive therapy proved superior to amitriptyline with phenelzine in 19 randomly allocated patients with depression previously treated ‘unsuccessfully with conventional psychotropic drugs at adequate doses’ (Reference Davidson, McLeod and Yone-LawDavidson 1978).
Side-effects
Most serious adverse events have occurred when a TCA has been added to an established MAOI treatment compared with the reverse sequence. It has been suggested that the safest option is to start MAOI and TCA simultaneously at low doses increasing slowly to a maximum of half that used with single-drug treatment (Reference White, Razani and SimpsonWhite 1982).
The most serious adverse reaction is serotonin syndrome (Table 1), which usually occurs very rapidly. Combination of TCAs with MAOIs was not advised owing to severe adverse reactions and fatalities (Reference Otte, Birkenhager and van den BroekOtte 2003). Imipramine and clomipramine appear to be particularly dangerous, with reports of serious adverse reactions, including serotonin syndrome. Reference Oefele, Grohmann and RutherOefele (1986) reported a fivefold increase in adverse reactions when clomipramine was combined with tranylcypromine compared with either drug alone or other TCA–MAOI combinations. Animal experiments suggest that trimipramine is the safest of the TCAs in combination with MAOIs. It is suggested that TCAs with weaker serotonergic properties might be safer with respect to serotonin toxicity.
The combination of an MAOI with a TCA might, at least theoretically, protect against the ‘cheese’ reaction. Tyramine uses the presynaptic noradrenaline transporter to enter the neuron, where it induces depolarisation-independent noradrenaline release. Antidepressants with noradrenergic reuptake inhibition properties will prevent tyramine entry and will therefore attenuate the response.
Other side-effects are due to the synergism of the two drugs and include orthostatic hypotension, dizziness, headache, urinary retention, weight gain and nausea, all of which can be caused by either drug alone.
TCA with moclobemide
There is a potential for synergism with the combination of dual reuptake inhibition from a TCA and monoamine oxidase inhibition from a monoamine oxidase A enzyme reversible inhibitor. One small RCT (n = 58) (Reference Tanghe, Steeman and BollenTanghe 1997), one open-label trial (n = 14) (Reference König and WolfersdorfKönig 1997) and a short report (n = 18) have published on this combination (Reference Steinberg, Jost and WeessSteinberg 1994). The RCT showed a non-specific trend towards faster onset of action in the combination group (amitriptyline and moclobemide), but also reported increased agitation. In the open-label trial, more than 50% of the sample responded to the combination when a dose of 300 mg/day of moclobemide was added to a TCA stabilised at an average dose of 180 mg/day trimipramine equivalents (Reference König and WolfersdorfKönig 1997).
Side-effects
Moclobemide is relatively free of any CYP inhibition effect. Agitation and inner restlessness were the most commonly described adverse events when combining TCAs and moclobemide. Hypomanic switches were reported in the RCT group of in-patients with treatment-resistant major depression (Reference Tanghe, Steeman and BollenTanghe 1997). Hypertensive crises may occur, especially in patients with pre-existing hypertension (Reference König and WolfersdorfKönig 1997).
TCA with mirtazapine or mianserin
Mianserin predominantly blocks α2-autoreceptors, leading to increased noradrenergic transmission. Its effect on α2-heteroreceptors present in serotonin neurons is mitigated by its direct α1-blocking effect. This reduces the serotonergic effect expected from such heteroreceptor blockade. Therefore, combining mianserin with TCAs that have a serotonergic profile might provide additive antidepressant efficacy.
There are two double-blind controlled studies of TCAs used in combination with mianserin (Reference Lauritzen, Clemmesen and KlysnerLauritzen 1992; Reference Medhus, Heskestad and TjemslandMedhus 1994). These reported encouraging results, although the numbers were small (total n = 57) and the treatment period was brief. In the first of the two (Reference Lauritzen, Clemmesen and KlysnerLauritzen 1992), imipramine was started at a low dose (25–50 mg/day depending on age), aiming for a plasma level of >200 nmol/l, and mianserin was given at a dose of 30 mg/day.
As far as we are aware, there are no studies that investigate the combination of TCAs with mirtazapine, although the principles behind the combination would be similar to those for mianserin.
Side-effects
No additional safety issues of the combination compared with a TCA alone were reported.
TCA with SNRI
Both TCAs and SNRIs act through noradrenaline and serotonin reuptake inhibition and therefore it is illogical to combine them. Venlafaxine might be useful in achieving an antidepressant ‘top-up’ effect for patients who require a higher TCA dose than they could tolerate, but there is no direct clinical evidence for this. Desipramine and venlafaxine may act via different noradrenergic reuptake mechanisms and systematic trials of this combination have been encouraged (Reference Gómez Gómez and Perramón TeixidóGómez Gómez 2000).
There is one small (n = 11) open-label trial of venlafaxine combined with a TCA (clomipramine or imipramine) in patients with depression, who had a partial response to TCAs but failed to respond to heterogeneous augmentation strategies. Of the sample, 82% responded, with 64% achieving full remission which in the majority was maintained at 2 years. A stable dose of around 200 mg/day of clomipramine or imipramine was used, to which venlafaxine was added and titrated from 75 to 300 mg in divided doses (Reference Gómez Gómez and Perramón TeixidóGómez Gómez 2000).
Side-effects
Reported side-effects with the combination include mild hypersomnia, sexual dysfunction after dose increases, constipation and weight gain. No significant changes in blood pressure, heart rate, blood analyses or electrocardiogram were described. Venlafaxine has little effect on CYP2D6 and therefore should not have a significant impact on TCA levels; dose adjustments in combinations may not be necessary. Venlafaxine may produce a modest increase in the desmethyl metabolite of imipramine, although the clinical significance of this is unclear.
SNRI combinations
The SSRI–SNRI and TCA–SNRI combinations have been considered in the previous sections.
Venlafaxine with mirtazapine
Venlafaxine and mirtazapine act synergistically to boost noradrenergic, serotonergic and dopaminergic transmission through monoamine reuptake inhibition and α2-blockade. Theoretically, this offers one of the most potent mechanisms of manipulating the monoamine system, leading to its nickname of ‘California rocket fuel’.
Three studies report on the combination of venlafaxine and mirtazapine, including a 12-week randomised controlled trial (STAR*D, n = 51), a 6-week open-label trial (n= 35) and a retrospective chart review (n= 32).
In the STAR*D study (Reference McGrath, Stewart and FavaMcGrath 2006), high-dose extended-release venlafaxine was combined with mirtazapine and compared with the MAOI tranylcypromine in adult outpatients with non-psychotic depression. Overall, 13.7% achieved remission (as defined by a score ≤7 on the Hamilton Rating Scale for Depression (HRSD)); these patients had previously failed to respond to three medication trials. Venlafaxine (extended release) was started at a low dose, built up to a mean dose of 210.3 mg/day in combination with mirtazapine gradually titrated to a mean of 35.7 mg/day.
In the open-label trial of out- and in-patients with depression who had not responded to adequate monotherapy with two antidepressants, the addition of mirtazapine (15–30 mg/day) to either an SSRI (n= 23) or venlafaxine (n= 12) led to remission in half of the patients. The decrease in HRSD scores in patients on venlafaxine was higher than in patients on SSRIs (P = 0.013) (Reference Aydemir, Taskin and DeveciAydemir 2005).
In the retrospective chart review, 32 patients with recurrent depressive disorder who had previous treatment trials (1–6 trials) received the combination of venlafaxine and mirtazapine: 50% showed improvement at 8 weeks (Reference Hannan, Hamzah and AkinpeloyeHannan 2007).
The combination of mirtazapine and venlafaxine (n = 4) was also included in the Reference Carpenter, Yasmin and PriceCarpenter et al (2002) study discussed earlier.
Serotonin syndrome has been reported even during a cross-taper. It is important to be aware of the potential for serotonin syndrome despite reports that mirtazapine may be less likely to cause serotonergic toxicity. Weight gain and sedation may be prominent and related to mirtazapine. In the STAR*D sample, 22.4% had a mild, 24.5% moderate and 6.1% severe to intolerable side-effect burden (Reference McGrath, Stewart and FavaMcGrath 2006).
SNRI with trazodone
This combination has been tried with a similar rationale to the SSRI–trazodone combination. A prospective 4-week semi-naturalistic study (n= 50) in in-patients with depression reported that although clinicians expected improvement of both insomnia and inner agitation with the addition of trazodone to venlafaxine, only insomnia improved (Reference Bertschy, Ragama-Pardos and MuscionicoBertschy 2005). Evidence is too scarce to comment further on this combination.
Venlafaxine with reboxetine
Employing the same rationale as SSRI–reboxetine combination, reboxetine has been added for patients not responding to venlafaxine alone in an open-label series – reasonable response rates have been reported (Reference Alamo, López-Muñoz and RubioAlamo 2007). Any synergism of such a combination is doubtful, as both drugs act via the same mechanism; the same effects could be achieved by a higher dose of venlafaxine alone, with more predictable pharmacokinetics. No serious adverse effects were reported in this series.
MAOI combinations
Combinations of SSRI–MAOI and TCA–MAOI have been considered in the previous sections. No studies were found for the MAOI–SNRI combination.
MAOI with trazodone
This combination has been tried with a similar rationale to the SSRI–trazodone combination. Two studies have reported on the use of trazodone for the treatment of insomnia in patients established on an MAOI. Both included a heterogeneous diagnostic sample. The first was an open pilot study (n= 48) and reported a sustained hypnotic effect in a large majority of the patients (Reference JacobsenJacobsen 1990). The second was a case series (n= 13) in which 69% of patients experienced a sustained benefit when a mean dose of trazodone 85 mg/day was added to an established mean dose of phenelzine 50 mg/day (Reference Nierenberg and KeckNierenberg 1989).
Side-effects
Side-effects included orthostatic hypotension, daytime sedation and mania in one patient with bipolar disorder. One patient experienced nocturnal myoclonus, which may have reflected a hyperserotonergic state. Serotonin syndrome can occur with this combination (Box 2).
BOX 2 Problems with the evidence base for combining antidepressants
-
• Weak evidence – very few randomised controlled trials
-
• Data from heterogeneous populations – various diagnosis, symptom profiles, severity and duration of illness
-
• Outcomes defined and measured variably response v. remission
-
• Duration of treatment before and after combinations varies widely
-
• Various methods of combination – different sequences with widely varying doses
Clinical implications
This review highlights the paucity of and problems with the evidence base for antidepressant combinations in the management of depression (Box 2). There are very few RCTs and an even greater scarcity of those with adequate size and study designs that are able to determine the efficacy of combinations v. monotherapy with the individual drugs alone (Table 2). It also highlights a number of combinations with established risks and toxicity, and indicates that some combinations are either illogical from a psychopharmacological perspective or unpredictable. Several combinations have a low benefit:risk ratio and should be avoided, and most should only be used with a second opinion and/or specialist advice and support.
The best evidence is for the combination of an SSRI with an NaSSA or trazodone – this combination received some support in the NICE guidelines (National Institute for Health and Clinical Excellence 2004). There is evidence that this combination shows greater efficacy than either drug alone, is well tolerated and carries a low risk of serious interactions. Nonetheless, it is mandatory to carefully monitor such combinations and avoid the routine use of high doses of both drugs. There is some evidence for TCAs with NaSSAs and for SNRIs with NaSSAs, but the evidence base is weak and these combinations cannot therefore be recommended in routine clinical practice.
Recent meta-analyses have shown stronger data for switching to a drug in a different class (Reference Papakostas, Fava and ThasePapakostas 2008) or augmentation of antidepressants with psychotherapy (Reference Pampallona, Bollini and TibaldiPampallona 2004), lithium (Reference Bauer and DopfmerBauer 1999) or atypical antipsychotics (Reference Papakostas, Shelton and SmithPapakostas 2007), suggesting that these strategies should be logical next steps in the management of treatment-resistant depression before employing a combination strategy. Because of the paucity of data and varying degrees of treatment resistance in studies to date, it is currently not possible to derive adequate algorithms for the management of treatment-resistant depression. For example, it is not clear where augmentation or combination strategies should fit in with respect to electroconvulsive therapy, which a number of studies have suggested gives the greatest degree of efficacy even in patients with treatment-resistant non-psychotic depression (Reference Husain, Kevan and LinnellHusain 2004).
The decision to employ a particular combination must be based on evaluation of each patient's clinical status (including the severity of key target symptoms). Patients should be informed about the state of the evidence base and enter into a trial of these combinations with this information fully explained and shared. Both the practitioner and the patient need to be aware of the potential risks of using a combination strategy as opposed to an alternative strategy and should set up an active monitoring system.
MCQs
-
1 A patient with treatment-resistant non-psychotic depression has just been started on a new antidepressant combination. He develops hyperthermia, agitation and diarrhoea. A possible diagnosis is:
-
a neuroleptic malignant syndrome
-
b serotonin syndrome
-
c ‘cheese’ reaction
-
d pseudocholinergic syndrome
-
e lithium toxicity.
-
-
2 The following combination has a plausible neurochemical basis:
-
a mirtazapine and venlafaxine
-
b venlafaxine and reboxetine
-
c TCAs and reboxetine
-
d SNRIs and SSRIs
-
e SSRIs and SSRIs.
-
-
3 The following drug could be potentially fatal if combined with an SSRI:
-
a phenelzine
-
b moclobemide
-
c trazodone
-
d mirtazapine
-
e reboxetine.
-
-
4 Trazodone is used in combination with SSRIs for:
-
a obsessions
-
b akathisia
-
c hallucinations
-
d insomnia
-
e myoclonus.
-
-
5 The following combination has been shown superior to the others listed in the management of patients with treatment-resistant depression:
-
a TCA with SSRI
-
b TCA with MAOI
-
c venlafaxine with mirtazapine
-
d moclobemide with SSRIs
-
e none of the above.
-
MCQ answers
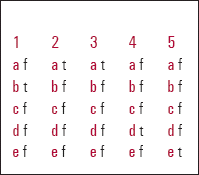
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | f | a | t | a | t | a | f | a | f |
| b | t | b | f | b | f | b | f | b | f |
| c | f | c | f | c | f | c | f | c | f |
| d | f | d | f | d | f | d | t | d | f |
| e | f | e | f | e | f | e | f | e | t |
TABLE 1. Clinically significant CYP450 interactions between antidepressants
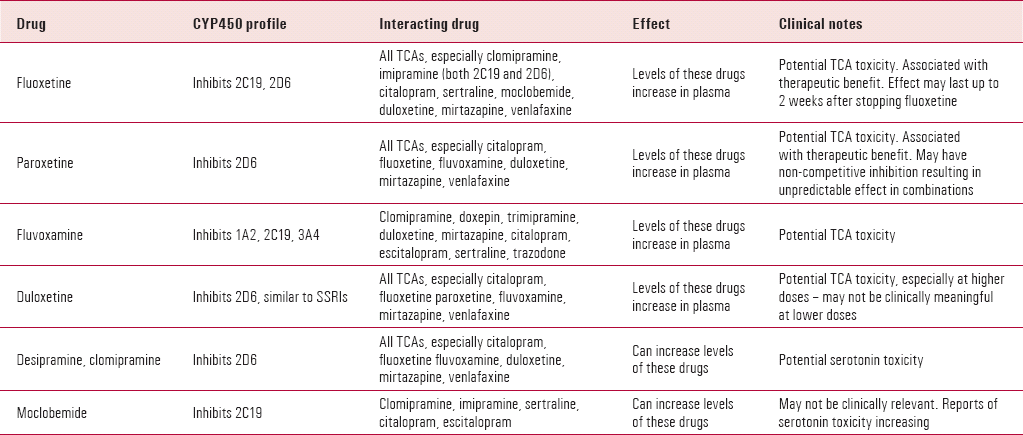
| Drug | CYP450 profile | Interacting drug | Effect | Clinical notes |
|---|---|---|---|---|
| Fluoxetine | Inhibits 2C19, 2D6 | All TCAs, especially clomipramine, imipramine (both 2C19 and 2D6), citalopram, sertraline, moclobemide, duloxetine, mirtazapine, venlafaxine | Levels of these drugs increase in plasma | Potential TCA toxicity. Associated with therapeutic benefit. Effect may last up to 2 weeks after stopping fluoxetine |
| Paroxetine | Inhibits 2D6 | All TCAs, especially citalopram, fluoxetine, fluvoxamine, duloxetine, mirtazapine, venlafaxine | Levels of these drugs increase in plasma | Potential TCA toxicity. Associated with therapeutic benefit. May have non-competitive inhibition resulting in unpredictable effect in combinations |
| Fluvoxamine | Inhibits 1A2, 2C19, 3A4 | Clomipramine, doxepin, trimipramine, duloxetine, mirtazapine, citalopram, escitalopram, sertraline, trazodone | Levels of these drugs increase in plasma | Potential TCA toxicity |
| Duloxetine | Inhibits 2D6, similar to SSRIs | All TCAs, especially citalopram, fluoxetine paroxetine, fluvoxamine, mirtazapine, venlafaxine | Levels of these drugs increase in plasma | Potential TCA toxicity, especially at higher doses – may not be clinically meaningful at lower doses |
| Desipramine, clomipramine | Inhibits 2D6 | All TCAs, especially citalopram, fluoxetine fluvoxamine, duloxetine, mirtazapine, venlafaxine | Can increase levels of these drugs | Potential serotonin toxicity |
| Moclobemide | Inhibits 2C19 | Clomipramine, imipramine, sertraline, citalopram, escitalopram | Can increase levels of these drugs | May not be clinically relevant. Reports of serotonin toxicity increasing |
TABLE 2. Summary of studies considered in this reviewa
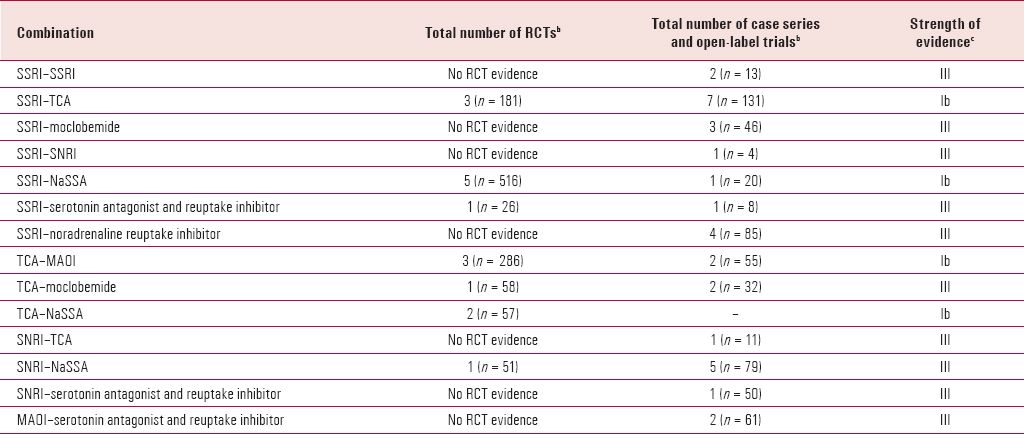
| Combination | Total number of RCTs b | Total number of case series and open-label trials b | Strength of evidence c |
|---|---|---|---|
| SSRI–SSRI | No RCT evidence | 2 (n = 13) | III |
| SSRI–TCA | 3 (n = 181) | 7 (n = 131) | Ib |
| SSRI–moclobemide | No RCT evidence | 3 (n = 46) | III |
| SSRI–SNRI | No RCT evidence | 1 (n = 4) | III |
| SSRI–NaSSA | 5 (n = 516) | 1 (n = 20) | Ib |
| SSRI–serotonin antagonist and reuptake inhibitor | 1 (n = 26) | 1 (n = 8) | III |
| SSRI–noradrenaline reuptake inhibitor | No RCT evidence | 4 (n = 85) | III |
| TCA–MAOI | 3 (n = 286) | 2 (n = 55) | Ib |
| TCA–moclobemide | 1 (n = 58) | 2 (n = 32) | III |
| TCA–NaSSA | 2 (n = 57) | – | Ib |
| SNRI–TCA | No RCT evidence | 1 (n = 11) | III |
| SNRI–NaSSA | 1 (n = 51) | 5 (n = 79) | III |
| SNRI–serotonin antagonist and reuptake inhibitor | No RCT evidence | 1 (n = 50) | III |
| MAOI–serotonin antagonist and reuptake inhibitor | No RCT evidence | 2 (n = 61) | III |





eLetters
No eLetters have been published for this article.