Sugars are undoubtedly the most important dietary factor in the development of dental caries(1–Reference Zero8) and dental caries is the most common chronic disease in the world(Reference Petersen, Bourgeois and Ogawa9). Treating caries is responsible for 6–10 % of total health costs, even though there has been a decline in dental caries levels in many countries(Reference Beaglehole, Benzian and Crail10, Reference Patel11). A significant relationship between sugars and caries persists despite the regular wide-scale use of fluoride toothpaste and fluoridated water(Reference Moynihan and Kelly4, Reference Marthaler5).
Recommendations by national governments and the WHO on a safe level of sugar intake suggest that intakes should be less than 10 % of energy intake (%E) based on the shape of the dose–response relationship between national sugar intakes and incidence of dental caries within one year post eruption in the permanent incisor teeth of 12-year-old children(1). That approach, first developed by Sheiham(Reference Sheiham6, Reference Sheiham7, Reference Sheiham12), was in keeping with Newbrun’s proposition from animal studies of a sigmoid-shaped curvilinear relationship between level of dietary sugars and caries incidence(Reference Newbrun13). That dose–response relationship became the accepted basis for establishing a limit on ideal sugar intakes and for monitoring dental caries in 12-year-old children as the basis for assessing acceptable levels of sugar consumption(Reference Sreebny14). The worldwide prevalence of dental caries is still unacceptably high in adolescents and adults despite the decrease in severity in 12-year-olds(Reference Patel11). Therefore, the objectives of the present paper were to examine: (i) the quantitative relationship between sugar intakes and the progressive development of dental caries; (ii) the validity of using a sigmoid-shaped curvilinear relationship between the level of dietary sugars and caries incidence in children; and (iii) whether the current dental approach mainly using topical fluoride or water fluoridation markedly reduces caries levels in adults. In the current paper, sugar intakes in different studies were considered as free sugars, added sugars, sucrose, non-milk extrinsic sugars or total sugars.
Methods
A critical in-depth review of previous international studies was conducted. The studies included had both sugar intakes monitored with detailed examination of the caries prevalence of individual teeth by trained professionals, taking account of the national state of fluoridation of water and the use of fluoridated toothpastes. Methods included reviewing relevant studies included in the most recent systematic review and meta-analysis conducted by Moynihan and Kelly(Reference Moynihan and Kelly4) on the relationship between levels of sugars and dental caries. A reanalysis of the dose–response between dietary sugars and caries incidence in teeth with different levels of susceptibility to dental caries in children was carried out using data from the classic Japanese studies conducted by Takeuchi and co-workers. Takeuchi analysed caries rates in each tooth type and by post-eruptive tooth age in large representative cohorts of Japanese children from before, during and after World War II(Reference Takeuchi15–Reference Koike20). Each tooth was monitored annually in children of known ages during the period when sugar intakes dropped dramatically in World War II to a low of 0·2 kg/capita per year, i.e. ≈0·1 %E, in 1946. These data are unique in relating the speed and differential tooth sensitivity to dental caries with different prevailing sugar intakes.
Analyses were undertaken of two lower-income countries, Tanzania and China, with sugar intakes below 15 %E. Data were also analysed from an Iraqi study where sugar intakes changed markedly over several years due to UN sanctions(Reference Jamel, Plasschaert and Sheiham21).
The diagnosis of caries was based on overt dental cavitation, despite the process of caries development having a long pre-cavitation phase(Reference Mejàre, Kallestal and Stenlund22, Reference Mejàre, Stenlund and Zelezny-Holmlund23). Nevertheless, for a conservative analysis, the review was confined to the quantification of cavitated caries incidence and accumulated rates.
Results
A scrutiny of a previous systematic review(Reference Moynihan and Kelly4) and an update on developments in 2012 and 2013, together with an analysis of FAO food balance sheets(24), revealed that sugar intakes in most countries are now well in excess of 10 %E, so either historical data or unusual settings were required to assess the full range of sugar intakes and their impact on caries development.
Reanalysis of Takeuchi studies
Takeuchi(Reference Takeuchi15–Reference Koike20) and co-workers noted marked differences in tooth susceptibility to caries: incisor teeth had about twice the resistance to caries compared with molar teeth. There was a log-linear relationship to sugar intakes and caries across the spectrum of exposures to sugars (Fig. 1). When the choice of teeth was standardised, the time since the teeth had erupted was also important (Fig. 2). The incidence of caries continued at very low sugar intakes even in teeth that had been erupted for 12 years, i.e. when children were entering adulthood. The cumulative effect of these processes was then evident in both susceptible (molar teeth, see Fig. 3(b)) and the most resistant incisor teeth (Fig. 3(a)) by the time 8 years had elapsed after tooth eruption, e.g. with children aged between 14 and 16 years on the maximum sugar intake of 30 kg/year (16·4 %E). Then 70 % of this group had dental caries. Even on 10 %E, equivalent to about 18·25 kg/capita per year, a third of these children had dental caries in their most resistant teeth, the incisors. The studies were conducted before, during and after World War II when per capita average sugar levels decreased from 15 kg/year (≈8·2 %E) to 0·2 kg (≈0·1 %E) and then increased to levels above 25 kg/year (≈13·7 %E)(Reference Takeuchi15).

Fig. 1 Regression lines of the correlation between annual sugars consumption and annual caries incidence rates by type of teeth(Reference Sheiham12). The average caries rates on a log-linear scale are shown for the two first molars (tooth number 6) with the bar at the top representing the molars in the upper jaw and the bar at the bottom representing those in the lower jaw; the average caries rates for tooth number 7 relate to the second upper and second lower molars. Tooth 1 is the central lower and tooth 2 the lateral lower incisors. Note the incisors are more resistant than the molars, which show the same incidence of cavitation at about half the sugar intake needed for the incisors. Sugar intakes of 5 kg/capita per year are equivalent to about 2·7 %E and 15 kg/capita per year to 8·2 %E, where %E is percentage of energy intake. (Adapted from Sheiham(Reference Sheiham12))

Fig. 2 The incidence rates (Mx) for different post-eruptive years, from 1 to 12 years, and annual sugar consumption for the upper and lower first molars (tooth number 6), showing the slowly increasing resistance of teeth to sugar-induced caries after their eruption but with a log-linear effect still evident in older teeth that have been exposed for 10–12 years(Reference Takeuchi15). Mx, the annual caries incidence rate=number of teeth newly attacked by caries during x years of post-eruptive age/number of sound teeth at x years of post-eruptive age. (Adapted from Takeuchi(Reference Takeuchi15))

Fig. 3 Cumulative numbers of upper central teeth (a) and first molar teeth (b) affected by caries per 1000 teeth plotted on a log scale, by post-eruptive tooth age and annual sugars consumption(Reference Takeuchi, Shimizu and Takehisa16). In 1971, when these data were published, the average national sugar intake in Japan from FAO food balance sheets was about 11·7 %E (≈21·4 kg/year), where %E is percentage of energy intake. (Adapted from Takeuchi et al.(Reference Takeuchi, Shimizu and Takehisa16))
Studies in populations with low sucrose intakes
Numerous studies from decades ago showed that in countries where sugar consumption was very low, dental caries was almost non-existent(Reference Sheiham25–Reference Fisher27). Sheiham reported that 98 % of Nigerians of all ages had no experience of caries in 1964(Reference Sheiham25). In 1938 sugar consumption of inhabitants of the Island of Tristan da Cunha was 1·8 g/capita per d (0·4 %E) and in 1937 the proportion of 13–19-year-olds with caries was 2 %. Sugar intakes increased to 150 g/d (30 %E) by 1966 and the prevalence of caries increased to 17·5 %. In 40–49-year-olds the increase was from 11 % to 32 %(Reference Holloway26, Reference Fisher27).
New analyses from low-income countries like Tanzania and China, with sugar intakes of 9·08 kg/capita per year (5 %E) and 6·9 kg/capita per year (3·8 %E), respectively, showed caries levels eight to nine times higher in adults than in children. The number of decayed, missing and filled teeth (DMFT) was 0·30 in Tanzanian and 0·53 in Chinese 12-year-olds compared with 2·9 and 4·5, respectively, in 35–44-year-olds.
Effects of wartime sugar rationing and sanctions
Meta-analyses confirm the statistical relationship between the average sugar intakes and the prevalence of caries(Reference Moynihan and Kelly4, Reference Downer28, Reference Masood, Masood and Newton29), reinforcing the ecological studies with in effect national interventions induced by wartime sugar rationing and sanctions on sugar imports. Iraq(Reference Jamel, Plasschaert and Sheiham21) showed the marked responsiveness of caries to prevailing sugar intakes: levels of dental caries in Iraqi children halved after UN sanctions that reduced sugars from 50 kg/capita per year (27·4 %E) before sanctions to 12 kg/capita per year (6·6 %E) five years later (Table 1)(Reference Jamel, Plasschaert and Sheiham21). Similar but less detailed observations come from World War II studies in Norway and Britain(Reference Toverud30–Reference Knowles32). Norwegian children aged 6–12 years consuming about 28·5 g sugar/d (5·7 %E) had lower caries levels than pre-war levels, but the prevalence was still high(Reference Toverud30). In Britain, wartime sugar consumption fell to 30 kg/capita per year (≈16·4 %E) and led to a 43 % decrease in caries in 12-year-olds but 74 % still had four or more decayed teeth. However, 51 % of Jersey children aged 3–7 years eating about 160 g sucrose/week (or ≈4·6 %E) until 1944 were caries free compared with 11 % of children evacuated from Jersey to England(Reference Knowles32). Using data from eighteen countries in 1959 on the relationship of sucrose consumption and average number of DMFT of 10–12-year-old children, Buttner(Reference Buttner33) revealed a high positive correlation: r=0·95.
Table 1 Levels of dental caries in Iraqi children before and after the UN sanctions (UNS) that reduced sugars from 50 kg/capita per year (27·4 %E) before UNS, to 12 kg/capita per year (6·6 %E) five years later(Reference Jamel, Plasschaert and Sheiham21)

%E, percentage of energy intake; dmft/DMFT, decayed, missing and filled teeth.
* Dental caries data for 6–7-year-olds include caries in the primary dentition (dmft), whereas data for older age group are for the permanent dentition (DMFT).
Dental caries in adults
Dental caries is commonly considered a disease of children. However, as will be shown here, it is apparent that more caries occurs in adults than in children. This indicates that a sugars-induced dental disease progresses despite the recent decline in dental caries in children(Reference Petersson and Bratthall34). Figures 1 and 2 apply to Japanese children over half a century ago where the water supply was not fluoridated and when fluoride toothpastes were unavailable. New analyses from Japan(Reference Kawashita, Kitamura and Saito35), but taking surveys of all age groups spanning the last half a century reveal that the rate of accumulation of caries continues in adulthood with a maximum accumulation evident in the fourth decade of life (Fig. 4). So the burden of caries is far greater in adults than in the 12-years-olds usually monitored in epidemiological studies. The progressive secular increase in dental caries in the 30–80-year-olds is consistent with the slowly increasing sugars content of the Japanese diet. In the young, however, there has been a declining incidence since about 1980 when sugar consumption levels decreased and fluoride toothpastes in Japan became increasingly available(Reference Kawashita, Kitamura and Saito35) (Fig. 4).
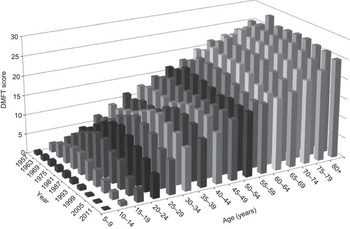
Fig. 4 Mean numbers of decayed, missing and filled teeth (DMFT) in Japan from nine national dental surveys conducted at 6-year intervals from 1957 to 2005 and presented sequentially for each age group from 1957 to 2005, showing the increase in DMFT with age and that most of the increase in DMFT occurred in adults(Reference Kawashita, Kitamura and Saito35). FAO food balance sheet data suggest sugar intakes of 7 %E in 1961 increasing to 11·2 %E in 1985 and with intakes of 9·6 %E in 2005, where %E is percentage of energy intake. (Adapted from Kawashita et al.(Reference Kawashita, Kitamura and Saito35))
Given the interactions between sugar-induced caries and the preventive role of careful repeated dental checks with plaque removal and improved dental hygiene and the use of toothbrushing, cohort analyses in affluent societies were sought. Figure 5 shows recent data from a longitudinal study in Dunedin, New Zealand where preventive dental work and specific advice on daily toothbrushing with fluoride toothpaste continued for 27 years in 955 children from 5 to 32 years of age(Reference Broadbent, Thomson and Poulton36). There is no suggestion of any diminution in the linear rate of caries development on sugar intakes varying from about 15·3 %E in 1973 to 17·5 %E in 2000. Thus despite the extensive repeated dental care and use of fluoride toothpaste, caries persisted and progressively increased with age in the majority of subjects.

Fig. 5 Trend lines showing increasing levels of caries of individuals when aged 5 years and followed through into adulthood until they are 32 years of age. Each line depicts the caries level of an individual, where DMFS is decayed, missing and filled surfaces(Reference Broadbent, Thomson and Poulton36). (Published with permission)
Even when high percentages of the water supplies have been fluoridated as in Australia(Reference Slade, Sanders and Do37) and Ireland(Reference Whelton, Crowley and O’Mullane38), there was an increase in DMFT with age and most of the disease was in adults, not children. In Australia, mean numbers of DMFT and decayed and filled tooth surfaces increased tenfold from the youngest to the oldest age groups. Adults with >75 % of lifetime exposure to fluoridation relative to <25 % of lifetime exposure had 11 % and 10 % fewer DMFT and reductions of 30 % and 21 % in decayed and filled tooth surfaces in the pre-1960 and 1960–1990 cohorts, respectively(Reference Slade, Sanders and Do37). In Ireland with fluoridated water supplying drinking water to 73 % of the population for over 50 years, the number of DMFT in 12-year-olds was 1·30 and in 35–44-year-olds was 15·0. Their 2000–2002 National Dental Survey showed that the numbers of DMFT of persons in non-fluoridated and fluoridated areas were 5·2 v. 4·6 in 16–24-year-olds, 16·0 v. 13·3 in 35–44-year-olds and 26·7 v. 25·9 for ≥65-year-olds, respectively(Reference Whelton, Crowley and O’Mullane38).
Discussion
The most remarkable data on the relationship between dietary sugars and caries in man come from the meticulous sequential studies in Japan(Reference Takeuchi15–Reference Koike20) where it became clear that the dose–response relationship is log-linear or arithmetically curvilinear even though the diagnostic level of caries used was cavitation; a late stage of caries(Reference Fejerskov39). There is no evident threshold for sugars below which there are no adverse effects. So in children there is a log-linear relationship in increased rates of caries even for sugar intakes between 2 kg/year (≈1·0 %E) and 5–7·5 kg/year (≈2·7 %-4·1 %E) in teeth erupted for 7–8 years. However, Fig. 5 emphasises that whatever the prevailing level of relatively high sugar intake, the actual annual accumulation of caries progresses linearly throughout life.
The unique feature of the detailed repetitive Japanese data proved important in showing differential susceptibility to caries by tooth type and post-eruptive tooth age. Lower first molars are the most susceptible and incisors the least(Reference Batchelor and Sheiham40, Reference Korhonen, Käkilehto and Larmas41) and the regression lines between incidence of caries and annual sugar consumption differed for each kind of tooth(Reference Takahashi18). At each level of sugar consumption incidence of caries was higher in lower than upper molars; upper incisors were the least susceptible (Fig. 1). Okuya(Reference Okuya19) showed that when annual per capita sugar consumption decreased from 15 kg (≈8·2 %E) to 10 kg (≈5·5 %E), caries in second molars decreased but did not become zero. The correlation between sugars and caries was +0·7 and was log-linear, not sigmoid as previously suggested. The lowest caries prevalence (25 %) in 6–11-year-olds occurred between 1949 and 1951 when sugar levels were <15 kg/year (<8·2 %E)(Reference Takeuchi, Pu and Shimizu17). These prevalences were similar to those in various European countries with wartime sugar rationing(Reference Sognnaes42).
The fact that the relationship to dental caries holds for increases and decreases in sugars consumption in any given community limits the possibility of confounders affecting the relationship and shows that there is no ‘safe’ level of sugars for caries, contrary to what Sreebny(Reference Sreebny14) suggested. Even the most resistant upper central incisors had caries at sugar intakes <5 kg/year (≈2·7 %E) after they had been erupted for four or more years. For the susceptible molar teeth, a sugar intake of 15 kg/year (≈8·2 %E) seems almost maximal in its caries impact with only 3–5 years’ exposure in Japanese children without much, if any, fluoride(Reference Takahashi18). Indeed, 8 years post eruption molar teeth show a doubling in caries rates between sugar intakes of about zero and 5 kg/year (≈2·7 %E). This curvilinear dose–response relationship is supported by a later study in Japanese 12-year-olds by Miyazaki and Morimoto(Reference Miyazaki and Morimoto43), who showed a positive log-linear correlation of 0·91 and caries increasing as sugar consumption rose to a peak at 29 kg/year (≈15·9 %E) in 1973.
Early data from the WHO dental database clearly showed that even at low sugar availability levels in the 1970s, in countries with sugar consumption of <50 g/d (<10 %E) such as Bangladesh, Ethiopia, Nigeria and in Togo, where sugar intakes were <20 g sugar/capita per d (<4 %E), the number of DMFT in 12-year-old children was still 1·5 or above. These rates were higher than in some industrialised countries.
There are numerous later studies showing a strong relationship between sugars consumption and caries rates in children(Reference Sreebny14, Reference Silverstein, Knapp and Kircos44–Reference Routtinen, Karjalainen and Pienihakkinen47). Extrapolating from such studies suggested that for each increase of 25 g/d (5 %E) in sugars, one tooth per child would become DMF(Reference Fejerskov39) in the short term. Similarly, Szpunar et al.(Reference Szpunar, Eklund and Burt45) found that each additional 5 g/d (1 %E) predicted a 1 % increase in caries in their short-term (3-year) longitudinal study in a fluoride-rich, low-caries environment of 11–15-year-old US children.
The WHO originally accepted Sreebny’s Reference Sreebny1982 proposition(Reference Sreebny14) that 50 g/d (10 %E) intake was acceptable when the number of DMFT was below 3 in 12-year-old children. With the then high level of dental caries in children in affluent societies in the 1970s, WHO considered that a goal of DMFT of 3 for dental health in 12-year-olds(48) was appropriate. Thus the concept of 50 g/d as a safe sugars level seems to have been the basis for several countries to agree a daily sugars load of 50 g as the acceptable limit. So the proposition arose that 10 % of daily energy from sugars was acceptable to both governments and WHO(1). This meant that there was then little concern for the fact that numerous publications from low- and high-income countries such as the UK showed a significant linear dose–response relationship between caries in children and sucrose availability and that caries was highly prevalent in countries with sugar levels below 10 %E. Unfortunately, the acceptability of 50 g/d (10 %E) sucrose limit was reinforced by the very selective choice of finding an increased cavitation rate in less susceptible teeth that had only been erupted for one year in 6–12-year-olds when sugar intakes were nearly 10 %E(Reference Sheiham12).
The progressive nature of caries. Why the majority of caries occurs in adults, not in children
A major shortcoming of most research on the relationship between sugars and caries is that the relationship in adults has been largely ignored, with all the conclusions being based on children’s studies. That is equivalent to drawing conclusions on the relationship between consumption of fats and non-communicable diseases by looking only at children. Caries rates have declined in children with hardly any change in the total amount of sugars available in the diet, so some dental authorities have concluded erroneously that sugars are not a major determinant of caries provided fluoride toothpaste is used diligently. However, the majority of caries occurs in adults, not in children, because the disease is cumulative and rates of caries in individuals track from early childhood to adolescence and then into adulthood(Reference Sheiham and Sabbah49). In China as well as in Japan the cumulative effect of caries is evident, with the number of DMFT being 0·53 in 12-year-olds in 2005 compared with 4·0 in 35–44-year-olds and 14·6 in 65–74-year-olds(Reference Xiaoqiu50) at a time when the availability of sugar corresponds to about 3·3 %E. Indeed, universally, the majority of caries occurs in adults, not in children(Reference Kawashita, Kitamura and Saito35, Reference Xiaoqiu50–Reference Bernabé and Sheiham53). In a new study of twenty-six countries, very much higher caries levels were observed in adults than in children in all the countries studied. There was a fivefold increase in number of DMFT in 35–44-year-olds compared with 12-year-olds, with greater proportions of adults than children affected in all those countries(Reference Bernabé and Sheiham53). When annualised, the combined coronal and root surface caries increments experience of older people was between 0·8 and 1·2 new surfaces affected per year when sugar intakes were in the range of 14–18 %E, and exceeded increments reported for adolescents(Reference Thomson54). So the major burden of dental diseases and costs associated with caries as well as periodontal disease occurs in adults.
One reason why most caries occurs in adults is that fluoride, the main reason for the recent decline in caries in children, does not stop the progress of the caries process on some susceptible tooth sites(Reference Groeneveld55). The role of fluoride in caries prevention is ascribed to its ability to induce fluoroapatite formation from solutions of calcium and phosphate. It enhances remineralisation as pH is lowered by sugars and inhibits demineralisation of the tooth enamel, as fluoroapatite is less soluble than hydroxyapatite. Small amounts of fluoride in solution around the tooth inhibit demineralisation more effectively than incorporated fluoride and have a much greater caries-protective potential than a large proportion of fluoroapatite in enamel mineral. Fluoride also interferes with acid production from cariogenic bacteria(Reference Fejerskov39).
The costs of dental caries
Dental diseases are the most common of all chronic diseases in both industrial societies and lower-income countries(Reference Petersen, Bourgeois and Ogawa9). Their impact on individuals and society includes pain, discomfort, social and functional limitation and handicap, and effects on the quality of life(Reference Slade56). So the financial impact is relatively high. The treatment of dental diseases costs 5–10 % of total health expenditure in industrialised countries, with WHO estimating oral diseases as the fourth most expensive diseases to treat in most industrialised countries(Reference Petersen, Bourgeois and Ogawa9–Reference Patel11).
Current analysis indicates that the treatment of caries in low-income countries by traditional restorative dentistry for the permanent dentition would cost between £1024 ($US 1618) and £2224 ($US 3513) per 1000 children of mixed ages from 6 to 18 years. The cumulative cost of treating caries in children exceeds the financial resources available for the whole health care of children in the majority of poor nations, even before the greater costs of caries affecting adults are considered(Reference Yee and Sheiham57).
Indirect costs and impaired quality of life from dental caries
The costs increase if we take into account the resulting loss of productivity from the 164 million hours of work lost each year in the USA due to dental diseases and dental visits(58). In summarising the costs Casamassimo et al.(Reference Casamassimo, Thikkurissy and Edelstein59) noted that in the Philippines toothache is the most common reason for absence from school, which is likely to have a substantial impact on the child’s learning and therefore on his/her future earnings. In Thailand, 1900 hours of school were lost per 1000 children in 2008 because of dental problems and treatment(Reference Pongpichit, Sheiham and Pikhart60). In Sri Lanka, 53 % of 6-year-olds and in South Africa, 88 % reported having experienced oral pain in their lifetime(Reference Ratnayake and Ekanayake61, Reference Naidoo, Chikte and Sheiham62).
Impacts on children’s growth
A most important impact that caries and its consequences have on children is that the disease process affects their growth and weight gain(Reference Sheiham63). Children aged 3 years old with caries weighed about 1 kg less than control children(Reference Acs, Lodolini and Kaminski64) because toothache and infection alter eating and sleeping habits, dietary intake and metabolic processes. Disturbed sleep also affects the stress-related glucosteroid production. In addition, there is fall in blood Hb levels stemming from the infection-induced depression of erythrocyte production. Two controlled clinical trials have shown that young children with many untreated caries lesions are more likely to be underweight. Extraction of the severely affected teeth led to increases in weight gain. In addition, the children’s quality of life was improved after extraction of carious teeth: they slept and ate better(Reference Monse, Duijster and Sheiham65, Reference Alkarimi, Watt and Pikhart66).
Globalisation and demographic changes
During the past decade, repeated scientific reports signal an alarming increase in the global prevalence of dental caries in children’s and adults’ primary and permanent teeth, as well as coronal and root surfaces(Reference Bagramian, Garcia–Godoy and Volpe67). This coincides with the increasing penetration of lower-income markets by transnational companies and the marketing of soft drinks, confectionery and sugar-containing foods to previously unexposed lower-income countries. The severity of caries has become so serious in developing countries that a new dental index of caries, the PUFA index, has been developed: P=Pulp involvement, U=Ulceration of mucosa from caries, F=Fistula from abscess due to caries, A=Abscess due to caries(Reference Monse, Heinrich-Weltzie and Benzian68).
It is also important to recognise that the demography of lower-income countries is changing rapidly as fertility rates decline and life expectancy increases. Our previous focus on children’s dental health, while proper, now needs to be reframed in a public health context where, given the huge pressures on health-care budgets and the burgeoning populations of older adults in Africa, the Middle East, Asia and Latin America, there needs to be a new emphasis on the role of sugar intakes in inducing major suffering, demands for health care and societal costs. Clearly what is needed is a radical approach to limiting sugar intakes as well as ensuring that fluoride use in water and/or toothpastes is still promoted. Fluoridation, however, cannot be considered a substitute for major efforts to reduce population sugar intakes.
Acknowledgements
Acknowledgements: The authors are grateful for the help of Dr Shinsho Fumiaki in providing them with a translation of the relevant papers from Japan that allowed them to undertake these new analyses. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflicts of interest: None. Authorship: The idea for the paper was conceived by W.P.T.J. A.S. provided the scientific material and content for the paper and obtained permission for reproducing the graphs. Both authors drafted the paper. Ethics of human subject participation: Ethical approval was not required.








