INTRODUCTION
Legionnaires' disease (LD) is considered to be a frequent cause of bacterial community-acquired pneumonia [Reference Mandell1, Reference Dunowitz, Mandell and Mandell2]. There are conflicting data regarding its incidence, but most studies have found Legionella spp. to be responsible for 2–5% of acute sporadic community-acquired pneumonia [Reference Almirall3–Reference Marrie7]. These cases are believed to be acquired by inhalation of aerosolized bacteria or by microaspiration [Reference Fields, Benson and Besser8]. Human-made aqueous environments (e.g. cooling towers, air conditioners) were rapidly identified as a source of infection following the recognition of the disease [Reference Dondero9]. Potable water has also been shown to be contaminated with Legionella spp. [Reference Arnow, Weil and Para10, Reference Joly11]. There is now strong evidence that domestic water distribution systems are important reservoirs for Legionella and are responsible for a substantial number of sporadic community-acquired legionellosis (SCAL) [Reference Stout12–Reference Lim16]. However, very little is known about the relative burden of disease attributable to this source.
Residential electric hot-water tanks are particularly prone to Legionella contamination [Reference Joly11, Reference Lee, Stout and Yu17, Reference Marrie18]. In a study conducted in 211 homes in the province of Quebec (Canada), with 178 electricity-dependent and 33 oil- or gas-dependent tank water heaters, 39% of the former were contaminated by Legionella, as opposed to none of the fossil-dependent ones [Reference Alary and Joly19]. In homes with contaminated tanks, close to half of the distal hot-water distribution sites (taps and shower heads) also grew the organism. In a large case-control study, electric heaters were associated with LD [Reference Straus13]. Importantly, studies that have investigated the domestic origin of SCAL have been conducted in regions with only a minority of electrically heated water systems.
Canada is the world's second largest hydroelectricity producer [20]. In the province of Quebec (population 7·8 million), most (~90%) of the residential hot-water heaters are electricity-dependent. LD is a notifiable disease in Canada. Between 1989 and 2004 its incidence has fluctuated between 0·13 and 0·35 cases/100 000 persons-years. In Quebec, the incidence was comparable to the Canadian average during that period [21]. By 2004 the incidence in Quebec increased, partly due to greater access to the urinary antigen detection test, and rose above the Canadian average to reach an incidence of 0·89 cases/100 000 persons-years for 2008. For 2009, the estimated incidence is 0·64 cases/100 000 persons-years (T. Smith, Public Health Agency of Canada, and R. Dion, personal communications). Most cases are thought to be sporadic. The last outbreak observed in Quebec was identified in 1996 [Reference Bolduc22]. Because of the sustained high incidence of LD in Quebec, as well as the high rate of domestic hot-water systems contaminated by Legionella spp. in this province, we hypothesized that the proportion of SCAL patients with contaminated domestic potable hot-water systems would be higher than that observed elsewhere.
From a cohort of culture-proven SCAL patients residing in homes with private domestic hot-water tanks, the hot-water distribution systems were prospectively investigated for Legionella contamination. Using molecular fingerprinting methods, we determined the microbiological and epidemiological link between the Legionella spp. recovered from the domestic environment and the isolates that caused the patients' pneumonia.
METHODS
Case selection
Eligibility
All adult patients (aged ⩾18 years) living in the province of Quebec with documented culture-positive SCAL identified during the 11-year study period, from June 1998 to June 2009, were eligible for the study. Patients had to own their hot-water tank to be included.
Definitions
A nosocomial case was defined as a confirmed case of LD diagnosed ⩾48 h following admission to an acute healthcare institution. All other cases were considered as community-acquired. Of these, no distinction was made at recruitment between travel-associated or not. An outbreak was defined as two or more geographically linked cases. Other cases were considered as sporadic.
Recruitment
In Quebec, suspected Legionella spp. isolates recovered from clinical specimen by various clinical microbiology laboratories are sent to the provincial public health laboratory [Laboratoire de santé publique du Québec (LSPQ), Montréal, Québec] for identification confirmation and serogrouping. As legionellosis is a notifiable disease, the LSPQ reports all confirmed cases to regional public health agencies (18 throughout the province territory). Notified cases are initially investigated by these agencies. During the study period, the agencies were instructed to assess the eligibility of the patients to participate in the study. First, patients or their family members were contacted by telephone and the study procedures were explained. Upon patients' agreement to participate and after screening for eligibility the patients' details were passed to the research team, at Hôpital Maisonneuve-Rosemont (HMR), in Montréal. Patients or their representatives were again contacted by telephone to confirm their desire to participate, review eligibility, and make arrangements for a home visit and water collection. Patients were interviewed in their homes and a limited standardized epidemiological questionnaire was completed. Water samples were collected during the same visit. Families of patients who had died were contacted and included in the study only if the next of kin could answer specific questions about the deceased's life habits.
Ethics
Research protocol was submitted and approved by the HMR ethics guidelines for human experimentation. A written informed consent was obtained from each patient or their legal appointee.
Epidemiological questionnaire
Patients were questioned for demographic information, health condition, potable water consumption habits and other potentially significant exposures (hot tubs, spa pools, travel) that may have occurred within 2 weeks prior to onset of their LD. Recent diagnosis of pneumonia or recent symptoms compatible with lower respiratory tract infection among co-inhabitants of their home were also sought.
Residential potable water sampling
Water samples were collected in the homes of participating patients from four different sites: hot-water tank, kitchen taps, shower head and bath taps. Water temperature was measured at all sites. For the three latter sites, two series of 500 ml of water were collected. A first 500 ml was collected immediately at opening; a second 500 ml was collected once the water, which was left running, reached a measured maximal temperature. From the tank heaters, only the first 500 ml of water were collected from the drainage valve. There were a total of seven samples per home. Residents were told not to use hot water for at least 8 h prior to sampling. Water samples were transported to the HMR microbiology laboratory and stored at 4°C for a maximum of 24 h prior to culture.
Processing and culture of water samples
All water samples were processed at the HMR microbiology laboratory. They were concentrated using high-speed centrifugation (13 000 g, 15 min). The supernatant was aspirated and discarded in order to leave 10 ml of concentrated water, which was re-homogenized by shaking. A tenfold dilution was prepared from this pellet. Then, 0·1 ml of each of the diluted and undiluted solutions was inoculated on buffered charcoal yeast extract (BCYE) medium (Quelab, Canada) and on buffered cefamandole, polymyxin, anisomycin (BMPA) selective medium (Oxoid, Canada). No decontamination treatment was performed. There were a total of four plates per sample (28 plates per home). Plates were incubated at 37°C in a 5% CO2 incubator with humidified atmosphere and were examined on days 3, 5 and 7.
Identification and serogrouping
For clinical isolates, isolation and identification were performed according to each hospital's laboratory procedures. Suspect isolates were sent to LSPQ, as part of normal standard practice (see Recruitment section above). For water sample isolates, identification of possible Legionella colonies was performed at the HMR microbiology laboratory, according to accepted criteria [Reference Edelstein and Murray23]. Briefly, morphologically compatible colonies were characterized by assessment of autofluorescence, Gram staining and subculture on sheep blood agar and BCYE media. Suspected Legionella isolates were sent to LSPQ for further testing. If more than one compatible morphotype was present in a sample, all were further analysed. Randomly, in a few samples with a single compatible morphotype, more than one colony were isolated and sent as distinct isolates, to assess the possibility of the presence of different strains with similar macroscopic aspect. At LSPQ, L. pneumophila strains were identified by immunofluorescence (Monofluo Legionella pneumophila IFA test, Bio-Rad Laboratories, Canada) and serogrouped by slide agglutination for serogroups 1, 4, 5, 6 (L. pneumophila antisera, Denka Seiken, Japan). For other, less frequent species or serogroups, additional microbiological testing was conducted at the National Microbiology Laboratory (NML, Canada), using gas chromatography, 16S rDNA gene sequencing, direct fluorescence assay and latex agglutination. One isolate that was difficult to serogroup was sent to the Center for Disease Control and Prevention (CDC, USA).
Molecular fingerprinting
When Legionella was recovered from a patient's home, the isolate speciation and serogrouping were paired and compared to the patient's clinical isolate. Matching isolates were studied by DNA macrorestriction followed by pulsed-field gel electrophoresis (PFGE) at LSPQ. Agarose plugs were prepared with Genepath group 5 reagent kit (Bio-Rad Laboratories). Purified whole-cell DNA was treated with the restriction endonuclease SfiI (New England Biolabs, Canada). PFGE was run in a electric field system (GenePath, Bio-Rad Laboratories) at 14°C and 6 V/cm, with an increasing switch time of 5·3 s to 34·9 s for 20 h. Interpretation was realized according to Tenover's criteria [Reference Tenover24]. Isolates from patient no. 2 were compared using restriction-endonuclease analysis (REA), also referred as restriction fragment-length polymorphism (RFLP), since this was the method used at LSPQ at the time these isolates were recovered in the domestic water samples.
Statistical analysis
Data were analysed using Stata v. 10.0 (StataCorp, USA). Proportions were compared with the χ2 test or, when numbers were small, with Fisher's exact test. Means were compared with the two-tailed Student's t test, using Welch's correction when applicable.
RESULTS
Patients' characteristics
A total of 331 cases of legionellosis were declared in Quebec during the study period. Of these, 112 (34%) were culture-confirmed. About two thirds of the patients from these culture-confirmed cases were not eligible, mainly because the patients did not own their hot-water tank, or their LD infection was probably nosocomial. Very few patients or their next of kin (<5%) refused to participate in the study.
Thirty-six patients were enrolled in the study. Male to female ratio was 2:1 (24 men, 12 women) and median age was 62 years (range 30–85 years). Case-fatality rate was 36% (13/36). All 36 patients, except one who spent only 24 h in an emergency room, were hospitalized due to the severity of their pneumonia. All had at least one recognized risk factor for LD. Multiple comorbidities were observed in several patients (Table 1).
Table 1. Legionella serogroup according to patient risk factors (n=36)
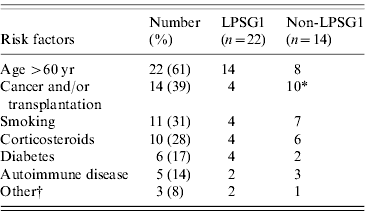
LPSG1, Legionella pneumophila serogroup 1; Non-LSPG1 includes non pneumophila species.
* P=0·003.
† Includes alcohol consumption (1), renal failure (1), chronic obstructive pulmonary disease (1).
Epidemiological questionnaire
Seven patients (19%) had spent at least one night away from home during the 2 weeks preceding onset of symptoms: five in a hotel (patient nos. 13, 24, 27, 29, 30) and two in a camping facility (patient nos. 21, 25). One patient had been in a spa pool (patient no. 28). No difference was found between the matching and non-matching cases with regards to water consumption habits including drinking tap-water, dish washing and showering (data not shown).
Clinical isolates
Most clinical isolates were L. pneumophila (33/36, 92%), predominantly of serogroup 1 (22/33, 67%). L. pneumophila isolates of patient no. 24 (clinical and environmental) could not be serogrouped with certainty (despite testing by NML and CDC). The serogrouping of another L. pneumophila isolate (patient no. 36) was not pursued further since no Legionella isolate was recovered from his home environment. Three non-pneumophila Legionella spp. were recovered in clinical specimens: L. cincinnatiensis, L. longbeachae and L. micdadei. Non-serogroup 1 L. pneumophila or non-pneumophila species were significantly associated with cancer and/or transplantation (OR 11·3, 95% CI 2·3–55·0, P=0·003) (Table 1).
Environmental isolates
Home water samples were obtained from each of the 36 participating patients' residences (Table 2). The median time to water collection from the patients' admission to healthcare facilities for their SCAL was 39 days (range 1–85 days). The mean delay was not different for the patients with matching isolates (40·6 days) than for those without matching isolates (40·7 days) (P=0·99). Legionella was recovered in 12 (33%) houses. Among contaminated systems the hot-water tank was the site most frequently positive (9/12, 75%; Table 2). Overall, 14 different Legionella strains were recovered (homes of patient nos. 11 and 32 had two different strains each). All were L. pneumophila. Five different serogroups were identified, but most were serogroup 1 (6/14, 43%; Table 3). One isolate was not characterized further than ‘LPSG other than 1, 4–6’, because that information was sufficient to eliminate similarity to the corresponding clinical isolate (patient no. 27). In houses colonized with two strains, each strain was recovered from distinct sites (Table 2). Further analysis of multiple colonies from plates with a single morphotype did not reveal unsuspected mixed culture. Potentially interfering bacteria were seen occasionally on BCYE media, but not on BMPA media.
Table 2. Individual patients' clinical and environmental isolates (n=36)
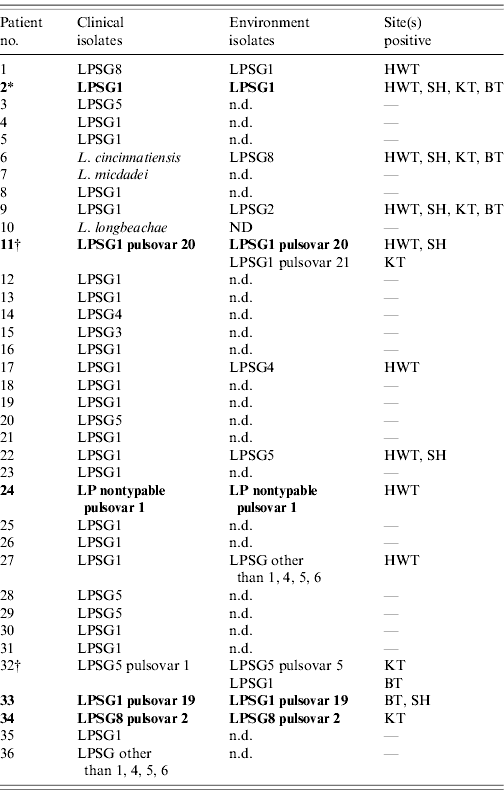
LPSG, Legionella pneumophila serogroup; n.d., not detected; HWT, hot-water tank; SH, shower head; KT, kitchen tap; BT, bath tap.
Bold rows indicate patients with matching isolates.
* Restriction fragment-length polymorphism.
† For homes with two different strains, positive sites for each are indicated on separate lines.
Table 3. Distribution of clinical and environmental isolates according to species and serogroup
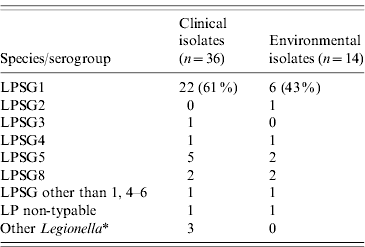
LPSG, Legionella pneumophila serogroup; LP, Legionella pneumophila.
* Includes L. cincinnatiensis (1), L. micdadei (1), L. longbeachae (1).
Matching isolates
Out of the 14 environmental isolates, identical species and serogroups corresponding to the clinical isolate were observed on six occasions (patient nos. 2, 11, 24, 32, 33, 34). Five pairs appeared to be the same strain by a fingerprinting method. RFLP profiles were identical for one case (patient no. 2). PFGE confirmed similarity in the four other patients (three indistinguishable, one closely related, according to Tenover's criteria).
Hot-water tanks and water samples
In 33 (92%) homes, hot-water tanks were heated by electricity. There were two oil- and one gas-dependent heaters. All the environmental isolates were found in electricity-dependent hot-water distribution systems (12/33, 36%). Water temperatures were available for 31 homes. To avoid variability introduced by sampling order or differences in water system structure, only the highest temperature measured at any site of a given home was considered as the hot-water temperature of the whole system. There was a significant relationship between hot-water temperature of the system and the recovery of Legionella (Table 4). The mean maximal temperature was higher in the fossil-dependent systems compared to the electricity-dependent systems, but the difference was not statistically significant (56·7 vs. 68·5°C, P=0·46). Mean water temperature collected at the drainage valves of heaters was 31·4°C.
Table 4. Legionella recovery according to water temperature
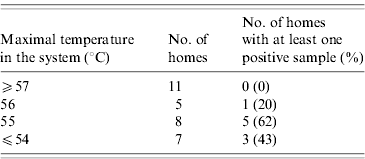
χ2 test for trend: P=0·013.
Seasonality
The occurrence of SCAL cases showed a seasonal distribution, with the majority observed during spring and summer. The highest numbers of cases were recorded in July and September (Fig. 1).
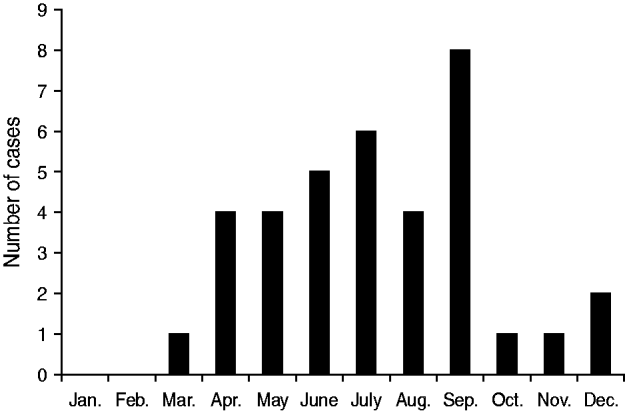
Fig. 1. Monthly variation in the number of culture-proven sporadic community-acquired legionellosis cases (n=36 patients).
DISCUSSION
Few studies have assessed the role of contaminated domestic water in the acquisition of SCAL using a prospective investigation with microbiological fingerprinting [Reference Stout12, Reference Straus13]. To our knowledge this is the first study to do so in a geographic region where the majority of hot-water tanks are electrically heated. Our study established that in 36 patients with culture-proven SCAL, 33% were living in a house where the residential hot-water system was contaminated with Legionella, mostly L. pneumophila. This contamination rate is at the high end of previously reported rates [Reference Arnow, Weil and Para10, Reference Lee, Stout and Yu17, Reference Stout25, Reference Borella26], and similar to previously reported rates in this province [Reference Joly11, Reference Alary and Joly19]. Despite this relatively high exposure rate, only 5/36 patients (14%) developed a pneumonia caused by a microbiologically similar Legionella isolate that contaminated their domestic hot-water distribution system. This is comparable to results previously reported among culture-proven cases in different geographic settings. In the Pittsburgh area, patients' homes were identified as the probable source of infection in 3/20 (15%) culture-confirmed cases of SCAL [Reference Stout12]. In two Ohio counties, homes of 3/42 (7·1%) culture-positive patients were contaminated with the same Legionella strain that caused infection in their residents [Reference Straus13].
Interestingly, we did not identify any other case of legionellosis in persons living in contaminated homes, as established by our questionnaire. Similarly during the duration of our study, we were not informed by any of the 18 regional health agencies of any other cases reported at the same civic address. Host risk factors probably explain this observation. Heudorf et al. in a case-control study reported that persons living in a building with a central hot-water tank heavily contaminated with Legionella spp. had anti-Legionella antibody titres twice as high as controls, but no difference in the rate of respiratory symptoms [Reference Heudorf27].
We observed a seasonal distribution in our study. Such an observation has already been reported in other Canadian studies [Reference Marrie7, Reference Ng28]. The reason for this pattern is currently unknown. Differences in water contacts (e.g. spa pools, hot tubs, cooling towers, fountains, sprinklers), changes in water ecology (abundance of free-living amoebas) or increase in travelling are possible explanations.
Legionella was not recovered uniformly from all sites sampled in contaminated homes in our study. Only three were positive at all sites and four had Legionella found strictly in the hot-water tank. Three of the systems with colonization limited only to the hot-water tank had a peripheral system maximum water temperature of 55°C. This proliferation at the central point of origin may be explained by the structural configuration of electric hot-water tanks. The position of the lower heating element in those vertical tanks is typically located a few centimetres above the bottom, thus creating a zone of lower temperature (contrary to fossil-dependent tank) at the very bottom of the tank, where sediments accumulate. It provides a permissive environment for biofilm formation and proliferation of microorganisms, including free-living amoebas and Legionella spp. [Reference Alary and Joly19]. In our study, the mean temperature at the lower drainage valve was markedly low (31·4°C). Inconsistencies in the recovery of Legionella at all sampled sites may also be explained by stagnant water niches secondary to the configuration of the plumbing system and peripheral accessories (e.g. shower heads, water spouts, etc.). It was not possible to obtain reliable information from the homeowners about the age of peripheral accessories.
Our data corroborate the association between Legionella colonization and water temperature. The World Health Organization recommends that hot-water temperature be maintained above 55°C [29]. It may be more critical for electric tanks, as our data showed that only a temperature of ⩾57°C completely prevents colonization. Concerns for skin burn when water heater temperatures are set at 60°C must be addressed. Anti-scald devices that control temperature at the exit points are among technologies of potential interest.
We recognize some limitations of our study. First is the limited number of subjects. Only about one tenth of the total cases and one third of culture-confirmed cases were included in the study. The duration of the study (11 years), number of regional health agencies involved (10/18 agencies), frequent changes that occurred in the human resources among these agencies and limited financial means all contributed to our incapacity to assure compliance in patient recruitment and to establish the exact number of patients approached and the precise reasons for their non-participation in the study. However, demographic profile in terms of age and sex and distribution of Legionella spp. were similar between the study participants and all declared cases and culture-confirmed cases (data not shown). Only cases living in Montreal were moderately over-represented among the study participants (P=0·06).
Culture-proven legionellosis probably represents the most severe form of the disease spectrum. All clinical Legionella spp. isolates identified in our patients were recovered following invasive diagnostic investigations, imposed by the severity of their illness. The high hospitalization rate (97%) and the high case-mortality rate (36%) observed in our culture-proven disease study population probably reflect some analytical limitations. Sputum cultures are seldom considered appropriate specimens for the isolation of Legionella by most laboratories, limiting the number of culture-proven legionellosis cases in less severe disease. During our study period, 78% of Legionella isolates recovered in the province of Quebec were from respiratory tract secretions obtained through invasive procedures (B. Lefebvre, personal communication). Among legionellosis cases notified in Quebec during that same period, case-fatality rates of patients diagnosed through invasive procedures were higher than in patients diagnosed with the non-invasive urinary antigen detection test (35·1% vs. 11·1%; RR 3·2, 95% CI 1·8–5·6, P<0·01) (R. Dion, personal communication).
The relatively low sensitivity of culture could also have limited the Legionella recovery in water samples. It is possible that low-level colonization have been overlooked. Nevertheless, culture remains a recommended method for environmental Legionella recovery and guidelines have been published to standardize its use [Reference Sehulster and Chinn30, 31]. It has the advantage of considering only viable organisms and was warranted for subsequent fingerprinting analysis. Recently, genotyping was achieved directly using DNA present in both environmental and clinical samples [Reference Luck32, Reference Kahlisch33]. These methods will allow culture-independent epidemiological studies in the future, and help circumvent the problem of competitive growth that might interfere with the recovery of Legionella.
Many typing methods have been described for Legionella spp., including monoclonal antibody subtyping, RFLP, PFGE, arbitrarily primed polymerase chain reaction (AP–PCR), sequence-based typing (SBT) and amplified fragment-length polymorphism (AFLP) [Reference Fields, Benson and Besser8, Reference Fry34, Reference Reimer35]. Both RFLP and PFGE have proved to be useful in many outbreak investigations. Even with highly discriminatory typing methods such as PFGE and AFLP, epidemiological association must be interpreted carefully. Isolates lacking any spatio-temporal relationship, but showing the exact same genotype, are being increasingly reported in both clinical and environmental samples [Reference Boccia36–Reference Thouverez38]. Epidemiological context and knowledge of geographical strain diversity must be considered. In our matching cases the questionnaire failed to identify any other plausible exposure. Moreover, the current collection of strains in Quebec shows a great diversity (B. Lefebvre, personal communication) of genotypes. Furthermore, two of our matching cases were infected by an extremely rare serogroup (patient nos. 24 and 34), which decreases the possibility of a match due to chance. Therefore, we believe that it is very likely that theses cases represent a real epidemiological association.
Finally, our study is limited by the occasional considerable lapse in time between the patients' illnesses and our epidemiological investigation. Median time between hospital admission and environmental sampling was 39 days. It can be speculated that water-system ecology is dynamic and that the causative Legionella could have disappeared from the domestic distribution systems in the mean time, thereby underestimating the epidemiological link. Whether this delay can significantly reduce the recovery of the culprit bacteria can hardly be assessed. However, no significant difference was noted between matched and unmatched pairs in relation to the delay in the investigation conducted in patients' homes. Further, in an outbreak investigated in Denmark, samples from the source were positive 6 weeks after the estimated transmission period [Reference Sonder39].
In conclusion, a high proportion (36%) of contamination by Legionella spp. of electricity-dependent hot-water distribution systems was confirmed in the homes of patients with documented SCAL. Five (14%) of the 36 patients in our study developed a pneumonia caused by microbiologically similar Legionella isolates that had contaminated their domestic hot-water distribution system. This is likely to represent the source of their infections. The proportion is similar to that previously reported, but this number may be underestimated. Interventions should be studied further and considered by public health authorities particularly in the setting of high-risk patients living in homes with domestic water distribution systems contaminated by Legionella.
ACKNOWLEDGEMENTS
The authors acknowledge Manon Lorange, Gabriela Martinez, and Louise Ringuette from the LSPQ, for identification confirmation and serogrouping of the environmental isolates; Dr Anne-Marie Bourgeault, Scientific Director of the LSPQ, for her support throughout this study; The National Microbiology Laboratory in Winnipeg for isolate characterization; Sylvie Bélanger for coordinating the administrative aspects of the study; Dr Jean R. Joly for his input and comments in the concept of this study.
This study was partially supported by Hydro-Québec.
DECLARATION OF INTEREST
None.







