LEARNING OBJECTIVES
After reading this article you will be able to:
• understand the epidemiology and prognosis of delirium in the acute hospital setting
• recognise that delirium often occurs in mixed presentations, which may require detailed longitudinal phenomenological profiling
• appreciate the current evidence-based approaches to delirium care in the acute hospital setting
Delirium is a major public health concern internationally; and as the biopsychosocial syndrome par excellence, its management can serve as an index of the quality of care an older patient receives in hospital (Jackson Reference Jackson, Gladman and Harwood2017). Delirium is often defined as an acute-onset neuropsychiatric syndrome, marked by changes to cognitive domains (e.g. attention and memory) and wider behavioural changes (e.g. sleep–wake cycle and motor disturbances), occurring in the context of medical illness (Inouye Reference Inouye, Westendorp and Saczynski2014). Delirium is a complex syndrome that has a highly heterogeneous and fluctuating phenomenological profile, which results in a wide differential diagnosis. The aetiology is multifactorial and includes both modifiable (e.g. acute critical illness) and non-modifiable (e.g. advanced age) risk factors (Vasilevskis Reference Vardy, Holt and Gerhard2012). In the acute hospital setting, it has a prevalence of 20% among general medical admissions, and this increases to over 50% in medical admissions of older people (Davis Reference Davis, Kreisel and Terrera2013; Inouye Reference Inouye, Westendorp and Saczynski2014). In more specialist clinical settings such as the intensive care unit (ICU) and palliative care, its prevalence can reach over 80% (Krewulak Reference Krewulak, Stelfox and Leigh2018; Hosie Reference Hosie, Davidson and Agar2013). It is associated with a variety of detrimental clinical outcomes, including increased length of hospital stay and increased risk of morbidity, mortality and dementia (Inouye Reference Inouye, Westendorp and Saczynski2014). Delirium is also a distressing experience for both patients and families. Indeed, qualitative research has reported on the experiential distress and resulting features of anxiety, depression and post-traumatic stress-like features that patients have following an in-patient episode of delirium (Grover Reference Grover, Ghosh and Ghormode2014; Martins Reference Martins and Fernandes2016). Families and caregivers are also affected by the presence of delirium and it can increase burnout and carer distress (Finucane Reference Finucane, Lugton and Kennedy2017). Following discharge, patients often face long-term consequences such as reduced adaptive functioning and an increased risk of needing institutional care (Jackson Reference Jackson, Wilson and Richardson2016). Despite all of these factors, delirium remains clinically underdetected and hence poorly managed in up to two-thirds of cases (Fong Reference Fong, Inouye and Jones2017). Psychiatrists’ skills in assessing complex psychopathology, coupled with specialist knowledge of pharmacological and non-pharmacological interventions, enable them to provide the necessary support required to help improve detection, optimise management and support recovery.
Assessment
Delirium phenomenology and diagnosis
At present there is no gold-standard biochemical test to accurately detect delirium (Toft Reference Svenningsen2019). Neuroimaging has a limited role clinically in delirium assessment, with current research highlighting the non-specific findings such as cerebral atrophy and neural dysconnectivity (Nitchingham Reference Nitchingham, Kumar and Shenkin2018). One study reported positive findings in only 14.5% of patients undergoing structural (magnetic resonance imaging and computed tomography) neuroimaging investigations (Hijazi Reference Hijazi, Lange and Watson2018). Therefore, in the absence of reliable biomarkers, the detection and assessment of delirium is informed largely by a review of the patient's clinical psychopathology/phenomenology.
Although there are an estimated 340 synonyms for delirium. depending on the clinical setting, the consensus from the DSM-5 and ICD-10 classification systems recognises all acute disturbances of global cognitive functioning as delirium (World Health Organization Reference Witlox, Eurelings and de Jonghe1992; American Psychiatric Association 2013). The recently available ICD-11 continues with this presentation of delirium as a clinical entity (World Health Organization 2019).
Delirium is a complex syndrome reflective of generalised neural dysfunction and yet lacks a pathognomonic feature. Accumulated research into the phenomenology of delirium indicates that there are four main domains of disturbed phenomenology: circadian integrity, executive cognition, orders of consciousness, and temporality (Fig. 1) (Hobson Reference Hobson and Voss2011; Franco Reference Franco, Trzepacz and Meagher2013; Leonard Reference Leonard, Adamis and Saunders2015). Circadian integrity is composed of two subdomains, motor behaviour and disturbances in the sleep–wake cycle (Fitzgerald Reference Fitzgerald, Adamis and Trzepacz2013). Executive cognition refers to such cognitive processes as attention, memory, language and orientation (Lindroth Reference Lindroth, Bratzke and Twadell2019; Mitchell Reference Mitchell, Shum and Mihala2018). The third domain, the orders of consciousness, are the functional modes by which executive cognition is enabled. They are divided into primary and secondary consciousness, but are experienced as an integrated whole. Primary consciousness refers to sensory and perceptual experience, and secondary consciousness encompasses metacognition, i.e. awareness of one's own thought processes. These aspects of consciousness serve as the modalities by which delirium, waking and sleep consciousness possess a phenomenological continuum (Hobson Reference Hobson and Voss2011). The fourth dimension is temporality and results from the convergence of the neurocognitive reserve of the individual and the influence of the pathological processes on this reserve (Cunningham Reference Cunningham and Maclullich2013). This domain is critical to detecting delirium in the acute hospital setting, given the plethora of research highlighting the heterogeneity in the temporal duration of delirium features (Kim Reference Kim, Kim and Kim2018). It is clinically essential to recognise the temporal pattern of delirium phenomenology. Delirium has an acute onset of features, with a prodromal change in mental state lasting an estimated 2–3 days and including lethargy, malaise and restlessness. A fluctuating course is driven by disturbed circadian regulation, with features worse at night, lasting days to weeks in most cases (Trzepacz Reference Trzepacz, Franco and Meagher2017). Taken together, an understanding of these domains is clinically relevant to the accurate psychiatric assessment of delirium, which includes both a detailed longitudinal analysis of its phenomenology and a focus on the temporal pattern of features and their severity (Adamis Reference Adamis, Sharma and Whelan2010).
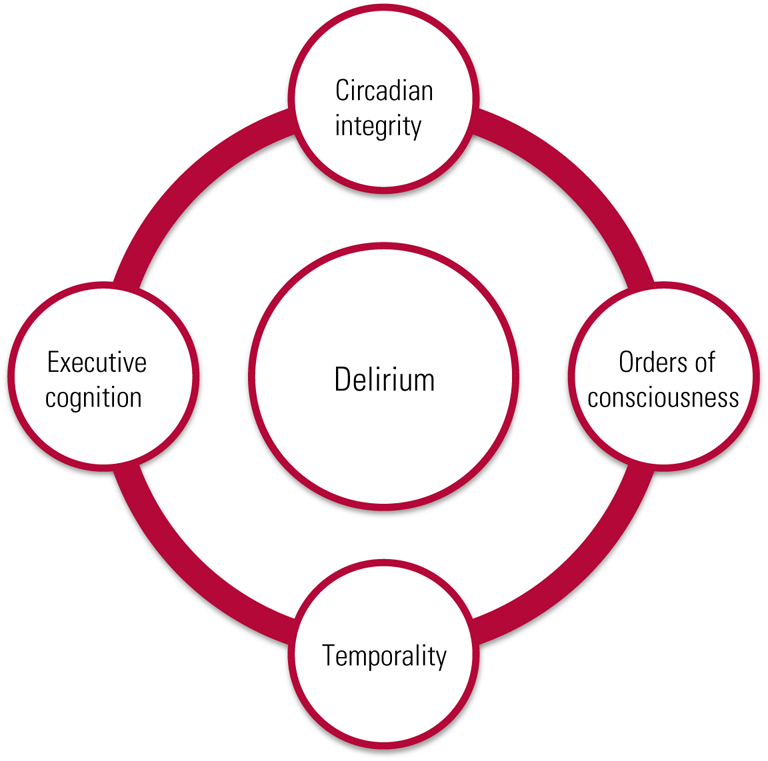
FIG 1 Multidimensional model of delirium phenomenology.
Subsyndromal delirium
Subsyndromal delirium is a state characterised by the presence of delirium symptoms, but without the criteria for full syndromal delirium. It is associated with outcomes that are intermediate between full syndromal delirium and no delirium (Dosa Reference Dosa, Intrator and McNicoll2007; Cole Reference Cole, Ciampi and Belzile2013). Although it is thought to include many features of full syndromal delirium, such as disturbances in motor behaviour, consciousness and sleep–wake rhythmicity, a comprehensive account has been impeded by the lack of clear diagnostic criteria (Boettger Reference Boettger, Nuñez and Meyer2018). Although the frequency of full syndromal delirium has been well described, the frequency of subsyndromal delirium is less well defined and is estimated to occur in approximately 7–50% of older adult in-patients. This wide range is likely to be due to the clinical population studied and definition applied (Ouimet Reference Ouimet, Riker and Bergeon2007; Bond Reference Bond, Dietrich and Shuster2012).
Overlapping phenomenology and complex presentations
The diagnosis and assessment of delirium is complicated by a wide differential, with depression and dementia being especially relevant. Delirium often exists in the context of neurocognitive disorders, with the prevalence of delirium superimposed on dementia reported at between 20 and 80% (Fong Reference Fong, Inouye and Jones2017). Delirium superimposed on dementia is associated with a significantly higher risk of mortality, the need for long-term institutional care and functional and cognitive decline compared with delirium alone (Avelino-Silva Reference Avelino-Silva, Campora and Curiati2017).
Although delirium is typically a transient disorder, it is increasingly recognised that delirium is often marked by incomplete recovery, with an estimated 20% of patients having persistent delirium that may last months (Cole Reference Cole, Ciampi and Belzile2008). In addition, dementia with Lewy bodies (DLB) is often described as the delirium–dementia continuum owing to the presence of shared features such as fluctuating cognition, visual hallucinations and disturbances in the sleep–wake cycle (Fong Reference Fong, Inouye and Jones2017). In a retrospective case-note study of patients undergoing review of dementia diagnosis at a tertiary referral unit, it was found that delirium was more closely associated with DLB than with Alzheimer's disease (Vardy Reference Trzepacz, Tousi and Cummings2014). A more recent study using electronic health records highlights the significantly higher occurrence of delirium in DLB than in Alzheimer's disease (FitzGerald Reference FitzGerald, Perera and Chang-Tave2019). There is unfortunately a lack of consensus regarding the differentiation between delirium and dementia, which has an impact on patient care and clinical outcomes (Richardson Reference Richardson, Teodorczuk and Bellelli2016). In the acute hospital setting, patients with complex neurocognitive features may be misdiagnosed and inappropriately treated with antipsychotics, which may lead to detrimental outcomes, given the high rate of neuroleptic sensitivity among people with DLB (Mueller Reference Mueller, Ballard and Corbett2017). Beyond the existence of dementia, older patients may present with mixed neuropsychiatric syndromes, including symptoms associated with depression, schizophrenia and mania. Given the non-specific manifestation of delirium phenomenology, specialist review by a psychiatrist is warranted to optimise patient care and recovery.
Delirium subtypes
Delirium can be categorised by clinically defined subtypes, with current evidence favouring the designation of subtypes based on motor activity profiles. There are currently four categories of motor subtype recognised: hyperactive, hypoactive, mixed and none (FitzGerald Reference FitzGerald2018). It has been reported that clinical motor subtypes of delirium differ in several ways, including detection rates, treatment experience, pathophysiology, duration of delirium episode and clinical outcome. Hypoactivity has been associated with metabolic causes and organ failure, whereas hyperactivity is more connected to substance-related delirium such as delirium tremens (Morandi Reference Morandi, Di Santo and Cherubini2017). Unfortunately, such studies have been found to have inconsistent findings due to heterogeneous methodology regarding motor subtype profiling (FitzGerald Reference FitzGerald2018). Despite these methodological limitations, hypoactive and mixed subtypes have been found to have a significantly poorer prognosis, with an estimated 1 in 3 patients dying during their hospital admission (Avelino-Silva Reference Avelino-Silva, Campora and Curiati2018). Hypoactive motor profiles have been found to have higher associated mortality independent of factors such as comorbidity, age, delirium and severity of dementia (Kiely Reference Kiely, Jones and Bergmann2007). The association between hypoactive delirium and elevated mortality may be reflective of delayed detection of delirium, and hence more prolonged episodes (González Reference González, Martínez and Calderón2009). Conversely, hyperactive delirium is associated with more frequent use of antipsychotics, higher detection rates and better outcomes (Meagher Reference Meagher, Leonard and Donnelly2011).
Methods to improve detection
The underdetection of delirium is the biggest challenge to optimising delirium management and recovery (Ritter Reference Ritter, Cardoso and Lins2018). It has been reported that delirium is particularly underdetected in the emergency department and that approximately 77% of patients with delirium in the emergency department continue to have delirium during their hospital admission (Han Reference Han, Vasilevskis and Chandrasekhar2017).
In clinical practice, assessing delirium requires a longitudinal perspective, which includes repeated assessments. Such a process can be informed by using validated tools to optimise the diagnostic ability of ward staff and provide reliable longitudinal assessments following review by a psychiatrist. There are an estimated 24 delirium detection tools available, and many have been translated into a variety of languages (Adamis Reference Adamis, Sharma and Whelan2010). In the acute hospital setting, both the Confusion Assessment Method (CAM) and the 4AT have been widely used as screening methods to help optimise delirium detection and monitor recovery. Although the 4AT is in widespread use in the UK's National Health Service (NHS) and has demonstrated high diagnostic performance, the CAM has been adapted and applied to a multitude of clinical settings, including the emergency department and ICU as well as numerous ward settings (Gélinas Reference Gélinas, Bérubé and Chevrier2018; Shenkin Reference Saczynski, Kosar and Xu2019). Of the rapid bedside tests of attention and arousal, the Observational Scale of Level of Arousal (OSLA) and the Richmond Agitation–Sedation Scale (RASS) have demonstrated high sensitivity and specificity for detecting delirium in older medical in-patients (Quispel-Aggenbach Reference Quispel-Aggenbach, Holtman and Zwartjes2018). At present there is no consensus regarding the optimum routine screening system for delirium or the best tool to use. These tools are dependent on the training of the individuals and begin to lose their utility when patients present with mixed neuropsychiatric conditions such as dementia and affective disorders. In these complex contexts, more detailed tools have been developed and validated to optimise detection, such as the Delirium Rating Scale – Revised-98 (Adamis Reference Adamis, Sharma and Whelan2010). This instrument detects the wide variety of presenting features of delirium, combined with severity scores for each item and global severity scores (Trzepacz Reference Toft, Tontsch and Abdelhamid2001). Again, this approach requires extensive training. In the context of subsyndromal delirium, there are no validated assessment tools, and such a diagnosis is based on specialist review by a psychiatrist, who may apply an operationalised algorithm adapted from the literature (Trzepacz Reference Trzepacz, Mittal and Torres2012). In the context of motor subtyping, several methods have been validated. These include the Delirium Motor Subtyping Scale (DMSS) and the abbreviated 4-item DMSS. Both these tools have been found to have high cross-sectional and longitudinal reliability (Fitzgerald Reference Fitzgerald, O'Regan and Adamis2016; Boettger Reference Boettger, Nuñez and Meyer2017). This is clinically helpful as it may enable ward staff to accurately detect subtypes and monitor their features longitudinally. Informal screening is consistently reported to be insufficient to accurately detect delirium in routine clinical practice, whereas validated screening tools and integrated diagnostic algorithms have demonstrated reliability and feasibility across several acute hospital settings (Grossmann Reference Grossmann, Hasemann and Graber2014; Maclullich Reference Maclullich, Shenkin and Goodacre2019). In real-world clinical practice each case of delirium is unique. Ward staff can be helped by their liaison (general hospital) psychiatry service to apply a suitable method for the particular patient to enable the necessary longitudinal monitoring of patient progress and recovery.
Management
Cognitive-friendly hospitals and policy development
The priority in approaching delirium in the acute hospital setting should be to optimise the conditions for its prevention. Indeed, comprehensive geriatric assessment with a view to reducing any modifiable risks for delirium is essential. Addressing polypharmacy is a key priority, especially rationalising any anticholinergic medications that may reduce cognitive function. Another priority is to identify and treat underlying causes and contributors. Delirium does not exist in isolation, but rather in a complex interaction of ward processes and acute illnesses. To provide optimum care for the ageing population, the concept of the cognitive-friendly hospital has emerged. The characteristics that are required for such a hospital according to Maclullich et al (Reference Maclullich, Anand and Davis2013) are outlined in Box 1. In the NHS clinical context, the National Institute for Health and Care Excellence (NICE) (2010) offers guidelines at a national level (Clinical Guidelines CG103) that can be adapted to the individual hospital setting. These guidelines describe the current evidence in four key domains: (a) risk factor assessment; (b) interventions to prevent and manage delirium; (c) delirium screening and assessment; and (d) information, support and communication. Unfortunately, national and international evidence reports on the persistent gap between delirium policy guidelines such as NICE CG103 and their application through associated quality standards (such as NICE QS63; NICE 2014) (Lamond Reference Lamond, Murray and Gibson2018).
BOX 1 Key characteristics of cognitive-friendly hospitals
• Guidelines for the prevention and management of delirium
• Routine delirium screening
• Delirium education for frontline staff, and for patients and their families
• Specialist services
(Maclullich Reference Maclullich, Anand and Davis2013)
Liaison psychiatry can offer a valuable contribution to local, regional and national policy development and hospital management structures with a view to improving care beyond the unit of the patient or ward. The Royal College of Psychiatrists has highlighted the role liaison psychiatry can offer by supporting ward staff to apply these guidelines in the real-world clinical setting (Royal College of Psychiatrists 2013, 2019a).
Multicomponent interventions and liaison psychiatry multidisciplinary teams
The current evidence recommends that multicomponent interventions are the first-line management and prevention strategy for delirium in the acute hospital setting. These interventions consist of nurse-led enhanced care plans that minimise and contain modifiable risk factors for delirium, such as dehydration, immobility, pain and malnutrition (Teale Reference Teale, Siddiqi and Clegg2017; Oberai Reference Oberai, Laver and Crotty2018). In addition to the liaison psychiatry service, the delivery of multicomponent interventions would be supported by other members of the multidisciplinary team such as physiotherapists (mobility recovery), dieticians (patient nutrition) and elder care physicians (comprehensive geriatric assessment).
In the largest and most up-to-date systematic review and meta-analysis on the subject, Hshieh et al (Reference Hshieh, Yang and Gartaganis2018) appraise the impact and implementation of multicomponent interventions on delirium detection and management in the acute hospital setting. In total, 44 studies set in a wide variety of international (USA, Europe and South America) acute hospital settings were included in the systematic review and 14 of those were included in the meta-analysis. The authors concluded that the evidence supported the implementation of multicomponent interventions in the acute hospital setting to reduce delirium incidence, the rate of patient falls, the length of hospital stay and the rate of transfer to long-term institutional care. Six of the included studies explored barriers and facilitators in implementing this approach to delirium care. The key facilitators included clinician leadership and changing organisational culture. However, the most prominent facilitator was the implementation of the policy measure to identify and empower an administrative champion (also known as a delirium champion). Ward-based nursing staff are in a key position to adopt this role, which could be supported by the liaison psychiatry service.
Given the prevalence and acute critical care needs of patients with delirium, nursing staff are in an important position to initiate multicomponent interventions and deliver routine delirium screening. However, neither NICE nor the Royal College of Nursing endorses any particular methods of implementing these interventions. International and national evidence suggests that, in the absence of routine patient screening or educational training for ward staff, patients with delirium may be missed and hence not treated appropriately (NICE 2014; Saczynski 2014; Yue Reference Yoon, Park and Choi2014). In qualitative and quantitative studies on nurses’ experience, perceptions and management of delirium, the most consistent barrier reported is the lack of detail on implementation of policy/guidelines regarding delirium detection and management. In particular, respondents reported a lack of guidance on how to apply key factors identified in the literature, which include: nursing screening and detection of delirium; training and education for nurses; and guidelines on management of delirium in the acute hospital setting (Fan Reference Fan, Guo and Li2012; Awad Reference Awad2019). Therefore, liaison psychiatry service input focused on these domains may be a suitable approach to operationalising professional delirium guidelines and delivering optimum delirium care for patients in the acute hospital setting. This approach would also help coordinate patient care and provide additional support to the role of family members/caregivers.
Delirium care can also benefit from input from the wider liaison psychiatry multidisciplinary team and, in addition to the role doctors can offer, liaison psychiatry nurses have invaluable roles in areas such as detection of delirium, monitoring treatment efficacy, role-modelling good care, and giving prescribing advice. Such input can be further supported by the provision of high-quality mediation skills to help ward teams coordinate care with patients and families. Finally, the use of assessments under the Mental Capacity Act, Deprivation of Liberty Safeguards (DoLS) and occasionally the Mental Health Act 1983 may be required to support management of patients with a delirium whose risks are significant. Such expertise can enable ward teams to support patients within the appropriate legal framework and protect patient rights.
Pharmacotherapy
Specialist knowledge of psychotropic medication is another key service that psychiatry can offer in the management of delirium. The current consensus regarding the pharmacological management of delirium proposes that psychotropic medication be used with caution and only for situations that have not yielded to non-pharmacological interventions (NICE 2010, 2014). Research has indicated that patients presenting with hyperactive delirium tend to be prescribed antipsychotics more frequently, likely owing to the distress and or the psychotic symptoms experienced (Meagher Reference Meagher, Leonard and Donnelly2011). Conventionally, the antipsychotic of choice is haloperidol, given its absence of anticholinergic side-effects (Yoon 2013). The use of antipsychotics is controversial, and there is little evidence to indicate that they should be used to treat delirium directly (Neufeld Reference Neufeld, Yue and Robinson2016; Burry Reference Burry, Mehta and Perreault2018). In congruence with this, there is no robust evidence to suggest that at-risk patients should be given antipsychotics prophylactically (Oh Reference Oh, Needham and Nikooie2019).
Generally, antipsychotics are associated with a wide variety of adverse effects, such as prolonged QTc, torsades de pointes, and extrapyramidal and anticholinergic side-effects (Huhn Reference Huhn, Nikolakopoulou and Schneider-Thoma2019). In the context of delirium, antipsychotics have been found to be relatively safe if given for the duration of the detected delirium, for example 3–7 days. In a prospective observational study of 2453 acutely admitted patients who experienced an episode of delirium, it was found that approximately 1% experienced an adverse event attributable to antipsychotic use. Of note, the authors found no deaths attributable to antipsychotic use (Hatta Reference Hatta, Kishi and Wada2014). However, caution must be used when DLB is suspected, particularly in the context of spontaneous Parkinsonism, rapid eye movement (REM) sleep behaviour disorder or visual hallucinations preceding acute cognitive decline (McKeith Reference McKeith, Boeve and Dickson2017). When required, antipsychotics should be prescribed for distress/agitation during the delirium episode where the patient may be a risk to themselves or others. They should be prescribed at the lowest effective dose for the shortest period and regularly reviewed for efficacy (NICE 2010, 2014). Psychiatry can offer additional advice and recommendations on the choice of antipsychotic if a patient's clinical profile requires a more careful consideration of its pharmacological properties, taking into consideration, for example, route of administration, comorbidities such as Parkinson's disease, cardiac history, and history of falls and hypotension. However, it is highly recommended that antipsychotics are reviewed prior to discharge and discontinued wherever possible, given the consistent finding that approximately a quarter of antipsychotics initiated for older patients in acute hospital settings continue after discharge (Herzig Reference Herzig, Rothberg and Guess2016; Loh Reference Loh, Ramdass and Garb2014).
Finally, there is no evidence to support the use of benzodiazepines in managing delirium not associated with alcohol withdrawal (Lonergan Reference Lonergan, Luxenberg and Sastre2009). There is limited emerging evidence to suggest that dexmedetomidine (an alpha-2 adrenergic agonist) may be a reasonable alternative to antipsychotics to manage agitation in ventilated ICU patients (Flükiger Reference Flükiger, Hollinger and Speich2018). More recent studies have proposed that novel substances such as melatonin may have clinical utility due to its effect on sleep, but there is insufficient evidence to endorse this in the mainstream approach to delirium (Chen Reference Chen, Shi and Liang2016).
Models of delirium care
As a psychiatry subspecialty, liaison psychiatry operates in the main via a ‘referral–response’ model, although models and composition of teams vary. This reactive (as opposed to proactive) model may risk missing opportunities for implementing preventive and early management strategies that may lead to improved outcomes for those at risk of delirium. The Royal College of Psychiatrists, for example, recommends that age-inclusive services have suitable embedded expertise to meet the specific needs of older people (Royal College of Psychiatrists 2019b). Interest is growing in more integrated models of liaison psychiatry, although the evidence base for clinical effectiveness and cost-effectiveness is in development. The ongoing HOME study based in the UK is a notable example of developing a more proactive approach to psychological medicine in the acute hospital setting (Walker Reference Vasilevskis, Han and Hughes2019).
Although this article, and indeed the majority of literature on delirium, pertains to the acute hospital setting, it should be acknowledged that delirium occurs in a range of settings and therefore psychiatrists need to acquire skills in delirium management as a core aspect of their training. In addition to this consideration, integrated care models outside the typical hospital ward model are something to aspire to and have been implemented in some settings: a good example is the management and recovery approach to post-operative delirium (McDonald Reference McDonald, Heflin and Whitson2018).
Recovery
The impact and experience of delirium
Owing to its wide-ranging impact, recovery from delirium must be considered from different perspectives, which include cognition, physical health and mental health. Recovery is also informed by each patient's particular journey through the acute hospital setting. For example, patients presenting with delirium to the emergency department tend to have significantly longer hospital stays, higher 30-day mortality rates and higher 30-day readmission rates (Kennedy Reference Kennedy, Enander and Tadiri2014). Such vulnerable patients have significantly higher rates of admission to the ICU and transfer to post-acute institutional care facilities on discharge from hospital (Han Reference Han, Zimmerman and Cutler2009; Kennedy Reference Kennedy, Enander and Tadiri2014).
In the ICU setting, delirium is associated with an increased risk of mortality both during admission and following discharge. In this setting, delirium is associated with an increased ICU length of stay and patients are significantly more likely to undergo tracheostomy. It is also associated with prolonged mechanical ventilation time and total hospital length of stay (Mehta Reference Mehta, Cook and Devlin2015). Post-operative delirium has been found to be associated with an increased rate of ICU stays that last more than 5 days, significantly higher rate of 30-day readmissions to acute hospital and higher rate of discharge to a permanent care home placement (Raats Reference Raats, van Eijsden and Crolla2015). When patients are discharged to a post-acute care facility following an episode of delirium it has been reported that they have significantly increased risk of mortality and higher 30-day acute hospital readmission rates (Kosar Reference Kosar, Thomas and Inouye2017). Taken together these clinical factors may have a detrimental impact on patient recovery in both cognitive and wider functional domains.
Not surprisingly, delirium is a distressing experience for patients, families, carers and healthcare staff (Grover Reference Grover, Ghosh and Ghormode2014; Martins Reference Martins and Fernandes2016). Qualitative research has reported on the experiential distress and resulting features of anxiety and depression that patients have after an in-patient episode of delirium (Whitehorne Reference Walker, Burke and Toynbee2015). In particular, post-traumatic stress disorder (PTSD) is emerging as a recognised consequence of delirium and it has been suggested that it should be more routinely followed up, given its potential impact on patient recovery and quality of life (Teale Reference Teale, Siddiqi and Clegg2013; Bolton Reference Bolton, Thilges and Lane2019). However, many patients do not report their symptoms as they feel that the delirium experience is evidence of a severe mental illness associated with stigma (Kim Reference Kim, Kim and Kim2017). As mentioned earlier, the presence of delirium can increase burnout and carer distress in families and caregivers (Finucane Reference Finucane, Lugton and Kennedy2017). Patient recovery can be optimised, however, by engaging with orientation strategies and emotional support, which can significantly ameliorate the experiential distress of delirium (Halloway Reference Halloway2014). Consultation with liaison psychiatry services may offer advice regarding suitable support that patients and their families may require following discharge from hospital. However, given the growing literature highlighting the need for follow-up in the context of PTSD, mood and anxiety symptoms, service development to set up brief post-discharge clinical liaison clinics may be warranted.
The interface between delirium and dementia
Recovery from delirium is an ill-defined concept in the literature, and although it is widely considered that delirium is often reversible, accumulating evidence suggests that it is often marked by incomplete resolution (Witlox Reference Whitehorne, Gaudine and Meadus2010; Adamis Reference Adamis, Devaney and Shanahan2014). Indeed, it has been found that, for each day a patient has delirium in the emergency department, a significantly worse long-term cognitive and functional outcome has been reported (Han Reference Han, Vasilevskis and Chandrasekhar2017). It has also been reported in a systematic review of 18 cohort studies that the proportions of patients with persistent delirium at point of discharge and at 1, 3 and 6 months are approximately 44.7%, 32.8%, 25.6% and 21% respectively (Cole Reference Cole, Ciampi and Belzile2008). According to Inouye et al (Reference Inouye, Westendorp and Saczynski2014), delirium can be a marker of the vulnerable brain, with a reduction in reserve capacity to withstand noxious insults. Therefore, delirium has a negative impact on the trajectory of normal cognitive ageing by adding a series of punctuated stages of decline and recovery associated with each episode of delirium (Fong Reference Fong, Inouye and Jones2017). These insults on cognitive function can consequently increase the risk in some vulnerable patients of developing dementia. This further reinforces the complex interface between delirium and dementia, and the post-discharge cognitive trajectory towards dementia that many patients with delirium may develop in the community (Fong Reference Fong, Davis and Growdon2015). To tackle this challenge, follow-up review of patients in the regional older people's mental health service (e.g. memory clinics) may help support patients and families to address the challenge and trajectory of cognitive impairment.
Paradigm, education and research
Good practice in the identification, assessment and management of delirium is a ‘life skill’ for medical professionals, especially those working with older people, but because the condition straddles the traditional separation of ‘mind’ and ‘body’ medicine it risks being neglected in more specialty-focused medical training programmes and therefore remaining underrecognised even in acute medical settings. Delirium may be most easily recognised when there is an obvious change in behaviour, psychiatric symptoms or difficulty in providing medical care. This may have contributed to a culture in which delirium is commonly conceptualised as a ‘psychiatric’ problem (albeit with an ‘organic’ underlying cause). An improvement in practice therefore requires a shift in culture whereby training in good delirium care is everybody's business and core to medical training throughout.
To optimise delirium detection, management and recovery in the acute hospital setting, further educational support and research approaches are warranted. Psychiatry as a discourse and as a practice can inform the multidisciplinary research required to improve the development of screening methods and routine profiling approaches across the clinical settings in which delirium is most manifest. At the centre of this issue is the role of phenomenology, which has often been cited as the rationale for the different approaches taken to manage delirium (Meagher Reference Meagher and Trzepacz1998). Indeed, future pharmacotherapy trials may adapt their approach to targeting specific features of delirium, namely positive psychotic symptoms or perhaps clinical subtypes (e.g. hypoactive versus hyperactive) to yield more innovative findings. Moreover, the complex interface between delirium and dementia requires further investigation with a view to creating more reliable methods of either differentiating between these two neurocognitive syndromes, or identifying methods that can measure the impact of delirium on the vulnerable brain. In particular, neuroimaging combined with detailed phenomenological profiling may yield such benefits, particularly in elucidating the interface between delirium and DLB.
Gaps in knowledge and training have consistently been cited as critical barriers to implementing best practice care for people with delirium. Knowledge pertaining to screening, risk factor detection and reduction, and management of distress/psychosis are often cited as recurrent themes. Congruent with this, the 2011 National Audit of Dementia in acute hospitals in England and Wales found that only one-third of staff felt they had received adequate training or guidance in dementia care (Royal College of Psychiatrists 2012). In addition, ward teams may be uncertain about the optimal approach to engage with the specific processes of discharge planning, specialist out-patient referrals, older adult mental health requirements, and more intimate discussions with patients and families regarding the experience and impact of delirium. Liaison psychiatry services are well positioned to work collaboratively with ward teams to deliver the quality and provision of such information and skills. However, it is important to reiterate the importance of liaison psychiatry services delivering specific expertise in managing the needs of older people as compared with those of the younger adult population. This has been highlighted by the Royal College of Psychiatrists in a recent position statement (Royal College of Psychiatrists 2019a). Such an approach may enable the ward team to enhance its capacity to implement best-practice guidelines when engaging with patients with delirium and to improve the general approach with this vulnerable patient cohort.
Discussion
Delirium is an acute-onset neuropsychiatric emergency that presents across multiple acute clinical care settings. This article has outlined the different forms of support psychiatry can offer (Fig. 2). Psychiatry teams can provide assessment in the context of complex neuropsychiatric phenomenology and its impact on patients’ mental health. Moreover, the liaison psychiatry service can work collaboratively with the ward team to optimise patient management and monitor its efficacy. Psychotherapeutic and general mediation skills can help support ward teams, families, carers and patients to aid in recovery from delirium. Old age psychiatrists are particularly skilled in dealing with the impact of delirium on cognitive functioning and advanced dementia. Indeed, the versatility of contemporary older people's mental health services enables psychiatry to offer a coherent and invaluable approach to delirium across domains.
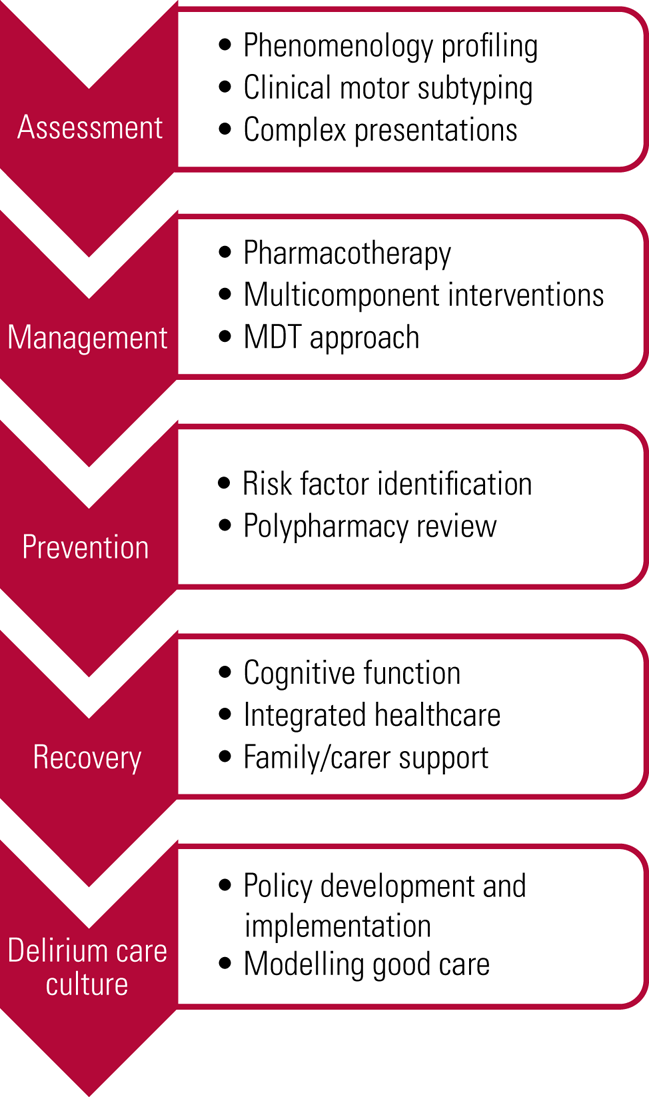
FIG 2 Psychiatry support for the multidisciplinary team (MDT).
Delirium is not a condition that exists in isolation and it is often the outcome of the general approach to care of the older patient in the acute hospital setting. Hence, a more multifaceted approach may have a positive impact on delirium prevention, detection and management. Therefore, robust policy and practice initiatives are required to reconstruct and adapt the hospital setting to address the acute care needs of the ageing population across various clinical settings. Psychiatry is in a key position to support the optimisation of care regarding delirium in the acute hospital setting and provide a meaningful contribution to the evolving elder care discourse. Although perhaps as important as any of the crucial clinical contributions that psychiatry can offer would be a change in perspective so that delirium is regarded as an index of the quality of care that patients can receive in the acute hospital setting.
Acknowledgements
We thank Professor John O'Brien for reviewing an early draft of this article.
Author contributions
The authors contributed equally to the conception, writing and final submission of this article.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bja.2020.44.
MCQs
Select the single best option for each question stem
1 The estimated frequency of antipsychotic prescriptions initiated for older patients in acute hospital settings that continue after discharge is:
a 25%
b 66%
c 1%
d 100%
e 40%
2 Of the following subtypes of delirium, the best clinical outcomes are for:
a hyperactive
b hypoactive
c hypoactive and mixed
d mixed
e hyperactive and mixed
3 A recommended first-line treatment in managing delirium is:
a an enhanced care plan
b single component interventions
c multicomponent interventions
d admission to a mental health ward
e watch and wait approach
4 As regards the pharmacotherapy of delirium:
a there is robust evidence to support the use of atypical over typical antipsychotics in managing delirium
b the use of antipsychotics is controversial and there is little evidence to indicate that they should be used to treat delirium directly
c typical antipsychotics such as haloperidol should be used prophylactically to reduce delirium
d risperidone is the antipsychotic of choice for managing delirium
e olanzapine is the antipsychotic of choice for managing delirium
5 The estimated proportion of patients with persistent delirium at 6 months post-discharge from hospital is:
a 41%
b 81%
c 21%
d 31%
e 11%
MCQ answers
1 a 2 a 3 c 4 b 5 c






eLetters
No eLetters have been published for this article.