Introduction
Tso Lhamo, a small Trans-Himalayan plateau in the state of Sikkim, in north-eastern India, supports populations of four of the eight ungulate species of the Tibetan plateau: Tibetan argali Ovis ammon hodgsoni, Tibetan gazelle Procapra picticaudata, southern kiang Equus kiang polyodon and blue sheep Pseudois nayaur. All four are on lists of nationally or globally threatened animals and several are declining in other areas of their range on the Tibetan plateau (Schaller, Reference Schaller1998). The IUCN Red List categorizes argali (Harris & Reading, Reference Harris and Reading2008) and Tibetan gazelle (Mallon & Bhatnagar, Reference Mallon and Bhatnagar2008) as Near Threatened but does not categorize Tibetan argali separately from other sub-species. Blue sheep (Harris, Reference Harris2008) and kiang (Shah et al., Reference Shah, St. Louis, Huibin, Bleisch, van Gruissen and Qureshi2008) have been assessed as of Least Concern but the assessors caution that the classification of the southern kiang as a separate sub-species requires investigation. All four species are listed under Schedule I, the highest protection category of the Indian Wildlife Protection Act of 1972. Tso Lhamo is home to India’s only population of the southern kiang (Shah, Reference Shah1994) and supports one of the country’s largest populations of Tibetan gazelle, a species on the verge of extinction in India (Namgail et al., Reference Namgail, Bagchi, Mishra and Bhatnagar2008). The country’s only other population of Tibetan gazelle, of c. 50, persists in Hanle, Ladakh (Bhatnagar et al., Reference Bhatnagar, Wangchuk and Mishra2006), in north-west India. The Tibetan argali population in Tso Lhamo is important, given the small population of 300–360 reported from the extensive Ladakh Trans-Himalaya (Fox et al., Reference Fox, Nurbu and Chundawat1991a; Namgail et al., Reference Namgail, Fox and Bhatnagar2009). The Tso Lhamo plateau also supports other animal species adapted to high-altitude cold deserts, including the snow leopard Uncia uncia, wolf Canis lupus chanko, Pallas cat Otocolobus manul and migratory waterfowl (Chanchani, Reference Chanchani2008). Nomadic Dokpa herders graze their yaks Bos grunniens and domestic sheep Ovis aries in this region.
Although the Tso Lhamo plateau is known regionally for its verdant pastures for grazing livestock, its value for threatened wildlife is largely unrecognized and it has not been gazetted as a protected area. This region forms India’s international boundary with the Tibetan Autonomous Region, China, and has hitherto remained closed to detailed wildlife research. Here we: (1) describe the distribution and status of wild ungulates on the Tso Lhamo plateau, (2) detail livestock herding practices in the region, (3) examine potential threats to the plateau’s wild ungulates, and (4) discuss conservation in the Tso Lhamo landscape. We focus in particular on the Tibetan argali and gazelle because of their threatened status in India.
Study area
Located in Sikkim’s North District, the Tso Lhamo plateau (c. 400 km2, elevation > 4,300 m; Fig. 1) forms the upper catchment of the River Teesta. Prominent topographical features in the northern parts of the study area include the Teesta basin or Chombo chu, scree hills that rise to c. 6,000 m, plateaus and glacial moraines. The southern portion of the area (65 km2) lies in a transition zone between the Greater and Trans-Himalaya, and is characterized by steep mountainous slopes, valleys and rocky outcrops. Total annual precipitation on the Tso Lhamo region plateau is > 500 mm (Paljor, Reference Paljor1995), winter temperatures fall to -28oC at Gyamtshona at 4,990 m, and winds of 30–50 km h-1 are common (P. Chanchani, pers. obs.). In the transition zone moist and dry alpine scrub (Rhododendron, Juniperus, Cotoneaster) and riverine scrub (Salix) and sedge meadows are prominent. Desert steppe (dominated by Stipa, Elymus, Carex, Ephedra, Artemisia, Androsace and Arenaria), marsh meadows (Kobresia, Pedicularis) and scrub formations (Lonicera, Potentilla) are major vegetation types on the plateau.

Fig. 1 The study area in the Tso Lhamo region of Sikkim, with the 10 survey blocks and the locations of herder camps and the vehicle (V) and foot (F) transects (Table 1). The shaded area on the inset indicates the location of the main figure in India. Survey blocks are: B1, Kongra la-Gyamtshona; B2, Chulung–Changri ridge; B3, Chombo chu; B4, Kerang–Oloten; B5, Khongyakma–Teesta Glacier; B6, Leten–Dongkung Giagong; B7, Lukrep–Chopta; B8, Lasher; B9, Chomodo–Bamchola; B10, Gurudongmar–Kanchangyao.
At present 15 Dokpa families (average family size 5.53; Paljor, Reference Paljor1995) occupy this region and herd a total of c. 1,000 yaks, 1,100 domestic sheep and 100 domestic goats Capra aegagrus hircus, of which half belong to the influential Lachenpas of Lachen township. The Dokpas follow a seasonal migratory grazing system, occupying the plateau in winter and the lower transition zone in summer. Because of its proximity to the Tibetan Autonomous Region, China, the area has been monitored by Indian defence forces for several decades.
Methods
Sampling and surveys for ungulates were carried out in winter (December 2006 to end of April 2007) and late summer (August–September 2007) for a total of 140 days, between 06.30 and 18.00 by a researcher and a field assistant (both familiar with the area and experienced at detecting and identifying mammals in this region). Following a 15-day reconnaissance we divided the study area into 10 blocks based on topographical features and vegetation (Fig. 1), and the area was further sub-divided into 130 1 × 1 minute grid cells to guide survey efforts. Survey methods included a combination of vehicle transects (n = 7, 1,006 km total length, including replicates), foot transects along fixed routes (106 km total length, including replicates; Fig. 1, Table 1), and foot surveys primarily to more remote locations not surveyed from transects (c. 450 km).
Table 1 Length, area visible, and number of replicates of the seven vehicle (V) and two foot (F) transects surveyed on the Tso Lhamo plateau in Sikkim (Fig. 1).

Replicate surveys of individual vehicle and foot transects were spaced by at least 1 day and were at various times of the day. Vehicle transects were carried out at a constant speed of c. 15 km h-1, and on foot transects the two observers maintained a constant pace, with one observer scanning either side. Observations were aided by a spotting scope (15–60x) and binoculars (10x). Locations of ungulates (both wild and domestic) were recorded with a global positioning system (GPS), either by visiting a site after animals had vacated it or by projecting the location of the sighted animals relative to that of the observer (by entering the distance and angle to the animals into a GPS). Sex and age of individuals were also recorded, and other habitat variables (topography, vegetation cover and composition) were recorded for the analysis of species–habitat relationships, the details of which can be found in Chanchani (Reference Chanchani2007).
Foot surveys were made of 33 1 minute grid cells (randomly selected from the total of 130 but in proportion to the area of each of the 10 blocks). For the foot surveys we walked in an approximately straight line, usually a diagonal across the grid, ensuring that each selected grid was thoroughly surveyed for ungulate presence. We attempted to survey each such grid square at least three times each in winter and summer. Animal locations and habitat variables associated with animal presence in an area were recorded as described above. In addition to observations recorded along transects and in the foot surveys, opportunistic sightings were recorded en-route to a survey area.
Over the duration of the study our surveys covered the entire study area. Habitat for argali, gazelles and kiang was only found in the northern plateau and logistic constraints resulted in this region being sampled more intensively than the south. Heavy snowfall and inclement weather in the winter resulted in differences in sampling effort between blocks because of occasional difficulties of access.
In addition to transect sampling we also attempted to enumerate the population sizes of argali, gazelle and kiang using counts carried out by a combination of vehicle and foot surveys over a large portion of the species’ habitat on 1 day each in winter (22 March 2007) and summer (10 September 2007). These counts provide a minimum population size for each species in the area, rather than a total count, because it was impossible for our small research team to survey the entire study area in a single day.
We interviewed eight herder families (from Tso Lhamo) and 13 regional government officials/NGO representatives who had some association with managing land or other resources in the region. These interviews helped us determine their perspectives on wildlife populations and challenges to conservation in the Tso Lhamo region.
Ungulate densities and population sizes were estimated from transect data, separately for winter and summer. Encounter rates were calculated by dividing the number of individuals encountered on each transect by the cumulative effort (distance covered) for that transect. To obtain an estimate of density (assuming perfect detectability) we first determined the area visible from each transect by manually delineating polygons on a satellite image of the study area (Landsat, 30 m resolution) using ArcGIS v. 9.1 (ESRI, Redlands, USA). These areas were based on our knowledge of the landscape and on plots of ungulate locations on the satellite images. The estimates of the areas surveyed from transects were then used to calculate densities along each transect (separately for each replicate, and then an average density across replicates for each transect). Densities are only reported for transects that were in areas we identified as potential habitat for these species (transition zone excluded for argali, gazelles and kiang). The density of animals for each transect were then calculated by combining densities from individual transects to obtain a pooled mean density. We did not estimate detection probabilities because of the difficulty of accurately measuring distances to observed animals, a problem often encountered with species such as argali (McCarthy et al., Reference McCarthy, Fuller, Ming, McCarthy, Waits and Jumabaev2008). We thoroughly scanned areas visible along transects and, while detection probabilities were < 1, we believe most animals present during transect surveys were detected; there was little vegetation cover and few rocky outcrops to conceal animals in the northern plateau region, where nine of the 10 transects were located.
Results
Abundance of wild ungulates
Argali and gazelles occasionally permitted us to approach within 15 m on foot but usually maintained a flight distance > 100 m. Gazelles and blue sheep were tolerant of vehicles but responded with greater apprehension to humans on foot. Kiang were the most intolerant of our presence and usually maintained a flight distance of c. 500 m. Total counts on 2 days, one each in summer and winter, gave mean minimum population sizes of 177 argali, 77 gazelles, 18 kiang and 73 blue sheep (values for these total counts are presented separately for each season in Table 2).
Table 2 Total numbers of groups observed, mean group size (± SD) and maximum group size, and maximum number of individuals counted in a single day for wild and domestic ungulates on the Tso Lhamo plateau in Sikkim (Fig. 1) in winter and summer. Clusters of animals resting or feeding at distances > 200 m were classified as separate groups. This summary has been compiled from observations from vehicle and foot transects, other foot surveys, total counts and opportunistic sightings. Maximum number of individuals counted in a single day are our total counts carried out over 1 day in each season.

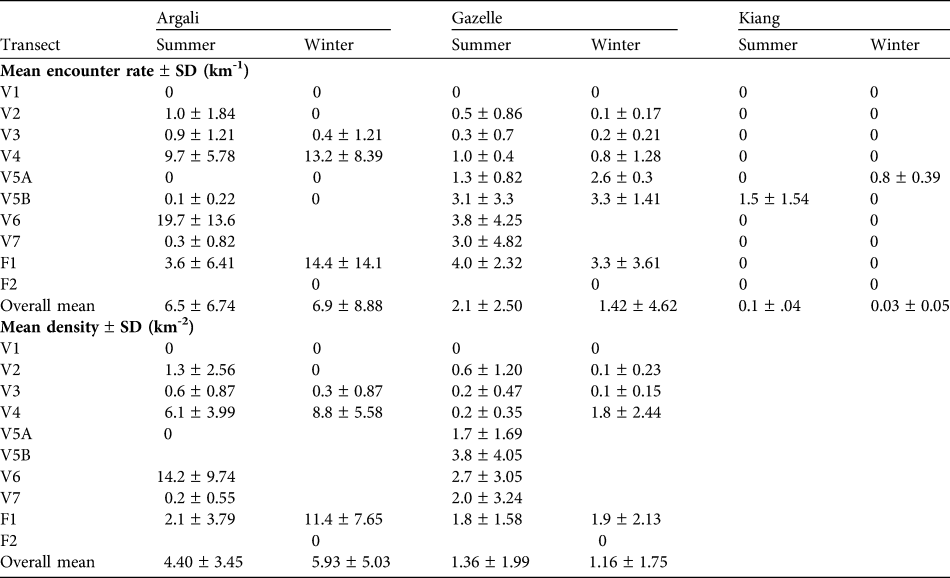
We analysed summer data for eight transects and winter data for five transects (Table 1). Transects V5, V6 and V7 were inadequately sampled in the winter and transect F2 was sampled only in the winter. Mean encounter rates and densities for argali, gazelle and kiang, for each transect, are presented in Table 3. No individuals were recorded on V1 and, as this area is not typical habitat of argali, gazelles or kiang, we omitted it from the analysis. We believe it is reasonable to extrapolate these estimates of density over an area of c. 55 km2, which is the sum of the areas visible from transects included in the analysis (see Table 1). We do not report estimates of encounter rates or densities for blue sheep because most observations came from foot surveys and ad-libitum observations, with very few detections along transects.
Table 3 Encounter rates and density estimates for argali, gazelle and kiang on the seven vehicle (V) and two foot transects (F; Fig. 1, Table 1) in winter and summer. For encounter rates a 0 indicates that no individuals were detected whereas a blank indicates that no surveys were conducted. For densities we have only analysed data for transects with > 2 replicates in each season; a blank indicates no survey or insufficient data. The pooled estimates of the mean and standard deviation exclude transect V1.
Distribution of wild ungulates
Of the four wild ungulate species in the region, kiang have the most conspicuous transboundary movement, with most observations being close to the international border in the Kerang area. All four species were present in the study area in both winter and summer, and our observations suggest that these populations are predominantly resident on the Tso Lhamo plateau. We repeatedly sighted certain argali and gazelle individuals (recognized by particular deformities) in the same area in both summer and winter. Our study confirms the local extinction of the chiru Pantholops hodgsonii, which was reported as present in this region by Hooker (Reference Hooker1854).
Based on observations recorded on transects and in foot surveys, total counts and opportunistic sightings, argali occupy a total of c. 65 km2, gazelles 75 km2, kiang 36 km2 and blue sheep 95 km2. Argali and gazelles primarily use the region north of the Chombo chu and kiang were never sighted south of this river. Argali and gazelles overlap in their distribution, with 91% of argali sightings in Blocks 1 and 2 (in an area of c. 42 km2) and 70% of the gazelles observed in these same blocks but using c. 18 km2 (Fig. 2, Table 4). The distribution of gazelles extends eastwards, with 17% of all observations in the Sesela-Oloten area (Block 4) and 5% in the Chomodo-Bamchola area (Block 9). Blocks 4, 5 and 9 also comprise the kiang’s habitat, with 83% of all observations in an area of c. 12 km2 (Fig. 2, Table 4). Of the four wild ungulates only blue sheep use the transition zone between the Greater and Trans-Himalaya, with concentrations in Blocks 7 and 8 on grassy slopes and moraines. Large groups of blue sheep were also seen in secluded moraines and nearby grassy meadows in Block 5 close to lake Gurudongmar and in Block 10 in the Khongyakma–Teesta Khangse glacier region (Fig. 2, Table 4).

Fig. 2 Locations, derived from a combination of vehicle and foot transects, foot surveys and opportunistic sightings (see text for details), where Tibetan argali Ovis ammon hodgsoni, Tibetan gazelle Procapra picticaudata, kiang Equus kiang polyodon, blue sheep Pseudois nayaur and domestic yak and sheep were observed in winter and summer in the Tso Lhamo region of Sikkim (Fig. 1). Locations are plotted on a Digital Elevation Model generated from an SRTM image (90 m resolution).
Table 4 Percentages of the total number of each wild and domestic ungulate species observed in each of the 10 survey blocks (Fig. 1), by season (S, summer; W, winter), with effort (number of visits to each block) and general terrain types. This analysis is compiled from all survey methods. Blocks with > 9% observations for a species are in bold.

* TH, Trans-Himalaya; TZ, Transition Zone (between Greater and Trans-Himalaya); sh, scree hills; pl, extensive high-altitude plateaus; vb, valleys and basins (basins have a lake or stream); mt, moraine and tallus (usually in the vicinity of high mountains and glaciers); as, alpine slopes and valleys (more vegetated than scree slopes of Trans-Himalaya)
Based on broad-scale analysis of habitat data associated with ungulate sightings from all survey methods, argali were found to be associated with sparsely-vegetated scree hills, with 80% of argali sighted in both seasons on slopes, ridges or gullies and a few in valleys and basins. Male groups (3–24 individuals) generally avoided broad valleys, although valley edges were often used when feeding. Gazelles frequently used valleys, basins and plateaus, with 66% of summer and 38% of winter observations in flat terrain. Kiang predominately used plateaus and gentle slopes and were not sighted on steeper slopes and seldom in river basins, which are frequented by humans and livestock. Blue sheep were mainly seen on rocky or grassy slopes in the transition zone. Interviews with local herders indicated that the distribution of blue sheep extends well beyond the boundaries of the study area, most notably into the alpine pastures of Muguthang and the Sebu-la regions in North Sikkim.
Population structure and group composition
Both argali and gazelles showed marked sexual segregation except in the winter rut period (which ends in February). Based on observation from all survey methods there appear to be c. 35 adult argali males (>2 years) in the study area. The single largest congregation of argali comprised 107 individuals, sighted near Kongra La in January 2007, and a total of seven congregations with >90 in each were sighted in the two sampling periods. The single largest congregation of gazelles (41 individuals) was recorded in September 2007. Kiang were usually sighted in small groups (2–7 animals). Blue sheep group sizes varied greatly, although groups observed on the plateau were generally larger than those observed in the transition zone. Further details on group sizes are given in Table 2.
Adult male:female ratios were 37:100 for argali and 23:100 for gazelles. The juvenile (>1 year):adult female ratio was 43:100 for argali and 38:100 for gazelles. Juvenile gazelles attain near-adult size in < 1 year, and some may therefore have been mistaken for adults. For argali, not all of the juveniles in large groups may have been observed from long distances, particularly when animals were resting.
Livestock grazing patterns
There are clearly demarcated and exclusive summer and winter grazing areas, with the Tso Lhamo plateau being the winter grazing area and the southerly transition zone comprising the summer grazing area (Blocks 7, 8 and proximate regions; Table 4). In 2006–2007 the herders and their livestock spent December–May in the winter grazing area. We observed that livestock used an area of c. 175 km2 in winter and c. 120 km2 in summer. Winter densities of yaks and sheep are c. 6 and 7 km-2, respectively, and summer densities are 8 and 9 km-2, respectively. Yak and sheep were seldom grazed in the same areas simultaneously and, typically, many hundreds of animals would congregate in a small area for a few days or weeks before moving on. Within these grazing areas varying forage availability and grazing restrictions along the international border cause some areas to be extensively used and others avoided. Based on the availability and quality of forage at different locations and snow deposition the herders arrive at a consensus and regulate the movement of their herds.
Animals were typically grazed between 07.30 and 17.30 and were confined to their corrals at other times as a protection from wolves and other predators. In the winter river courses and moist basins dominated by sedges (Kobresia pygmaea and Kobresia schoenoides) and desert steppe areas with grasses and herbs such as Stipa orientalis and Oxytropis sp. were favoured feeding areas.
Factors influencing persistence or loss of Tso Lhamo’s wildlife
Based on personal observations and interviews with herders and government officials we identified small fragmented populations, transboundary concerns (lack of coordinated conservation across the international border), and anthropogenic disturbances as the main threats to this region’s ungulate populations (Table 5).
Table 5 Threats to wildlife on the Tso Lhamo plateau (Fig. 1), identified and assessed from personal observations, literature and interviews with herders, government officials and NGO representatives.

Discussion
Our surveys of wild and domestic ungulates on the Tso Lhamo plateau are not unbiased censuses. The density estimates for summer and winter may not be strictly comparable because of unequal effort and our survey in the kiang’s range in Block 5 is inadequate. Nonetheless, our density estimates for argali and gazelle corroborate the minimum population sizes we tallied in total counts and are therefore probably reasonable indicators of population size. We surveyed a large portion of the study area on multiple occasions and thus believe our distribution maps accurately delineate areas used by ungulates during the study period.
Our study establishes that the densities of argali and gazelles on Tso Lhamo may be amongst the highest in the Indian and Nepal Trans-Himalaya. The unusually large congregations of c. 100 argali (Ganguly-Lachungpa, Reference Ganguly-Lachungpa1996; P. Chanchani, pers. obs.) are unique to Sikkim (Table 2). The localized distribution of the four wild ungulate species within the study area suggests that terrain and landscape influence habitat selection (Pulliam et al., Reference Pulliam, Dunning and Liu1992). Species-specific anti-predator strategies, habitat size and carrying capacity (Singer et al., Reference Singer, Zeigenfuss and Spicer2001), the availability of favoured forage species and anthropogenic disturbance, including livestock grazing, probably also influence the distribution of these species.
Ungulates in Tso Lhamo appear to be faring better than populations elsewhere in the Trans-Himalaya. Nonetheless, argali, gazelle and kiang populations in the region are small, a factor that can affect population persistence (Pimm et al., Reference Pimm, Jones and Diamond1988; Harris & Loggers, Reference Harris and Loggers2004). Based on precedents in the region (Schaller, Reference Schaller1998; Harris et al., Reference Harris, Pletscher, Loggers and Miller1999; Bagchi et al., Reference Bagchi, Mishra and Bhatnagar2004; Mishra et al., Reference Mishra, Van Wieren, Ketner, Heitkonig and Prins2004; Namgail et al., Reference Namgail, Fox and Bhatnagar2007, Reference Namgail, Bagchi, Mishra and Bhatnagar2008; Shrestha, 2007), and anticipating the threats of increased anthropogenic disturbance and stochastic events on small populations, the wild ungulates of Tso Lhamo will probably decline. The local extinction of the chiru Pantholops hodgsonii and an apparent decline in India’s only population of the southern kiang (authors, pers. obs.) highlight the vulnerability of the region’s wild ungulates. Kiang appear to be particularly sensitive to disturbance and their present distribution appears to be reduced in comparison to the range described by Shah (Reference Shah1994).
The region’s ungulates have undoubtedly benefited from the non-hunting ethos prevalent among herders and the defence forces, founded in the local belief that there is an occult danger to hunters in this region. Even domestic animals are only slaughtered at lower elevations to the south and not on the Tso Lhamo plateau. During the winter the distributions of wild and domestic ungulates overlap, both spatially and temporally in some locations, and particularly in areas that are wind-swept and snow free. Wild and domestic ungulates are thus likely to be competing for forage. Well regulated seasonal migratory herding practices have, however, reduced the potential for competition. The absence of livestock in the summer probably benefits wild ungulates both because they have access to nutritious forage and because they face fewer disturbances in this season, when the young of argali and gazelles are born. Restrictions on livestock grazing, imposed by the military in certain areas, which are the regions with the highest densities of wild ungulates, are probably beneficial to wild ungulates.
The creation of a protected area such as a Conservation Reserve (land owned by the government where conservation is prioritized in consultation with local communities), which would permit human habitation and livestock grazing, would facilitate conservation whilst reinforcing local respect for wildlife. Based on our findings we recommend that certain areas, within Blocks 1, 2, 4, 5 and 9 in particular, be protected from undue anthropogenic disturbances. Our findings and recommendations have been noted by Forest Department officials, army officers, local government officers and NGO representatives. A project to curb the feral dog population in the region has been initiated, and some NGOs have initiated collaborative programmes with the village committee in Lachen to promote sustainable tourism. We plan to monitor ungulate populations and contribute to conservation through collaborations with government and non-governmental agencies in the region.
Our study also highlights the need for deliberation on how ecologically important areas along international borders should be managed. The creation of transboundary reserves may benefit the conservation of threatened species (Schaller & Kang, Reference Schaller and Kang2008), and the creation of preserves with connectivity has helped maintain mammalian populations that are more resilient to stochastic events (Noss & Cooperrider, Reference Noss and Cooperrider1994). Connectivity between habitats has facilitated dispersal of various species of mountain ungulates (Hutchins & Geist, Reference Hutchins and Geist1987), and metapopulation behaviour facilitated the re-establishment of argali in Ladakh (Fox et al., Reference Fox, Nurbu and Chundawat1991b). A better understanding of distribution, abundance and resource use across this landscape, over a longer time span, is required for conservation planning. Ultimately, only transboundary conservation initiatives will facilitate the long-term survival of wild ungulates in the Tso Lhamo landscape.
Acknowledgements
This study was funded and supported by the Wildlife Institute of India and WWF India (Sikkim Programme), and facilitated by the Department of Forest, Environment and Wildlife Management, Government of Sikkim. We gratefully acknowledge generous support from the Indian Army and Indo-Tibet Border Police. P.T. Bhutia, C.B. Karma and Wangden are thanked for spirited assistance in the field. S. Tambe and K. Ramesh and D. Ghose are thanked for their valuable support and inputs during the study. PC also acknowledges guidance and support from N. Bhutia, U. Lachungpa, S. Worah, R., S. and N. Chanchani, A. Harihar, I. Agarwal, A. Biviji, N. Page, Q. Qureshi, Y.V. Bhatnagar, N. Shah and N. Gupta. Suggestions from two anonymous referees greatly improved this article.
Biographical sketches
Pranav Chanchani’s primary interests are landscape ecology, conservation biology and sustainable development. Gopal S. Rawat has conducted research on vegetation and wildlife ecology in India for over 2 decades, with an emphasis on the Himalayan and Trans-Himalayan regions. Surendra P. Goyal has been working on the ecology and biology of ungulates and carnivores for 30 years and presently focuses on the use of non-invasive techniques in carnivore ecology.









