Introduction
Multiple sclerosis (MS) is a chronic neuroinflammatory and neurodegenerative demyelinating disease implicating both cell-mediated and humoral immunity. The role of B-cell mechanisms has emerged as a focal point in better understanding the disease’s pathophysiology. As a result, the CD20 B-cell surface molecule has become the target of several of the most effective disease-modifying therapies (DMTs). Reference Florou, Katsara and Feehan1 The anti-CD20 therapy rituximab was first approved in 1997 for hematologic cancers followed by its introduction to autoimmune disease with rheumatoid arthritis in 2006. It is considered an off-label option in MS and was previously evaluated as a DMT. Reference Hawker, O’Connor and Freedman2 Ocrelizumab, a B-cell depleting humanized anti-CD20 monoclonal antibody, was subsequently approved for MS. Reference No authors listed3 More recently, other novel anti-CD20 agents such as ofatumumab and ublituximab have also shown efficacy versus teriflunomide. Reference Hauser, Bar-Or and Cohen4,Reference Steinman, Fox and Hartung5
The OPERA I/II and ORATORIO trials served as the pivotal clinical trials demonstrating benefit of ocrelizumab use in the relapsing remitting MS (RRMS) population with modest reduction in the proportion of patients with progression shown in the primary progressive MS (PPMS) trial driven primarily by younger patients with more inflammatory activity. Reference Hauser, Bar-Or and Comi6,Reference Montalban, Hauser and Kappos7 Aside from infusion-related reactions, infections were reported as the most common adverse event in these studies (56.9% and 71.4% respectively). The majority of reported infections were respiratory and urinary tract infections, of which serious infections represented a minority (1.2% and 6.2% respectively). The ORATORIO trial illustrated an increased risk in the PPMS population. Reference Montalban, Hauser and Kappos7
Reduced B-cell differentiation into plasma cells and decreased antibody production is believed to be a key role player in infection risk. Multiple classes of antibodies or immunoglobulins exist including but not limited to, IgM, IgA and IgG. Reference Schroeder and Cavacini8 IgM is mainly produced in the primary immune response making it useful in facing acute exposure to various pathogens. Slightly higher in serum quantity, IgA is found in high levels in mucosal linings and in secretions such as saliva and breast milk. IgG represents by far the most common and extensively studied subtype and is synthesized mostly in the secondary immune response to pathogens with multiple functions ranging from antibody-dependent cell-mediated cytotoxicity to complement activation. Reference Dycha, Bartusik-Aebisher and Aebisher9 While normal ranges can vary from one center to another, we utilized the same lower limits of normal as the initial clinical trial data to facilitate comparisons (see Methods section). Long-term follow-up studies on the clinical trials have brought to light abnormally low immunoglobulins or hypogammaglobulinemia as a potential adverse event of ocrelizumab. More specifically, a 7-year follow-up study on the patients in the OPERA and ORATORIO trials revealed a proportion of patients who experienced decreased immunoglobulin levels of which serious infection risk seemed to correlate. Reference Hauser, Bar-Or and Comi6,Reference Montalban, Hauser and Kappos7 This association appeared to be strongest with IgG. Reference Hauser, Kappos and Montalban10,11 A similar study revealed similar risk in a long-term rituximab-treated MS population. 12 Hypogammaglobulinemia has been found to occur with other DMTs used in MS however to a much lesser extent than anti-CD20 therapy. Reference Tsao, Otani and Bove13
In this study we examined a real-world cohort of RRMS and PPMS patients treated with ocrelizumab and being followed at our tertiary care MS clinic with the goal of identifying patients who develop hypogammaglobulinemia, serious infections and the risk factors associated.
Methods
Cohort and data collection
The study population consisted of all the patients with either PPMS or RRMS being followed at the MS clinic at the IRDPQ (Institution de Réadaptation en Déficience Physique) center and the affiliated Enfant-Jésus hospital in Quebec City (QC, Canada) who had received at least one infusion of ocrelizumab (of which the initial treatment was divided into two doses as stated below). The treatment was administered in standard fashion with the first infusion being divided into two 300 mg intravenous doses two weeks apart. Subsequent doses were 600 mg per 6 months with a few delays according to specific patient differences and contexts. A secondary list provided by the Roche patient assistance program COMPASS enabled verification of inclusion of all treated patients. Patients who received at least one dose of ocrelizumab were included regardless of whether treatment was suspended or terminated at the final review date. Local institutional research ethics board approval was obtained prior to initiation of the data collection phase.
A chart review was completed using clinical database software at the IRDPQ center. Data such as patient demographics, disease characteristics at time of treatment initiation, hospitalization and serious infection during treatment were identified. Laboratory values were obtained using the provincial Quebec Health Record platform and identification of serious infections was done using a combination of the single center clinical database software and the electronic medical records of the CHU de Québec comprising 5 affiliated hospital centers.
Study outcomes and variables
One author (SN) systematically and retrospectively searched the hospital and provincial database, for every patient, to collect data on: age, sex, MS clinical phenotype (RRMS, PPMS), number of previous DMTs, date of MS diagnosis, disease duration, Expanded Disability Status Scale (EDSS) at time of first infusion, serious infections, and follow-up duration. Individual MS clinical phenotypes were categorized according to the diagnosis given in the chart by the patient’s neurologist using the 2017 McDonald Criteria. Reference Thompson, Banwell and Barkhof14 The data was collected by SN who was not involved in the care or charting of the patients. Follow-up duration was measured as being the time from the first infusion administration to the final review date (August 1st, 2021). The number of infusions and the reason for suspending or stopping ocrelizumab treatment was also noted. Suspension was defined as delaying of treatment beyond the regular 6-month interval for reasons such as pregnancy, losing a patient to follow-up or treatment risk decision-making made by the clinician.
The primary objective of this study was to characterize the frequency of hypogammaglobulinemia. For each patient, baseline immunoglobulins (IgM, IgA, IgG) were measured (if available) as well as subsequent periodic measures at the time of infusions. The term hypogammaglobulinemia was used if IgM < 0.40 g/L, IgA < 0.70 g/L and IgG < 5.65 g/L based on the threshold values used by Hauser et al. Reference Hauser, Kappos and Montalban10 to facilitate comparisons with clinical trial data. CD19 and CD20 levels were always measured as well as complete blood counts. Of note, laboratory tests were conducted within a few weeks or on the same day of treatment infusion. This was further emphasized during the SARS-CoV-2 global pandemic so as to reduce additional visits.
The secondary objective was to characterize serious infection rate. Serious infections were characterized as infections requiring either a hospitalization or an antimicrobial treatment after a visit in the emergency department.
Statistical analysis
The study population characteristics and the treatments received were described via central tendency and dispersion measures (95% confidence interval, mean, median, standard deviation and quartiles) for continuous variables and with relative frequencies for categorical variables. The Student T test or Wilcoxon Mann-Whitney test was used for quantitative variables while Exact Pearson Chi-Squared tests were used for qualitative variables to compare characteristics according to MS phenotype.
The immunoglobulin subtype quantification over time and cumulative incidence of hypogammaglobulinemia according to predefined thresholds at each infusion was analyzed. Univariate logistic regression models were used, and odds ratios were calculated to evaluate the association of various risk factors and laboratory values with hypogammaglobulinemia at baseline and each subsequent treatment. The mean rate of annual variation of each immunoglobulin subtype was estimated using a linear regression model with a generalized estimating equation (GEE) method.
Poisson regression models enabled calculation and comparison of serious infection rates according to MS phenotype and with respect to presence or absence of hypogammaglobulinemia.
A Kaplan-Meier curve was used to evaluate survival without serious infection. The survival analysis was conducted based off the total patient treatment exposures including those with suspended and restarted regimens. Those who only had one round of treatment without further laboratory and follow-up data were not included (6 patients). A Cox regression multivariate analysis allowed for evaluation of various risk factors (MS phenotype, sex, age, number of previous treatments, disease duration, EDSS) on this survival.
Analyses were conducted using the SAS 9.4 analytic software. A p value < 0.05 was considered as being statistically significant.
Results
Patient demographics
We identified 266 patients with MS who received at least one ocrelizumab treatment. The majority of patients received between 3 and 5 infusions (Table 1). When considering suspended and restarted infusions, the mean follow-up time was 2.05 years (2.41 for PPMS, 2.00 for RRMS). At the study cutoff date, 20 patients had stopped treatment and 15 patients had a treatment suspension. Reasons for stopping treatment included: treatment failure (10 patients), treatment intolerance (7 patients) diagnostic uncertainty (1 patient), one patient due to chemotherapy initiation after the discovery of a metastatic colorectal cancer and one patient due to pregnancy followed by treatment switch. Reasons for treatment suspension included pregnancy (11 patients), fear of Covid-related complications (2 patients), repeated infections (1 patient) and one lost to follow-up. Of the patients with treatment suspension, 7 restarted their infusion at a later date.
Table 1: Demographics and clinical characteristics at baseline
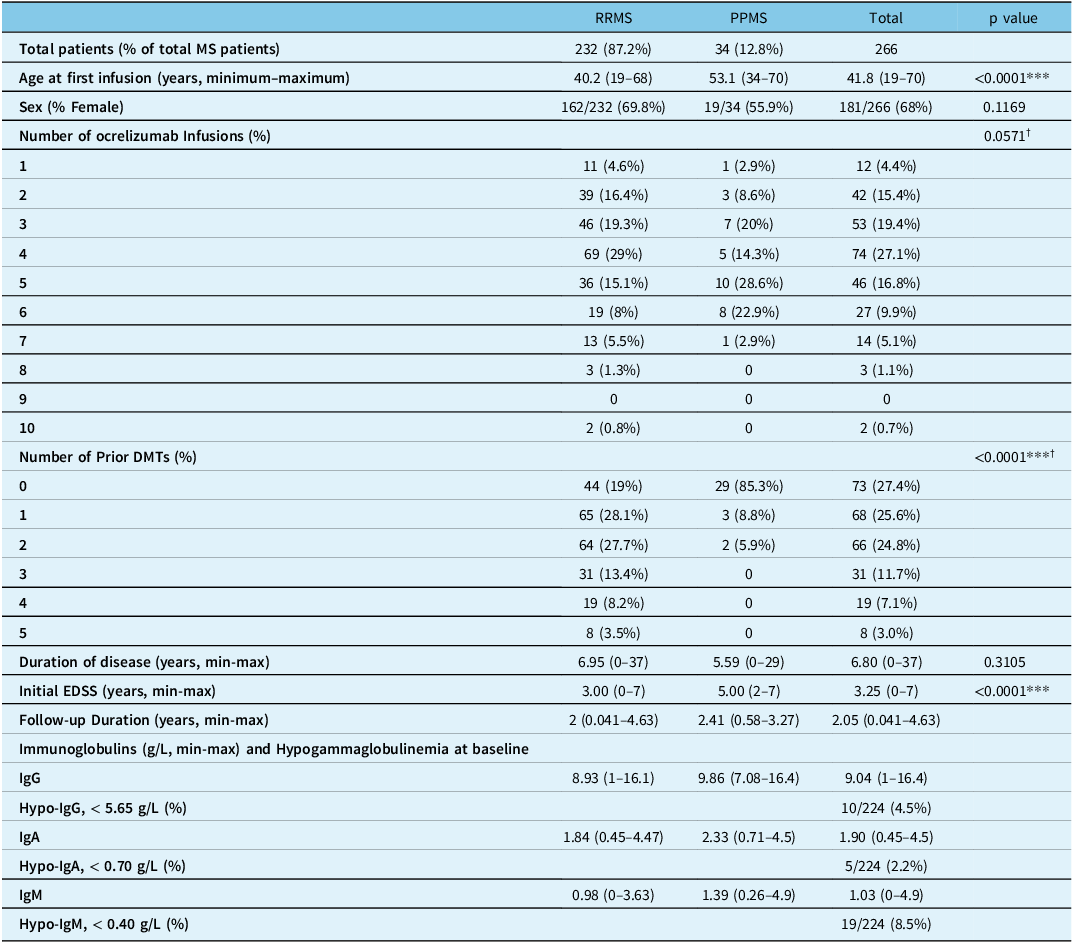
MS = Multiple Sclerosis; RRMS = Relapsing Remitting Multiple Sclerosis; PPMS = Primary Progressive Multiple Sclerosis; DMT = Disease Modifying Therapy; EDSS = Expanded Disability Scale.
*** = statistically significant (p < 0.05).
† p-values represent comparisons between RRMS and PPMS patients for the number of ALL ocrelizumab infusions and of ALL prior DMTs.
Table 1 illustrates the demographics and baseline bloodwork values. Of note, 68% (181/266) of patients were female and 87.2% were of the RRMS phenotype. PPMS patients were significantly older than RRMS patients (53.1 vs 40.2 years old, p < 0.0001). There was a significant difference in the usage of prior DMTs between MS phenotypes as the majority of patients with PPMS were treatment-naïve (85.3%) contrary to RRMS patients (19%). Initial EDSS scores were significantly higher in the PPMS group (5 vs 3, p < 0.0001).
No baseline immunoglobulin levels were measured for 16% (42/266) of patients. Absent immunoglobulin data at least once at time of infusion occurred in 18% (49/266) of patients. Very few patients had baseline immunoglobulin levels compatible with hypogammaglobulinemia (IgM 8.5%, IgA 2.2%, IgG 4.5%). There were no significant differences in baseline CD19/20 levels and complete blood count values (not shown) across MS phenotypes.
Hypogammaglobulinemia
At least one individual hypogammaglobulinemia event occurred after at least one treatment in 32.8% for IgM (< 0.40 g/L), 3.5% for IgA (< 0.70 g/L) and 4.2% for IgG (< 5.65 g/L). The reported rates of individual hypogammaglobulinemia at the time of each infusion up to 6 treatments is represented in Table 2. Of note, IgM hypogammaglobulinemia occurred earlier whereas IgA and IgG hypogammaglobulinemia became slightly more frequent over time. The immunoglobulin levels per treatment reflected this pattern and the grouped mean level for all 3 immunoglobulins never went below the inferior limit of normal threshold (Supplementary Material Table e-1, e-2, e-3). No significant differences were detected between PPMS and RRMS immunoglobulin levels.
Table 2: Hypogammaglobulinemia prevalence at each ocrelizumab treatment

GEE regression revealed a significant decrease in immunoglobulin rate over time for IgM (−0.1892 g/L/year, –0.2103 – –0.1681, p = < 0.0001), IgA (–0.0602, –0.0826 – –0.0377, p = < 0.0001) and IgG (–0.1761, –0.2789 – –0.0733, p = 0.0015).
Variables such as age, duration of disease, initial EDSS and number of prior DMTs were not associated with the occurrence of a hypogammaglobulinemia event during ocrelizumab treatment (Supplementary Material e-4, e-5, e-6). There was a significant association between having baseline hypogammaglobulinemia and subsequent hypogammaglobulinemia events while under treatment (IgM p < 0.0002, IgA p < 0.0001, IgG p < 0.0001). There was no association between the above-mentioned demographic variables with complete blood count values and CD19/20 levels in simultaneous analyses.
Serious infections
There was a total of 21 serious infections in 17 patients (4.65 per 100-person-years) including 8 urinary sepsis cases and 1 simple urinary infection, 4 cellulitis, 2 pneumonias, 1 influenza case, 1 SARS-CoV-2 infection, 1 Shingles, 1 appendicitis, 1 diverticulitis and 1 finger abscess. Only two patients had recurring infections including one patient with 4 episodes of urinary sepsis and another one with 2 cases of pyelonephritis. There was a higher rate of serious infections in PPMS patients compared with RRMS patients (12.33 vs 3.36 per 100-person-years, p = 0.0038) (Table 3).
Table 3: Serious infection risk
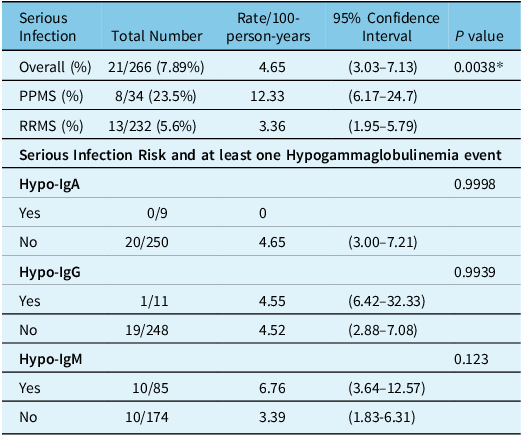
In the second part of the table, serious infections in patients who either had or did not have (yes, no) a hypogammaglobulinemia (IgA, IgG, IgM) event are shown. Totals not adding up to 266 patients are due to missing lab values.
* P value is for the comparison between serious infections in PPMS and RRMS.
Having a single hypogammaglobulinemia event was not significantly associated with serious infection for both IgM (6.76 with and 3.39 without hypogammaglobulinemia, p = 0.123) and IgG (4.55/100-person-years with and 4.52/100-person-years without hypogammaglobulinemia, p = 0.9939) (Table 3). No serious infections occurred for the 9 patients with IgA hypogammaglobulinemia events.
77 patients had lymphopenia after 1st treatment (29.3%) although the majority (74/77 patients) were of grade I or II severity (defined as 0.8–0.999 and 0.5–0.799 × 109/L). Similarly, more patients had neutropenia following the 1st treatment (23 patients, 8.8%) which were also all of grade I or II severity (Supplementary Material e-7, e-8)
A Kaplan-Meier survival curve for serious infections revealed an 87.96% survival rate without serious infection at 54 months post-treatment initiation (Fig. 1). Cox Regression analysis did not reveal any significant associations between serious infection and other variables. With this analysis, PPMS patients tended to have higher rates of serious infection, but contrary to the Poisson regression model, it was not statistically significant (RR 2.075, 0.353–12.209, p = 0.4196). Women tended to have less serious infections compared to men although this was also not significant (RR 0.46, 0.170–1.250, p = 0.1278).
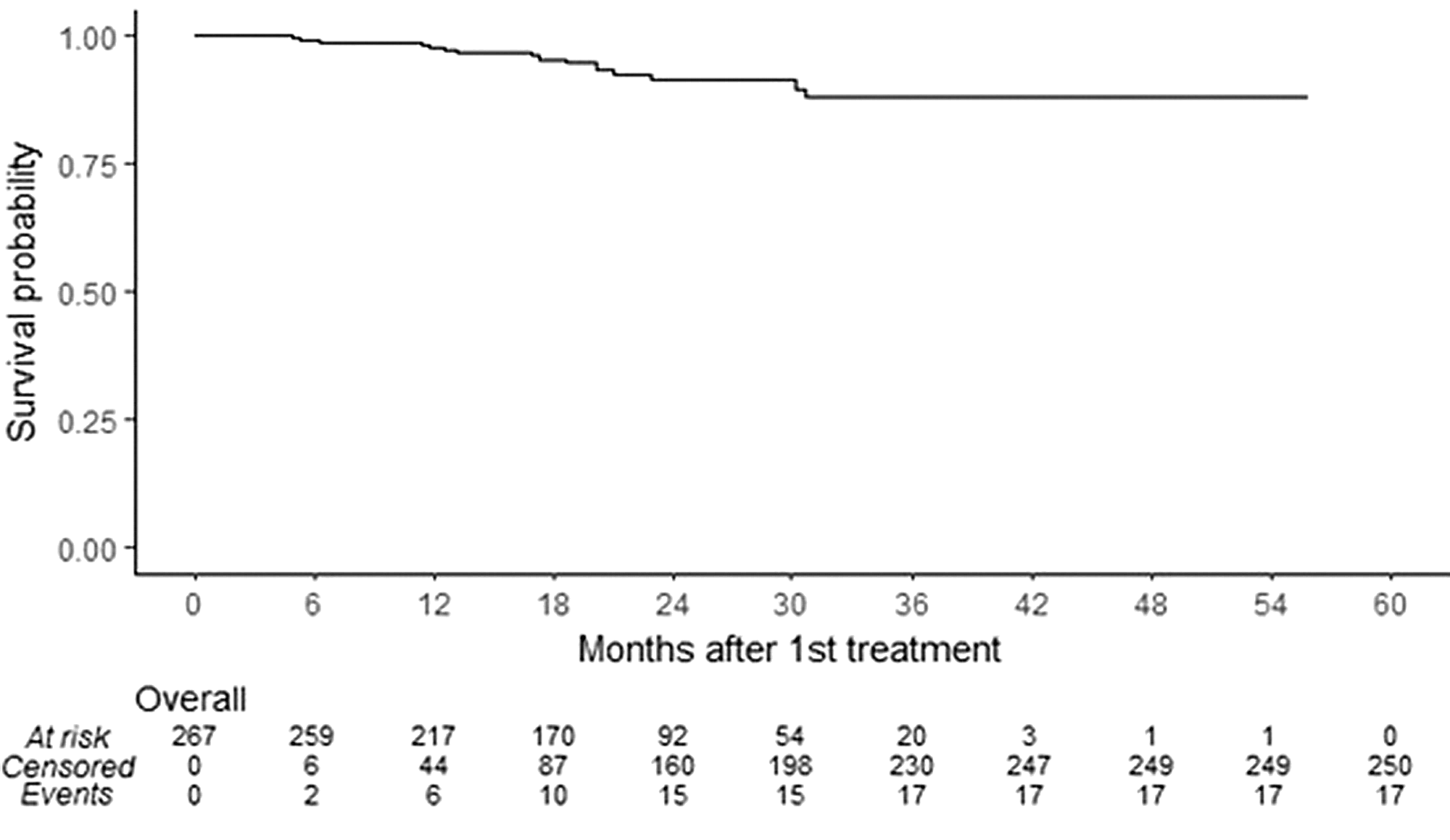
Figure 1: Total at-risk episodes represent all patients having received at least one treatment of ocrelizumab and those resuming treatment after temporary suspension. Of the total 273 episodes, 6 were excluded due to lack of follow-up time.
Discussion
This study represents the largest real-world cohort to date examining the presence of hypogammaglobulinemia and serious infection in ocrelizumab-treated MS patients. We showed in this cohort that at least one individual hypogammaglobulinemia event occurred in 32.8% of patients for IgM, 3.5% for IgA and 4.2% for IgG. We also demonstrated a significant rate of decrease in all immunoglobulins subtypes over time. Moreover, there was a total of 21 serious infections in our cohort of 266 patients with a higher rate in the PPMS subgroup. We did not find any association between hypogammaglobulinemia and serious infection risk.
Hypogammaglobulinemia is a known risk of MS therapies and especially anti-CD20 agents. A study of 1845 patients revealed that anti-CD20 therapy represents the bulk of this risk accounting for nearly 90% of hypogammaglobulinemia cases. Reference Tsao, Otani and Bove13 Moreover, it was initially detected as a potential adverse event of ocrelizumab therapy in the original pivotal trials. Reference Hauser, Bar-Or and Comi6,Reference Montalban, Hauser and Kappos7 It has already been known to be a significant potential consequence of rituximab therapy. Reference Chisari, Sgarlata and Arena15 Similar findings have been found in MS studies confirming risk of hypogammaglobulinemia with rituximab and interaction with serious infection risk. 12,Reference Perriguey, Maarouf and Stellmann16
Rates of hypogammaglobulinemia have been shown to vary across different real-world cohorts ranging roughly from 3% to 40% but with similar distinction between rate of decreases across immunoglobulin subtypes and with IgM tending to have higher incidence of this complication. Reference Habek, Piskač and Gabelić17–Reference Seery, Sharmin and Li20
Our study is consistent with prior data indicating that there is a cumulative treatment effect leading to increasing risk of hypogammaglobulinemia over time for patients continuing ocrelizumab therapy. After 7 years of follow-up on the original trial data, a mean decrease of –0.78g/L was found for IgM and –0.33g/L/year for IgG with similar values for IgA. Reference Hauser, Kappos and Montalban10 The rates detected in our study were higher for IgM but lower for IgG and IgA. The differences may be partially explained by shorter follow-up time especially given that IgG and IgA levels seem to decrease more slowly over time which was also seen in our study.
Our study did not show any associations between multiple risk factors and hypogammaglobulinemia aside from having hypogammaglobulinemia at baseline. A study of more than 500 patients treated with anti-CD20 therapy (17.6% switching to ocrelizumab therapy) suggested that higher cumulative doses of treatment also influenced hypogammaglobulinemia risk. Reference Vollmer, Vollmer and Corboy19 Age increased lymphopenia but not hypogammaglobulinemia risk. Reference Vollmer, Vollmer and Corboy19 A 14-year rituximab Neuromyelitis Optica (NMO) study also revealed baseline hypogammaglobulinemia, duration of treatment, mean annual dose as risk factors as well as history of mitoxantrone use and elevated body mass index. Reference Kim, Park and Kim21
Infections have been known to be elevated in MS patients. Reference Castelo-Branco, Chiesa and Conte22,Reference Wijnands, Kingwell and Zhu23 Moreover, anti-CD20 agents appear to be amongst the DMTs placing patients in the highest category of risk for infections. Reference Luna, Alping and Burman24 Associations with more severe SARS-CoV-2 infection have similarly been found with ocrelizumab. Reference Sormani, de Rossi and Schiavetti25 In our study, 7.89% patients had a serious infection with higher rates detected in the PPMS subgroup (23.5%). While the higher risk in PPMS patients is consistent, these represent higher rates than those detected in the OPERA and ORATORIO trials. Reference Hauser, Bar-Or and Comi6,Reference Montalban, Hauser and Kappos7 This could be due to multiple cohort-specific reasons including better baseline functional (EDSS) status and fewer comorbidities in the clinical trials and a stronger representation of treatment-naïve patients specifically in RRMS patients in OPERA. Moreover, it is possible that our more permissive study definition of serious infection influenced the results.
Trial and associated real-world data for rituximab has shown rates of serious infection to be within the 4.5-10% range with higher values found in the NMO population. Reference Hawker, O’Connor and Freedman2,Reference Chisari, Sgarlata and Arena15,Reference Perriguey, Maarouf and Stellmann16,Reference Kim, Park and Kim21 Real-world ocrelizumab data is limited however more recent studies have shown rates similar to ours ranging from 2.7 to 8.3% rate of hospitalization due to infections with varying representations of MS phenotypes. Reference Habek, Piskač and Gabelić17,Reference Oksbjerg, Nielsen and Blinkenberg18,Reference Seery, Sharmin and Li20 The majority of infections in our study were urinary infections (8 urinary sepsis and 1 simple urinary tract infection) consistent with the MS population known to have high rates of bladder dysfunction with risk of bacterial colonization. Of the total 21 serious infections, one PPMS patient had 4 episodes of recurring urinary sepsis partially explaining the increased rate in the PPMS subgroup.
Higher serious infection risk in progressive MS patients has been reported in multiple national databases often being attributed at least in part to the older age, higher disability, longer DMT usage and higher number of comorbidities in this subgroup. Reference Knapp, Hardtstock and Krieger26,Reference Brand, Smith and Piehl27 While our cohort did reveal higher risk in PPMS patients, other co-factors such as age, sex, EDSS, disease duration and total number of DMTs did not show a link. Insufficient power compared to large national database studies could explain this. Moreover, it is possible that the intrinsic nature of the debilitating PPMS phenotype renders patients more vulnerable to infection, insufficiently captured by EDSS alone. Differences in comorbidity burden could also have played a role as this is known to be higher in progressive patients and is itself linked to a more elevated risk of infection. Reference Knapp, Hardtstock and Krieger26,Reference Langer-Gould, Smith, Gonzales, Piehl and Li28
There was no association between hypogammaglobulinemia and serious infection in this cohort. This could partially be explained by a lack of power or the nature of the analysis which did not involve analyzing risk during specific hypogammaglobulinemia epochs like in the pivotal trials. Long-term follow-up data did show an association between low IgG (<5.65g/L) and serious infection. Reference Hauser, Kappos and Montalban10 Increased rates of infections have already been linked to hypogammaglobulinemia in the context of anti-CD20 therapy with rituximab. Reference Perriguey, Maarouf and Stellmann16,Reference van Vollenhoven, Emery and Bingham29,Reference Barmettler, Ong and Farmer30 Several real-world ocrelizumab studies have shown mixed results with one demonstrating reduced infection risk with elevated IgG and IgA but others showing no interaction. Reference Habek, Piskač and Gabelić17,Reference Oksbjerg, Nielsen and Blinkenberg18,Reference Seery, Sharmin and Li20 Other variables like age, sex and EDSS show inconsistent associations with infection risk probably reflecting the non-uniform risk profile across MS patients.
The SARS-CoV-2 pandemic has put a larger emphasis on better understanding infection risk and optimizing infection prevention strategies. Moreover, it has been shown that anti-CD20 agents subject patients to higher risk of severe SARS-CoV-2 infection. Reference Sormani, de Rossi and Schiavetti25,Reference Stastna, Menkyova and Drahota31 While there are no current specific MS studies looking at hypogammaglobulinemia’s impact on SARS-CoV-2 evolution, small non-MS cohorts do suggest higher rates of severe infection, superinfections, need for mechanical ventilation and prolonged disease course. Reference Scarpa, Dell’Edera and Felice32 While our cohort had low rates of SARS-CoV-2 infection, likely in the context of heightened precautionary measures and the timing of the data collection, we believe this further emphasizes the importance of monitoring immunoglobulin levels, especially in people with MS treated on ocrelizumab who are experiencing recurrent and/or severe infections.
We note that a very small minority of patients in our cohort had baseline hypogammaglobulinemia (of which the majority were IgM). There are currently no guidelines on ocrelizumab initiation strategies in this context. We believe that for minor decreases in immunoglobulins it could be reasonable to retest levels to see if they normalize. However, in contexts with more significant levels of hypogammaglobulinemia before treatment, it might be safer to consider alternative DMT options.
There are several limitations in this study. This study has an observational retrospective design. The follow-up duration was similar to other real-world studies but considerably shorter than the safety data from pivotal trials, potentially explaining differences in rates of hypogammaglobulinemia, especially for slowly declining subtypes like IgG and IgA. Timing between infusions was considerably affected for several patients during the SARS-CoV-2 pandemic which could have underestimated the rates of hypogammaglobulinemia and infection. A significant proportion of patients had missing baseline data with a higher representation coming from patients with treatment initiation at the beginning of the follow-up period. Serious infection definition varies significantly between studies which could explain discrepancies and there is possibility of reporting bias considering data was only obtained from one hospital center’s electronic medical records. The pandemic and its associated precautionary measures may also have created a protective effect resulting in a lower-than-normal infection risk. It is important to note that evaluating only serious infection risk allowed us to avoid underestimating non-serious infection risk given these circumstances.
Conclusion
Overall, our large real-world cohort confirms the risk of hypogammaglobulinemia across all immunoglobulin subtypes with a cumulative effect for MS patients under ocrelizumab therapy. We also showed significant serious infection risk, worse in the PPMS subgroup emphasizing the need to be more cautious when prescribing anti-CD20 therapy in this population. There was no interaction found between hypogammaglobulinemia and serious infection risk. These real-world studies add much needed generalizability to the pivotal trial data and emphasize the risks associated with anti-CD20 therapy.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2024.21.
Author contribution
SN: literature search, data collection and interpretation, project planning, study design, manuscript writing. PB: project oversight, data interpretation, project planning, study design, manuscript editing.
Funding statement
Funding was obtained via the CMDP (Conseil des Médecins, Dentistes et Pharmaciens) of the CHU de Québec. This study was not industry sponsored.
Competing interests
Dr. Steven Nobile: nothing to disclose.
Dr. Philippe Beauchemin: Dr Philippe Beauchemin was invited to advisory board meetings by Biogen Canada, Alexion Canada, Roche Canada, EMD Serono, Sanofi Genzyme, Bristol Myers Squibb. He received honorary fees from Novartis Canada, Roche Canada, Pendopharm Pharma, EMD Serono, Alexion Canada and Bristol Myers Squibb.






