INTRODUCTION
Iatrogenic infections related to outpatient care are becoming a growing concern [Reference Maki and Crnich1], as the proportion of ambulatory care patients in the healthcare system increases [Reference Jarvis2]. To widen the meaning of ‘nosocomial infection’, healthcare-associated infections (HAIs) are defined as infections that patients acquire during the course of receiving treatment for other conditions within the whole spectrum of healthcare [Reference McKibben3]. However, sparse data are available on the risk of HAIs in the ambulatory care setting, which explains the absence of infection control guidelines specific to the outpatient setting. Most epidemics in the outpatient setting have been associated with common source transmissions from contaminated solutions or equipment, usually a medical device, a multidose vial, or an intravenous solution [Reference Hutin4, Reference Hutin, Hauri and Armstrong5]. Contaminating microorganisms, e.g. rapidly growing mycobacteria (RGM), can resist conventional antiseptics [Reference Tiwari6, Reference Pitombo, Lupi and Duarte7] and take advantage of shortcomings in aseptic practices [Reference Siegel8]. RGM outbreak reports related to various interventions, such as injections [Reference Tiwari6, Reference Zhibang9–Reference Yuan11], alternative medicines, including mesotherapy [Reference Carbonne12–Reference Munayco15] and acupuncture [Reference Leao16–Reference Tang18], and cosmetic procedures [Reference Furuya19–Reference Toy and Frank21] are increasing, and are usually concluded using microbiological and epidemiological evidence.
In January 2005, a few malignant cases of recurrent subcutaneous abscess in the gluteal region were reported in Icheon City, South Korea. However, the probability of post-injection abscess outbreak was not raised until late March 2005, when an additional 30 similar cases had been reported. The Icheon Public Health Center (IPHC) launched a thorough investigation to reveal the size of the epidemic and the route of transmission. All suspicious cases were found to have received an intramuscular (i.m.) injection at a single clinic within the city, during the last quarter of 2004. Because the possibility of contaminated injection was considered first, the remaining injection medications at the clinic were able to be recovered and evaluated at the Korea Food and Drug Administration (KFDA) in April 2005; however, no microorganism was found in the medications. Despite this negative result, the possibility of contaminated medication caused great concern to the authorities involved.
Thus, efforts were made to rule out this possibility promptly by conducting an epidemiological investigation immediately after the outbreak was first detected. A comprehensive outbreak investigation was conducted to describe the epidemiological, clinical and microbiological characteristics. Although Mycobacterium massiliense was revealed as the causative microorganism of the outbreak at the initial stage of the investigation, the infection source still remained obscure because all the isolated M. massiliense was from clinical specimens [Reference Kim22]. There-fore a case-control study was conducted to identify the route of transmission and the host factors that precipitated the outbreak. The aim of this study is to describe the outbreak investigation and to provide the possible route of contamination and risk factors for the outbreak of post-injection abscess formation.
METHODS
A professional epidemiological investigation team, consisting of epidemiologists, microbiologists, physicians (including infectious disease specialists), and staff of the local public health authorities, was assembled to investigate the epidemiological, clinical and microbiological characteristics of this outbreak with the cooperation of Korea Centers for Disease Control and Prevention (KCDC) on 1 June 2005, which was about 6–8 months after the medications had been administered. Hence, we performed a comprehensive epidemiological investigation including microbiological tests and clinical research in cooperation with the KCDC and the KFDA.
Case definition and case findings
More than 30 suspected cases were reported at the outbreak identification, all having a common history of i.m. injection at the same ambulatory clinic. Therefore the clinic was suspected of being related to the vehicle of the outbreak. A complete list of patients who visited the clinic from 1 August 2004 to 31 December 2004 was available. Of these, patients with symptoms and signs of a self-reported injection site abscess or suspected through the clinic's medical records were defined as suspected cases. In addition, other information was used to minimize the misclassification of cases: (1) medical records of other local clinics and hospitals with patients exhibiting symptoms and signs of an injection site abscess, (2) suspicious RGM soft tissue infection in Icheon from 1 August 2004 to 31 May 2005 searched in the Korean Medical Insurance Corporation (KMIC) claims database, with ICD-10 codes (International Classification of Disease, 10th revision) A184, A318, A319, L010, L022, L023, L024, L028, L029, L03, L031, L033, L038, L039, L048, L208, L21, L239, L24, L25, L309, L508, L701, L84, L929, M725, M791, R10, R21, R50, T808, T814, T818, Z470, Z488, and Z98. A nationwide public service announcement was released publicizing the outbreak and encouraging residents of the Icheon area that had experienced adverse events after an i.m. injection during the last half of 2004 to enrol in the epidemic survey.
All suspected cases were requested to attend for further evaluation and those with a confirmed abscess or persistent induration at the site of injection by physical examination and ultrasonography were defined as a confirmed case.
Data collection and laboratory investigation
All suspected patients were contacted by telephone, informed of the post-injection abscess outbreak, and asked whether they had experienced any signs or symptoms related to the i.m. injection and were advised to visit the IPHC for confirmation.
The structured questionnaire composed of items including clinical history, details of any gluteal abscess, and exposure information with regard to possible risk factors, demographic factors, abscess characteristics, past medical history, family history, social history, personal hygiene, medication use, and others were used. Risk factors of skin infection (e.g. recent skin injury, being overweight, elderly, skin infection, oedema, other systematic condition of weakened immune system or being treated with corti-costeroid medication) were covered by this questionnaire. Standard operating procedures for interviews and physical examinations were adopted to standardize the data collection process. To collect accurate information on medication exposure, especially with respect to injections, investigators reviewed the medical records of all study participants. Dates of patient visits, names of medications, routes of administration, and other items were recorded.
Specimens from 18 patients were sent to the Department of Microbiology at Seoul National University College of Medicine, and subsequently M. massiliense was isolated from 12 specimens.
Site visits and interviews
A site visit was made on 4 June 2005, which was 5 months after the first case report, and the clinic was closed 4 months before the initiation of this investigation. We collected environmental samples as recommended by KCDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) [Reference Sehulster and Chinn23]. Injected drugs, needles, and alcohol swabs were collected and submitted for microbiological investigation. Injection drugs and normal saline (NS) with the same batch numbers as those used in the clinic were recovered. We interviewed three nurses who worked at the clinic to obtain detailed information on the constitution of injections and the routine procedure used for i.m. injections. Interviewees were asked to reconstruct normal practice and to provide samples from hands, nails, skin, and nasal cavities.
Control selection and data collection
Controls were selected from clinic patients confirmed to be without abscess from 1 August 2004 to 31 December 2004. Each case had three controls matched for age (±5 years) and sex. We conducted the same physical examination and ultrasonography on controls to rule out asymptomatic abscess, and the same information collection procedure was conducted as for cases.
Data management and statistical analysis
We inputted data from completed questionnaires and medical records into a computerized database with double-data entry. All missing values and outliers were compared manually with the original data. After this validation process, the database was locked.
Baseline characteristics of cases and controls were compared by Wald test using the conditional logistic regression model. Body mass index (BMI) groups were categorized according to the WHO Asia-Pacific guidelines for obesity [Reference Kanazawa24], using Korean standards for physical development for paediatric and adolescent patients [25].
We considered exposure to the NS used to reconstitute a powdered drug as a risk factor but there was no written record of NS use. Therefore, we applied exposure to medications supplied in vials that required dilution with NS as a surrogate of NS exposure. We then calculated the risk for abscess formation associated with the administration of medication supplied in vials using a conditional logistic regression model. All potential confounding factors were selected with P<0·2 from univariate analysis, and included in the multivariate analysis. All tests were two-sided and a significance level of 0·05 was used throughout. Statistical analysis was conducted using the SAS System for Windows (release 9.1, SAS Institute Inc., USA).
RESULTS
Characteristics of cases and controls
We found that 2984 patients had visited the clinic from 1 August 2004 to 31 December 2004. Of these, 44 and 34 patients were screened, respectively, by medical record of the clinic and other nearby local clinics. Additionally, one case was found through the KMIC database who had been exposed to ribostamycin injection at the clinic while visiting her daughter who lived in Icheon before returning home to Pusan. Each patient who visited the clinic during the period, including not only the suspected cases but also all others, was contacted by telephone to inform on the epidemic outbreak and to screen by telephone survey. Of 86 suspected cases, 77 patients were identified as cases by physical examination and ultrasonography. Because this post-injection infection of the outbreak was characterized as a cold nodule situated in the gluteal area, cases were unable to identify the date of illness onset. Therefore an epidemic curve was drawn with date of identification instead of onset date (Fig. 1).

Fig. 1. Epidemic curve for 77 confirmed cases and longitudinal distribution of injection dates for 198 participants with a record of intramuscular injection in Icheon City, South Korea, 2005 (date of identification could be different from the onset date because the epidemic outbreak was characterized by cold abscess). From June 2004, 500–1000 ml normal saline (NS) bottles were used until switching back to 20 ml NS after 18 November 2004.
Baseline characteristics of the study participants are presented in Table 1 and the only disease-related characteristic different in cases and controls was obesity (P=0·04).
Table 1. Comparison of host factors among 285 participantsFootnote *
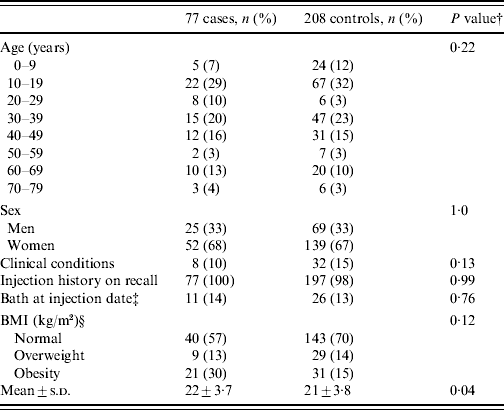
BMI, Body mass index.
* The characteristics, including injection history, described in this table are based on self-reports. All numeric information except frequency are expressed as two significant figures or less.
† P values for the Wald test were calculated using the conditional logistic regression models.
‡ Of 285 participants, 30 were excluded because of missing data for bathing history at injection date.
§ Twelve participants were excluded because of missing BMI data.
Site visits and interviews
The clinic was the only primary local clinic located in large apartment complex where around 1500 residents lived, with 100–200 patients visiting the clinic each day. The clinic had been cleaned by clinic employees 4 months before our site visit. Tap water was negative for any RGM, while M. porcinum and M. fortuitum were isolated from samples obtained from a fish tank located in the waiting room. All environmental samples from the clinic area, including the injection table, medication depot, and rest room were negative for M. massiliense. One physician with an internal medicine subspeciality who was the director and three nurses were at the practice. The nurses constituted and injected the medication to the patients. Samples from hands, nails, and nasal sinus cavities of the three nurses tested negative.
Disposable syringes and needles were not reused between patients. Alcohol swabs were used within 1 week of preparation. Injection drugs, NS, syringes, and needles from the clinic were microbiologically tested and found negative for M. massiliense. In addition, medications and NS with the same lot numbers from neighbouring medical facilities were free of M. massiliense contamination.
During the reconstruction of the i.m. injection protocol, nurses were found to have used 500–1000 ml NS for injection instead of 20 ml NS. They also admitted that they had used 500–1000 ml NS as diluent with no defined expiry date, and falsified receipt of 500–1000 ml NS into 20 ml NS to conceal that they had used 500–1000 ml NS for i.m. injection diluent. Moreover the syringe and needle that was used to extract NS from the multidose bottle was reused repeatedly. This practice had started in June 2004, but ceased in November. From 18 November 2004, just after the outbreak was recognized by the physician at the clinic, the nurses switched back to 20 ml NS thereby concealing any possible recognition of the cause of the outbreak. The timing of the NS diluent change showed good consistency with the cessation of new cases (Fig. 1). In participants with a single injection (participants with multiple injections were excluded because the time point of injection is confusing), NS injections for cases were between 27 September 2004 and 21 November 2004, while only a few controls were exposed to NS injection (data not shown).
Risk of i.m. injection
We found that six medications (ribostamycin, pheniramine, diclofenac, tiropramide, gentamicin, lincomycin) were intramuscularly injected in the gluteal region and we observed that ribostamycin and pheniramine were more frequently injected in the case group. Ribostamycin and pheniramine were commonly co-prescribed for common cold in the clinic (Table 2). To estimate the risk of abscess formation, we included age, sex, and BMI in the model as possible confounders considering biological plausibility and statistical associations. We found that 71 (92%) cases and 78 (60%) controls were exposed to ribostamycin and 50 (65%) cases and 57 (27%) controls were exposed to pheniramine, and the adjusted odds ratios (aORs) with ribostamycin (aOR 48, 95% CI 11–210) or pheniramine (aOR 5·9, 95% CI 3·0–12) were significant. However, when ribostamycin and pheniramine were considered concurrently, pheniramine was found not to be a signifi-cant risk factor of post-injection abscess formation (aOR 8·7, 95% CI 0·44–170). No increased risk was attributed to the other injections (Table 2). This finding was consistent when the practice of vial injection and ampoule injection was adapted (data not shown).
Table 2. Risk for abscess formation by injection medication and container type for 285 participantsFootnote *

NS, Normal saline; aOR, adjusted odds ratio; CI, confidence interval.
* Injection histories described in this table are based on medical records. All numeric information except frequency are expressed as two significant figures or less.
† Univariate aOR was estimated using conditional logistic regression model matched by age (±5 years) and sex.
‡ Multivariate aOR was estimated adjusting body mass index (BMI). Of 285 participants, 12 were excluded because of missing data for BMI.
DISCUSSION
We report here on an outbreak of M. massiliense abscesses related to a deviation from safe injection practice. In our analysis, ribostamycin showed the strongest association with the epidemic, but the causative agent was not isolated from injected medications recovered from the clinic or neighbouring medical facilities. The practice of antibiotic overuse for viral respiratory tract infection treatment is well known [Reference Gaur, Hare and Shorr26, Reference Sanz27]. Use of antimicrobials for viral upper respiratory infection at the clinic was not supported by any clinical practice guideline or any written protocols. The clinic physician who trained and practised before the dissemination of the guidelines that restrain antibiotic use for upper respiratory infection might have been unfamiliar with that guideline. The risk for an RGM infection is greatly increased by the use of multidose NS.
Pheniramine reduces nasal secretions and therefore was frequently co-prescribed with ribostamycin for upper respiratory infection symptoms. In the present study, pheniramine administration was independently found to be significantly associated with abscess formation, even though it was supplied in ampoule form. However, pheniramine was ruled out from a possible transmission component after losing its statistical significance when considered concurrently with ribostamycin. This finding was consistent when risk for abscess was compared between injection with NS, without NS, and both.
Moreover, we found a strong statistical association between NS container type used and abscess formation, and subsequently, a temporal relationship was found between the two using data from the clinic's medication account book and medical records. In other words, when the 20 ml NS container was used there was no report of post-i.m. abscess formation, whereas when the 500–1000 ml container was used there were many instances. Furthermore, when the 20 ml container supply was readopted, no post-i.m. abscess complications were reported. This temporal relationship is further evidence that NS worked as a vector of this outbreak.
Accordingly, at this stage of the investigation NS and ribostamycin were suspected of being causes of the outbreak. However, negative microbiological test results for both NS and ribostamycin indicated that neither was contaminated when first supplied to the clinic. Further, the fact that an intensive search for cases using the national database resulted in finding cases only from that particular clinic supported the view that contamination from outside the clinic was responsible. Considering the i.m. injection practice and the temporal relations referred to previously, we suspected that the NS in multidose containers was contaminated by syringes reused to access the bottles. This situation is similar to a report on a RGM epidemic published in 2009, which attributed NS contamination by M. abscessus to the practice of leaving needles in 100 ml multidose bottles of NS; ribostamycin was also found to have a high odds ratio and a negative microbiological test result [Reference Yuan11].
Although this statistical association strongly supported the ‘contaminated drug’ hypothesis, the absence of hard laboratory evidence was a limitation of our study. The outbreak investigatory process was initiated 6 months after the first case was reported, and because we started collecting data 4 months after the clinic had been closed, there was little chance of obtaining appropriate samples for microbiological investigation. This situation prevented our ruling out or confirming components of the transmission pathway, such as the actual vehicle involved. However, we did find during the early period of the investigation that M. massiliense isolates had identical genotype patterns (data not shown), which suggested that the outbreak originated from a common source [Reference Kim22]. Although environmental specimens were negative for M. massiliense and clinic employees were concealing evidence, the KMIC database enabled us to identify cases and adopt a statistical approach with minimal selection bias; moreover, medical records proved exposure with minimal information bias. However, the KMIC database only contains information on medications covered by national health insurance, and patients who did not visit any other medical institution or were treated for another diagnosis could not be detected.
Our investigation shows that the conventional epidemiological method contributes to the identification of real routes of transmission in cases with sparse microbiological evidence. Utilization of a secondary database with record linkage was helpful to minimize selection bias and information bias during the investigation. However, it took about 2 months to decide whether KFDA or KCDC should be in charge of the epidemiological investigation, since this event was the first case in which a medication was suspected to be the causative agent of the outbreak. Due to the difficulty in forming the joint investigation team including two different agencies, the epidemiological investigation was initiated after the clinic director had closed the clinic. Learning from this outbreak investigation, KFDA and KCDC introduced a manual defining the role and responsibilities of each agency for collaborative investigations involving an outbreak suspected to be associated with a medication or medical device. Moreover, continuous interest in an epidemic caused by drugs produced an epidemiological investigation unit as a formal structure in the National Institute for Drug Safety Management, which is a newly established organization within KFDA for pharmacovigilance.
In conclusion, epidemiological evidence strongly suggested that this post-injection outbreak was caused by saline contaminated with M. massiliense. The experience of this outbreak investigation provided an opportunity to integrate the regulatory workforces and improve the system to combat the next epidemic caused by a medical practice.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korean Center for Disease Control. The funders had no role in designing, conducting, and reporting this epidemic investigation or in preparing the manuscript.
DECLARATION OF INTEREST
None.





