Introduction
Major depressive disorder (MDD) is a serious psychiatric condition and one of the leading causes of illness-induced disability worldwide.Reference Murray, Vos and Lozano 1 - Reference Culpepper, Muskin and Stahl 3 According to the World Health Organization (WHO), over 300 million people suffer from depression worldwide. 4 The WHO has ranked MDD as the third leading cause of burden of disease worldwide and projected that the disease will rank first in contributing to the global disease burden by 2030. 5
While antidepressant therapy is the mainstay of pharmacological treatment for MDD, the effectiveness of standard antidepressants remains suboptimal. 6 - Reference Rush, Trivedi and Wisniewski 8 Naturalistic studies such as the STAR*D have shown that only one-third of patients with MDD achieve remission after a single course of antidepressant treatment, and even after a year of 4 sequenced treatments with different antidepressants, only about two-thirds of the patients with depression achieved remission.Reference Trivedi, Rush and Wisniewski 7 , Reference Rush, Trivedi and Wisniewski 8
Another major limitation associated with antidepressant use is their delayed onset of action. It usually takes 4 to 6 weeks for the full therapeutic effect to manifest.Reference Santarsieri and Schwartz 9 - Reference Artigas, Bortolozzi and Celada 12 This lag in antidepressant action may have negative consequences, such as increased risk of suicide attempts (presumably due to a mismatch in patients’ improvement of symptoms; their lack of energy may be resolved while they are still depressed with negative thoughts), psychosocial dysfunction, and poor quality of life.Reference Wells, Stewart and Hays 13 , Reference Machado-Vieira, Salvadore, Luckenbaugh, Manji and Zarate 14
Ineffective early treatment may also lead to treatment noncompliance, thus resulting in poor outcomes or treatment failure.Reference Voineskos, Daskalakis and Blumberger 15 - Reference Ho, Chong, Chaiyakunapruk, Tangiisuran and Jacob 17 In addition to lack of efficacy, approximately 23% to 36% of patients with MDD being treated with antidepressant therapy discontinue treatment due to adverse effects.Reference Demyttenaere, Enzlin and Dewe 16 , Reference Hu, Bull and Hunkeler 18 About 15% of patients show early worsening of anxiety and 64% experience insomnia within the first 2 weeks of selective serotonin reuptake inhibitor (SSRI) treatment.Reference Hu, Bull and Hunkeler 18 , Reference Gollan, Fava and Kurian 19 Such adverse events that appear early during the treatment course, or late adverse effects such as sexual dysfunction and weight gain, may further contribute to treatment noncompliance and discontinuation.Reference Hu, Bull and Hunkeler 18 - Reference Ferguson 22
Therefore, faster-onset antidepressants with improved tolerability that are devoid of initial activating/anxiety-inducing effects are greatly needed to improve the treatment of MDD.
Interest in early onset of action has been heightened by the studies of treatment resistant patients with second line agents such as ketamine, esketamine, other N-methyl-d-aspartate (NMDA) antagonists, and neuroactive steroids,Reference Artigas, Bortolozzi and Celada 12 , Reference Machado-Vieira, Salvadore, Luckenbaugh, Manji and Zarate 14 , Reference Voineskos, Daskalakis and Blumberger 15 so it seems timely to clarify whether the once-a-day (OAD) formulation of a conventional antidepressant─trazodone may also have an early onset of action for first line treatment of MDD.
Trazodone belongs to the class of serotonin antagonist and reuptake inhibitors that has been available since the early 1970s for the treatment of MDD.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 - Reference Settimo and Taylor 25 It is a multifunctional and multimodal drug with dose-dependent pharmacological actions.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 - Reference Stahl 26 Trazodone has the strongest binding affinity for the 5-HT2A receptors, and it functionally acts as an antagonist. It binds with moderate affinity to serotonin transporters (SERT). It also acts as a partial agonist at 5-HT1A receptors and an antagonist at 5-HT2C receptors. Other clinically relevant pharmacological actions of trazodone include blockade of histamine H1 receptors and α1 adrenergic receptors with affinity higher than that of SERT.Reference Settimo and Taylor 25 , Reference Stahl 26 It is well known among clinicians that trazodone exerts sedative/hypnotic effects by blocking 5-HT2A receptors, α1 adrenergic receptors, and H1 receptors.Reference Stahl 26 At proper doses, starting from 100 to 150 mg/day, trazodone can significantly block SERT like SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs), thus exploiting its potential as an antidepressant.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 , Reference Settimo and Taylor 25 , Reference Stahl 26 In fact, several comparative studies have demonstrated that the efficacy of trazodone is comparable to that of other antidepressants, including tricyclic antidepressants, SSRIs, and SNRIs, but these studies did not compare early onset of action.Reference Beasley, Dornseif, Pultz, Bosomworth and Sayler 27 - Reference Florkowski, Gruszczynski and Galecki 32
The antidepressant efficacy of trazodone has been shown to be significantly correlated to its steady-state plasma levels.Reference Monteleone and Gnocchi 33 Therefore, the formulations of trazodone with different release rates can show a variable course of effectiveness in the medium- and long-term treatment of a depressive episode. This means that for trazodone, the specific pharmacokinetic profile of the formulation used is crucial to address individual patients’ needs.
Trazodone is available in 3 different formulations: immediate-release (IR), prolonged-release (PR), and OAD tablets.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 , 34 , 35 The IR formulation has a rapid onset and short duration of action. Therefore, the need for multiple daily dosing, and the presence of daytime sedation associated with high trazodone peak plasma levels, limits the use of the IR formulation as an antidepressant.Reference Stahl 24 - Reference Stahl 26 The PR formulation is characterized by an absorption boost as soon as it is administered and reaches the C max after around 2.75 hours 36 ; this necessitates a second administration during the day to maintain a proper plasma level for antidepressant efficacy with an increased risk of daytime sedation. The OAD formulation provides a controlled release of trazodone over 24 hours without the early high peak plasma concentration seen with the IR and PR formulations (Supplementary Figure S1), thus generating an antidepressant effect with improved tolerability.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 , Reference Settimo and Taylor 25 , Reference Stahl 26 , Reference Mihara, Yasui-Furukori and Kondo 37 Moreover, the once-daily dose of the controlled-release formulation also simplifies the dosing regimen for patients and may improve treatment compliance.Reference Yildiz, Pauler and Sachs 38 , Reference Demyttenaere 39
In this study, we systematically reviewed results from different randomized clinical trials of trazodone OAD formulation vs active comparator or placebo to determine whether trazodone OAD shows an early antidepressant effect after 7 days of initiating treatment. We also show some recent in vitro proprietary receptor-binding data for trazodone, which largely corroborates the preexisting literature, and discuss putative mechanisms for the faster onset of antidepressant action of trazodone OAD.
Methods
Search strategy
A comprehensive search of PubMed was carried out to conduct a systematic review of studies reporting early antidepressant response in patients treated with trazodone OAD formulation within 1 week of initiating treatment. The search was limited to (1) randomized clinical trials that evaluated the use of only one formulation of trazodone for the treatment of depressive symptoms in patients with MDD and (2) studies published in the last 15 years from 2005 to 2020. The year 2005 was chosen as the lower limit because it was the year that the provisional U.S. patent application for trazodone OAD was filed.Reference Gervais, Smith and Rahmouni 40 The medication was first approved for use by the Food and Drug Administration in 2010 and in Europe in 2014.Reference Češková, Šedová, Kellnerová and Starobová 41 , Reference Khouzam 42 The articles included in this review were identified using the following search terms: ((trazodone [Title/Abstract]) AND (once a day [Title/Abstract])) OR ((trazodone [Title/Abstract]) AND (once daily [Title/Abstract])) OR ((trazodone [Title/Abstract]) AND (once-a-day [Title/Abstract])). The studies of trazodone IR and PR formulations were excluded from this review because their pharmacokinetic profiles are distinct from that of the OAD formulation, and the doses needed to generate an antidepressant response may be associated with adverse effects, such as sedation, that may limit their use as antidepressants.Reference Stahl 26 , Reference Monteleone and Delrio 43
Data extraction
The data in the included studies were extracted into a standardized Microsoft Excel spreadsheet. The following data were extracted: mean scores on the factor composition of HAM-D17 at all study visits including baseline, sample size at each study visit for the HAM-D17 factor analysis, incidence of adverse events reported by ≥5% of the patients, and discontinuation rates.
Data analysis
The mean score change from baseline in the HAM-D17 total score and HAM-D17 factors (anxiety/somatization, cognitive disturbance, retardation, and sleep disturbance) was analyzed at each postbaseline visit using an analysis of covariance (ANCOVA) model where baseline served as a covariate and treatment and pooled centers were sources of variation.Reference Fagiolini, Albert and Ferrando 44 , Reference Sheehan, Croft and Gossen 45 Analysis of variance (ANOVA) was used in cases where the statistical assumptions of ANCOVA were not met.Reference Fagiolini, Albert and Ferrando 44 Two-sided 95% confidence intervals (CIs) were calculated for the differences in mean score change from baseline for trazodone OAD vs placebo, as well as trazodone OAD vs venlafaxine XR, for the same HAM-D17 outcomes analyzed by ANCOVA or ANOVA.Reference Fagiolini, Albert and Ferrando 44 For trazodone OAD vs venlafaxine XR, the noninferiority was accepted if the upper limit of the 95% CIs for the difference between treatments did not surpass the threshold of 3, which represented the maximum difference associated with no clinical relevance.Reference Fagiolini, Albert and Ferrando 44 Trazodone vs placebo was analyzed similarly, except it was treated as a superiority study.
Results
Search results
Our search term criteria yielded articles that only studied the effects of trazodone OAD formulation. Of the 18 articles studying trazodone OAD formulation, only 2 studies were randomized controlled trials (RCTs) that evaluated the antidepressant efficacy of only 1 formulation of trazodone in patients with MDD and reported results at Day 7 after treatment initiation. The results from the 2 RCTs studying trazodone OAD formulation are therefore reviewed here. Both the studies were randomized, double-blind trials in patients with MDD, which showed that trazodone OAD is an effective antidepressant (at a starting dose of 150 mg/day). One of the studies compared trazodone OAD vs placebo and the other compared it to an active comparator, venlafaxine XR.Reference Fagiolini, Albert and Ferrando 44 , Reference Sheehan, Croft and Gossen 45 In both studies, enrollees were instructed to undergo a washout period according to a taper schedule that encompassed 5 elimination half-lives of their specific medication.Reference Fagiolini, Albert and Ferrando 44 , Reference Sheehan, Croft and Gossen 45 Here, we present an in-depth review of both the studies and discuss the results as they relate to trazodone OAD’s early effects on depression.
Early improvement of depressive symptoms with trazodone OAD formulation
In a study of 412 patients with MDD, Sheehan et al found that patients treated with trazodone OAD (dose range during the first week: 150-225 mg/day) showed significantly greater reduction in the mean HAM-D17 total score compared to placebo within 1 week of treatment (intent-to-treat [ITT] population: trazodone OAD −5.6 points vs placebo −3.9 points [95% CI, −2.4; −0.4]; Per protocol (PP) population: trazodone OAD −6.0 points vs placebo −4.2 points [95% CI, −2.6; −0.4]. The difference between the treatment groups in both the ITT and PP populations was statistically significant (P < .05). The corresponding percentage reduction at Day 7 in the mean HAM-D17 total score in the trazodone OAD group was 24% compared to 17% in the placebo group for the ITT population (Percentage reduction in the HAM-D17 total score in the PP population: trazodone OAD 26% vs placebo group 19%). This significantly higher improvement in the depressive symptoms was sustained in the patients treated with trazodone OAD until the study endpoint (Day 56) compared to patients receiving placebo (Figure 1A and Supplementary Figure S2A).Reference Sheehan, Croft and Gossen 45 , Reference Di Dato, Calisti, Comandini, Di Loreto and Cattaneo 46

Figure 1. Data are shown as mean percentage change in the Hamilton Depression Rating Scale (HAM-D17) total score from baseline in the intent-to-treat (ITT) population. (A) Sheehan et al: mean HAM-D17 total scores at baseline were 23.2 points and 22.4 points for trazodone once-a-day (OAD) and placebo, respectively. 95% confidence intervals [CIs] for differences in mean score change from baseline between trazodone OAD vs placebo at Day 7 were [−2.4; −0.4] and at Day 56 were [95% CI, −3.4; −0.4]. (B) Fagiolini et al: mean HAM-D17 total scores at baseline were 23.7 points and 23.8 points for trazodone OAD and venlafaxine XR, respectively. 95% CIs differences in mean score change from baseline between trazodone OAD vs venlafaxine XR at Day 7 were [−1.5; −0.2] and at Day 56 were [95% CI, 0.4; 2.9]., *P < .05; **P < .01, mean score change from baseline between trazodone OAD vs placebo or venlafaxine XR.
Similarly, Fagiolini et alReference Fagiolini, Albert and Ferrando 44 showed that trazodone OAD (dose during the first week: 150 mg/day) showed faster onset of antidepressant action compared to an SNRI, venlafaxine XR (dose during the first week: 75 mg/day) in 324 patients with MDD. A significantly greater reduction in the mean HAM-D17 total score was observed in the trazodone OAD group compared to venlafaxine XR within 7 days of treatment (ITT population: trazodone OAD −4.3 points vs venlafaxine XR −3.5 points [95% CI, −1.5; −0.2]; PP population: trazodone OAD −4.4 points vs venlafaxine XR −3.5 points [95% CI, −1.6; −0.2]; P < .05). This improvement in the depressive symptoms corresponded to an 18% reduction in the HAM-D17 total score in the trazodone OAD group vs 15% in the venlafaxine group for the ITT population (percentage reduction in the HAM-D17 total score in the PP population: trazodone OAD 19% vs venlafaxine group 15%). After the first week, the dose for the trazadone OAD group was increased to 300 mg/day. In contrast to Day 7, at the study endpoint (Day 56), both trazodone (median dose of 300 mg/day) and venlafaxine XR (median dose of 75 mg/day) showed similar overall antidepressant efficacy. (Figure 1B and Supplementary Figure S2B).Reference Fagiolini, Albert and Ferrando 44 , Reference Di Dato, Calisti, Comandini, Di Loreto and Cattaneo 46
This early antidepressant efficacy observed with trazodone OAD is consistent with the evidence from studies of other trazodone formulations for the treatment of MDD. Results from double-blind, randomized clinical trials of trazodone IR and PR formulations demonstrating its faster onset of antidepressant action are summarized in Table 1.Reference Beasley, Dornseif, Pultz, Bosomworth and Sayler 27 , Reference Weisler, Johnston and Lineberry 30 , Reference Di Dato, Calisti, Comandini, Di Loreto and Cattaneo 46 , Reference Zhang, Xie and Li 47
Table 1. Summary of Randomized, Controlled Trials of Trazodone (IR and PR Formulations) in Patients with MDD Demonstrating Its Early Onset of Action

Abbreviations: DSM, Diagnostic and Statistical Manual of Mental Disorders; MDD, major depressive disorder; IR, immediate-release; ITT, intent-to-treat; PR, prolonged-release.
a HAM-D21.
b Last observation carried forward.
c Graphical extrapolation.
Efficacy of the trazodone OAD formulation for improvement in the HAM-D17 factors
Depression is a multifaceted disorder, which is characterized by depressed mood along with several significant psychological, cognitive, and behavioral components. Different antidepressants, based on their pharmacological profile, may initiate improvement in various clinical components at different rates.Reference Katz, Koslow and Frazer 48 , Reference Katz, Tekell and Bowden 49 Therefore, we also reviewed the efficacy of trazodone OAD in improving individual depression dimensions as assessed by the scores on 4 HAM-D17 factors: anxiety/somatization, cognitive disturbance, retardation, and sleep disturbance. The scores on the 4 HAM-D17 factors are comprised of the following items: anxiety/somatization—anxiety (both psychic and somatic), somatic symptoms (both general and gastrointestinal), hypochondriasis, and insight; cognitive disturbance—feelings of guilt, suicide, and agitation; retardation—depressed mood, work and activities, retardation, and genital symptoms; sleep disturbance—insomnia early, insomnia middle, and insomnia late.
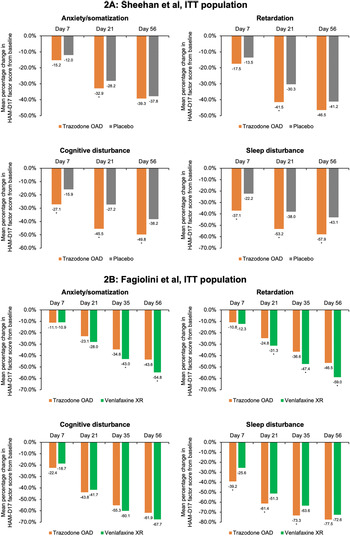
In the placebo-controlled study, conducted by Sheehan et al,Reference Sheehan, Croft and Gossen 45 trazodone OAD showed a trend for early improvement at Day 7 in the HAM-D anxiety/somatization factor compared to placebo; however, this difference was statistically significant only at Day 21 for both the ITT [95% CI, −1.0; −0.1] and the PP [95% CI, −1.3; −0.2] populations (P < .05) (Figure 2A and Supplementary Figure S3A). In the active-comparator study by Fagiolini et al,Reference Fagiolini, Albert and Ferrando 44 both trazodone OAD and venlafaxine showed similar improvements in the anxiety/somatization factor at Day 7, and the difference between the groups was statistically significant in the favor of venlafaxine XR at Days 35 [95% CI, 0.1; 1.0] and 56 [95% CI, 0.3; 1.3] for the ITT population and only at Day 56 [95% CI, 0.0; 1.0] for the PP population (P < .05) (Figure 2B and Supplementary Figure S3B).
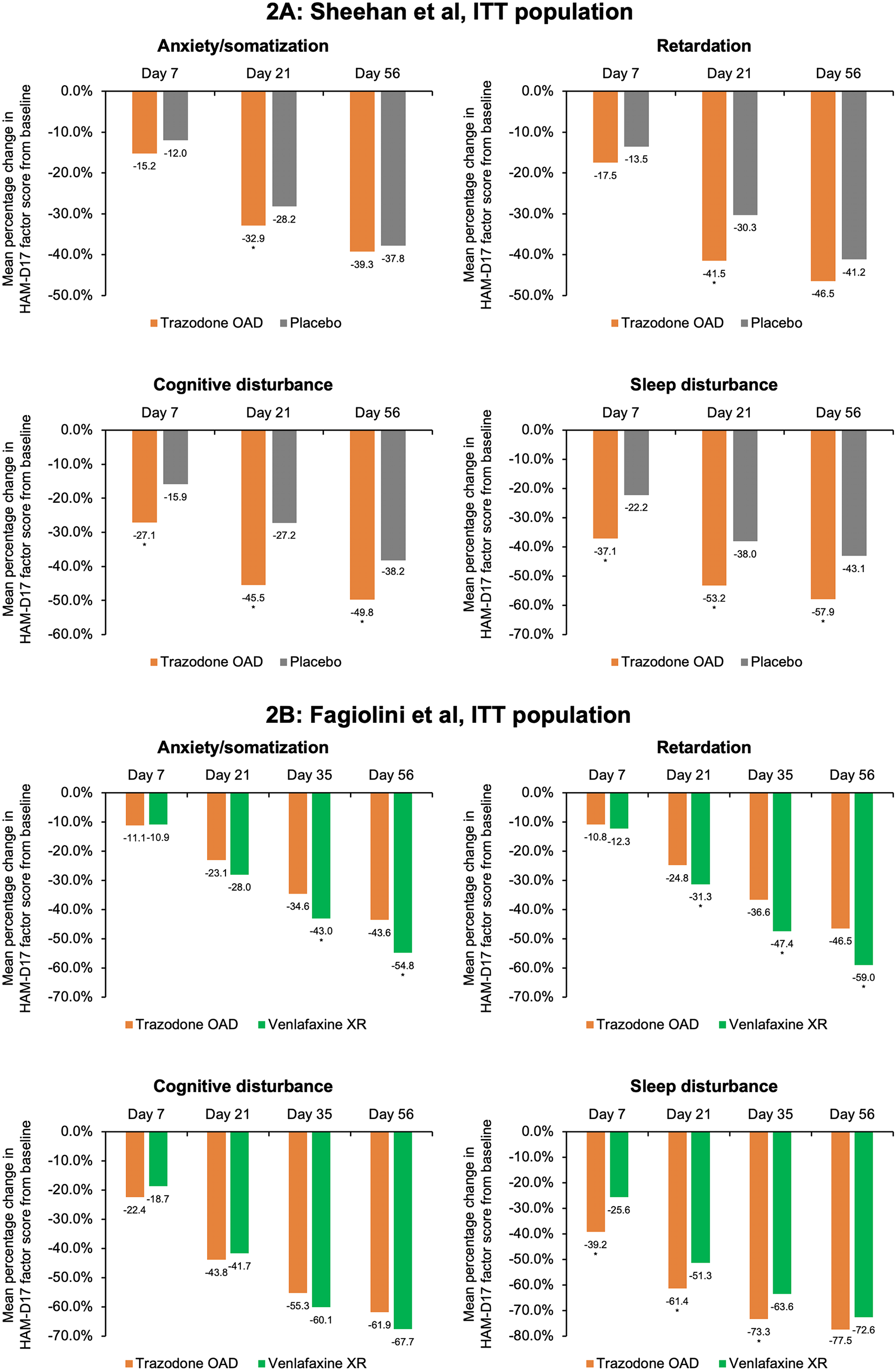
Figure 2. Mean percentage change from baseline in the factor composition of Hamilton Depression Rating Scale (HAM-D17) for the intent-to-treat (ITT) population. (A) Sheehan et al study. Ninety-five percent confidence intervals (CIs) were calculated for mean score change from baseline in trazodone once-a-day (OAD) vs placebo-treated patients for anxiety/somatization at Day 7 [−0.5; 0.3], Day 21 [−1.0; −0.1], and Day 56 [−1.0; 0.2]; for retardation at Day 7 [−0.6; 0.1], Day 21 [−1.4; −0.5], and Day 56 [−1,1; 0.0]; for cognitive disturbance for Day 7 [−0.7; −0.2], Day 21 [−0.8; −0.2], and Day 56 [−0.8; −0.2]; and for sleep disturbance for Day 7 [−1.0; −0.3], Day 21 [−1.1; −0.3], and Day 56 [−1.1; −0.3]. (B) Fagiolini et al study. 95% CIs were calculated for mean score change from baseline in patients treated with trazodone OAD vs venlafaxine XR for anxiety/somatization at Day 7 [−0.3.; 0.3], Day 21 [0.0; 0.8], Day 35 [0.1; 1.0], and Day 56 [0.3; 1.3]; for retardation at Day 7 [−0.2; 0.4], Day 21 [0.1; 0.8], Day 35 [0.4; 1.3], and Day 56 [0.4; 1.4]; for cognitive disturbance for Day 7 [−0.3; 0.0], Day 21 [−0.2; 0.3], Day 35 [−0.1; 0.3], and Day 56 [0.0; 0.4]; and for sleep disturbance for Day 7 [−1.1; −0.4], Day 21 [−0.9; −0.2], Day 35 [−0.8; −0.2], and Day 56 [−0.5; −0.1]. *P < .05, mean score change from baseline between trazodone OAD vs placebo or venlafaxine XR.
For the HAM-D cognitive disturbance factor, trazodone OAD showed statistically significant (P < .05) early improvement compared to placebo at Day 7 for the ITT [95% CI, −0.7; −0.2] and the PP [95% CI, −0.6; 0.0] groups, which was sustained until the end of the study for both populations in the study by Sheehan et alReference Sheehan, Croft and Gossen 45 (Figure 2A and Supplementary Figure S3A). In the study by Fagiolini et al, Reference Fagiolini, Albert and Ferrando 44 both trazodone OAD and venlafaxine XR showed similar reduction in the cognitive disturbance scores for both the ITT and PP populations (Figure 2B and Supplementary Figure S3B).
In the Sheehan et alReference Sheehan, Croft and Gossen 45 study, trazodone OAD showed a trend for early improvement in the HAM-D retardation factor at Day 7, but the difference between trazodone and placebo was only statistically significant at Day 21 [95% CI, −1.4; −0.5] for the ITT population (P < .05). However, for the PP population, treatment with trazodone resulted in significantly greater reduction in the retardation factor within 1 week of treatment compared to placebo [95% CI, −0.9; 0.0], which was maintained at all study visits until the analyzed endpoint. (P < .05) (Figure 2A and Supplementary Figure S3A). In the Fagiolini et alReference Fagiolini, Albert and Ferrando 44 study, both trazodone OAD and venlafaxine XR showed similar reduction in the retardation factor scores at Day 7 for the ITT [95% CI -0.2; 0.4] as well as PP [95% CI, −0.3; 0.3] populations. However, the difference between treatment groups was statistically significant (P < .05) in the favor of venlafaxine at Days 21 [95% CI, 0.1; 0.8], 35 [95% CI, 0.4; 1.3], and 56 [95% CI, 0.4; 1.4] for the ITT population and at Days 35 [95% CI, 0.2; 1.1] and 56 [95% CI, 0.2; 1.1] for the PP population (Figure 2B and Supplementary Figure S3B).
In the Sheehan et alReference Sheehan, Croft and Gossen 45 trial, trazodone OAD showed statistically significant (P < 0.05) early improvement in the HAM-D sleep disturbance factor at Day 7 compared to placebo for both the ITT [95% CI, −1.0; −0.3] and PP [95% CI, −1.0; −0.1] populations. Trazodone OAD also showed a greater reduction (P < 0.05) in this measurement at Day 7 as compared to venlafaxine XR for both the ITT [95% CI, −1.1; −0.4] and PP [95% CI, −1.1; −0.3] populations.Reference Fagiolini, Albert and Ferrando 44 This greater improvement in the sleep disturbance factor observed with trazodone was sustained at all the post-baseline visits until the study endpoint, except for Day 56 in the ITT population in the Fagiolini et al study, where trazodone treatment showed a trend for improvement in sleep disturbance; however, the difference between treatment groups did not achieve statistical significance (Figure 2A,B and Supplementary Figures S3A,B).Reference Fagiolini, Albert and Ferrando 44 , Reference Sheehan, Croft and Gossen 45
Safety and tolerability of the trazodone OAD formulation
Trazodone OAD formulation is well tolerated for the treatment of MDD. The severity of adverse events in both the studies was mild to moderate. The most common adverse events (≥5% in any treatment group) in both the studies were headache, somnolence, dizziness, dry mouth, and nausea (Table 2.). Additionally, the incidence of serious adverse events was low (Fagiolini et al: 3/165; Sheehan et al: 3/202). More patients in the trazodone OAD discontinued treatment due to adverse events in both studies. The most common adverse events that led to treatment discontinuation in the trazodone OAD group were dizziness, sedation, somnolence, and electrocardiogram (ECG) abnormality/ECG QT prolongation. 36 , Reference Fagiolini, Albert and Ferrando 44 , Reference Sheehan, Croft and Gossen 45
Table 2. Summary of Adverse Events Observed with Trazodone once-a-day (OAD) in the Placebo- and Active-Comparator Studies
Discussion
Early onset of antidepressant action is a desirable therapeutic outcome, and the emphasis on this action by novel antidepressants with NMDA antagonist and neuroactive steroid mechanisms for second-line treatment of resistant depressed patients highlights the need as well for early onset of action for first line treatment of MDD. This systematic review of 2 randomized, double-blind clinical trials evaluating the efficacy and safety of trazodone OAD formulation in patients with MDD suggests that trazodone OAD can result in an early antidepressant effect within 7 days of initiating treatment. However, the limited number of studies in this review warrant future clinical studies assessing the onset and time course of improvement with trazodone OAD treatment to confirm our findings. Trazodone OAD treatment resulted in statistically significant reduction in the total HAM-D score within 7 days of starting treatment compared to placebo or an active comparator (venlafaxine XR).Reference Fagiolini, Albert and Ferrando 44 - Reference Di Dato, Calisti, Comandini, Di Loreto and Cattaneo 46 At the study endpoint (Day 56), this improvement in depressive symptoms was sustained in the placebo-controlled study with the trazodone OAD treatment. However, in the active-comparator study at Day 56, venlafaxine XR showed greater antidepressant efficacy compared to trazodone OAD in the ITT population and no significant difference was observed in the PP population (the study population with a higher-level treatment compliance); in fact, the severity of depression in both trazodone OAD as well venlafaxine XR groups decreased from moderate to mild in the both the ITT and PP population.Reference Fagiolini, Albert and Ferrando 44 Interestingly, other studies have also shown that while venlafaxine may be a comparable or even more effective antidepressant at the study endpoint, treatment with trazodone may result in an early onset of antidepressant effect.Reference Cunningham, Borison and Carman 29 , Reference Cipriani, Furukawa and Salanti 31 , Reference Florkowski, Gruszczynski and Galecki 32
In fact, this early antidepressant efficacy of trazodone OAD was also observed in studies of other trazodone formulations (including the IR and PR formulations).Reference Beasley, Dornseif, Pultz, Bosomworth and Sayler 27 , Reference Weisler, Johnston and Lineberry 30 , Reference Zhang, Xie and Li 47 Overall, the percentage reduction of the HAM-D score after 7 days of treatment with trazodone (all formulations) ranged from 18% to 33%.Reference Beasley, Dornseif, Pultz, Bosomworth and Sayler 27 , Reference Weisler, Johnston and Lineberry 30 , Reference Fagiolini, Albert and Ferrando 44 , Reference Sheehan, Croft and Gossen 45 , Reference Zhang, Xie and Li 47 While the pharmacokinetic profiles of the IR and PR formulations may limit the treatment tolerability and compliance in patients due to the need for multiple dosing during the day, the early antidepressant response observed with trazodone regardless of the formulation further lends supporting evidence for early response in depression with trazodone treatment. This early antidepressant efficacy of trazodone OAD has also been observed in routine clinical practice. In an observational study conducted by Češková et alReference Češková, Šedová, Kellnerová and Starobová 41 in 8 psychiatric centers treating patients with moderate to severe depression, trazodone OAD formulation demonstrated statistically significant decreases in the overall Montgomery–Åsberg Depression Rating Scale score of patients at Week 1 after initiating the treatment. Most patients reported improvement in the overall severity of their illness as assessed by the Clinical Global Impression-Severity scale after 6 days of treatment, thus demonstrating that the early improvement observed with trazodone was clinically meaningful.Reference Češková, Šedová, Kellnerová and Starobová 41 , Reference Posternak and Zimmerman 50 This suggests that early onset of antidepressant action is a specific characteristic of trazodone.
Early improvement with antidepressants has important clinical implications. Several pooled analyses of RCTs and prospective naturalistic studies have demonstrated that early improvement in depressive symptoms is a clinically useful predictor of sustained response and lack of early improvement can predict nonresponse.Reference Stassen, Angst, Hell, Scharfetter and Szegedi 51 - Reference Lam 55 In fact, in the study by Sheehan et al,Reference Sheehan, Croft and Gossen 45 early improvement with trazodone OAD formulation was further characterized by a greater number of HAM-D responders compared to placebo. The ability to know as early as possible that a patient will or will not respond to an antidepressant allows the clinicians to encourage the ultimate responders to keep taking the medication and adjust the treatment plan for the ultimate non-responders to minimize their time spent on ineffective treatment.
In addition to trazodone OAD’s fast onset of action in improving the overall core symptoms, we also reviewed early improvements in specific depressive symptoms. The sleep disturbance factor showed the greatest improvement at Day 7 across the ITT and PP populations in both the placebo-controlled and active-controlled studies of trazodone OAD formulation.Reference Fagiolini, Albert and Ferrando 44 , Reference Sheehan, Croft and Gossen 45 This early improvement observed with trazodone OAD compared to placebo sustained until the end of the study duration. Trazodone’s efficacy for improvement in insomnia in patients with MDD, particularly early in the therapy, has also been previously reported in other randomized, double-blind studies comparing trazodone to placebo or other standard antidepressants.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 , Reference Cunningham, Borison and Carman 29 , Reference Weisler, Johnston and Lineberry 30 , Reference Munizza, Olivieri, Di Loreto and Dionisio 56 , Reference Kasper, Olivieri, Di Loreto and Dionisio 57 A post-hoc analysis of the placebo-controlled study by Sheehan et alReference Sheehan, Rozova, Gossen and Gibertini 58 further showed that the antidepressant efficacy of trazodone OAD was independent of the early improvements observed in the sleep disturbance factor. Thus, this suggests that treatment with trazodone OAD can provide early depressive symptom relief including the early and sustained benefit on insomnia.
Insomnia is reported in more than 90% of the patients with MDD.Reference Stahl 24 It is one of the most frequent residual symptoms that persists after treatment with standard antidepressants, resulting in relapse and recurrence.Reference Stahl 24 , Reference Yildiz, Pauler and Sachs 38 , Reference Krystal 59 Therefore, an antidepressant such as trazodone OAD, with the ability to reduce sleep disturbance, may improve overall outcomes in patients with MDD.
The sedative/hypnotic property of trazodone suggests that trazodone may also confer anxiolytic benefits.Reference Sheehan, Rozova, Gossen and Gibertini 58 Our review of the placebo-controlled study of trazodone OAD showed a trend of early improvement in the anxiety/somatization factor, but the change in the HAM-D factor score from baseline to Day 7 was not statistically significant.Reference Sheehan, Croft and Gossen 45 While systematic studies demonstrating the efficacy of trazodone for treating anxiety are limited, several studies comparing the efficacy of trazodone and other antidepressants have shown that trazodone can alleviate symptoms of anxiety with the first week of treatment.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 , Reference Munizza, Olivieri, Di Loreto and Dionisio 56 , Reference Haria, Fitton and Trazodone 60 A randomized double-blind study comparing the trazodone PR formulation to sertraline for treatment of MDD demonstrated early onset of anxiolytic activity for patients taking trazodone, compared to those taking sertraline, within 1 week of treatment as assessed by the reduction in the Hamilton Anxiety Rating Scale (HAM-A).Reference Munizza, Olivieri, Di Loreto and Dionisio 56
Many data for binding affinity of trazodone with different neurotransmitter targets (receptor and transporters) are already available in literature.Reference Settimo and Taylor 25 , Reference Stahl 26 , Reference Roth and Driscol 61 In Figure 3, we show some recent in vitro proprietary data for binding affinity of trazodone for its targets.Reference Mangano, Mancini, Durando and Ragni 62 Trazodone shows high to medium affinity (pKi 6-8) on a series of receptors subtypes. Trazodone has the highest binding affinity for 5-HT2A receptors. Most affinities are comparable to other reported in literature, but interestingly, a higher affinity for 5-HT7 with respect to previously published data were found (not substantially different from SERT), supporting the hypothesis that a pharmacological activity on this receptor could be exerted at therapeutic concentrations for antidepressant activity.Reference Mangano, Mancini, Durando and Ragni 62

Figure 3. Relative binding affinities of trazodone for neurotransmitter receptors and transporters. 5-HT, 5-hydroxytryptamine (serotonin) receptors (different subtypes); alpha, alpha adrenergic receptors (different subtypes); H, histamine receptor (subtype); SERT, serotonin reuptake transporter.
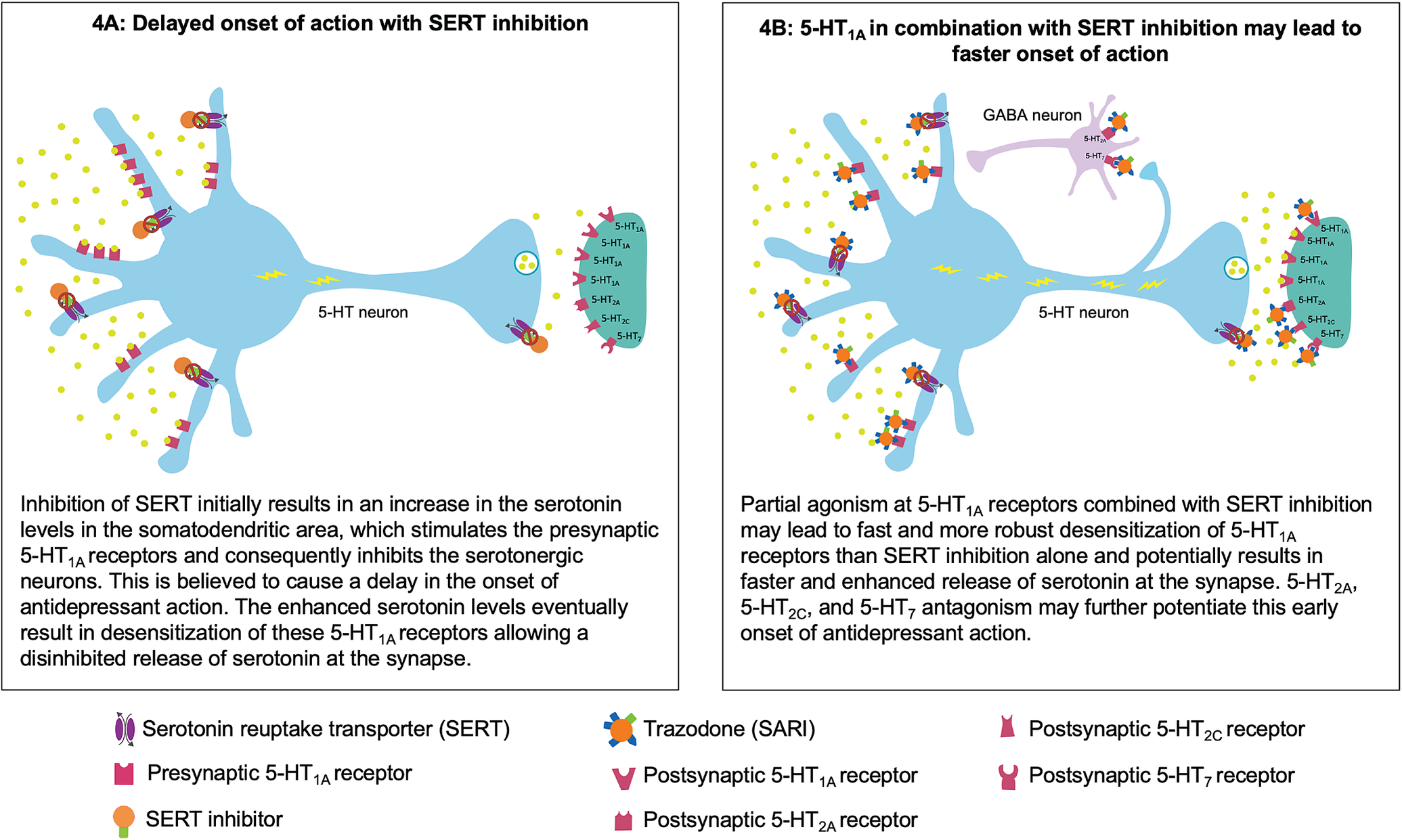
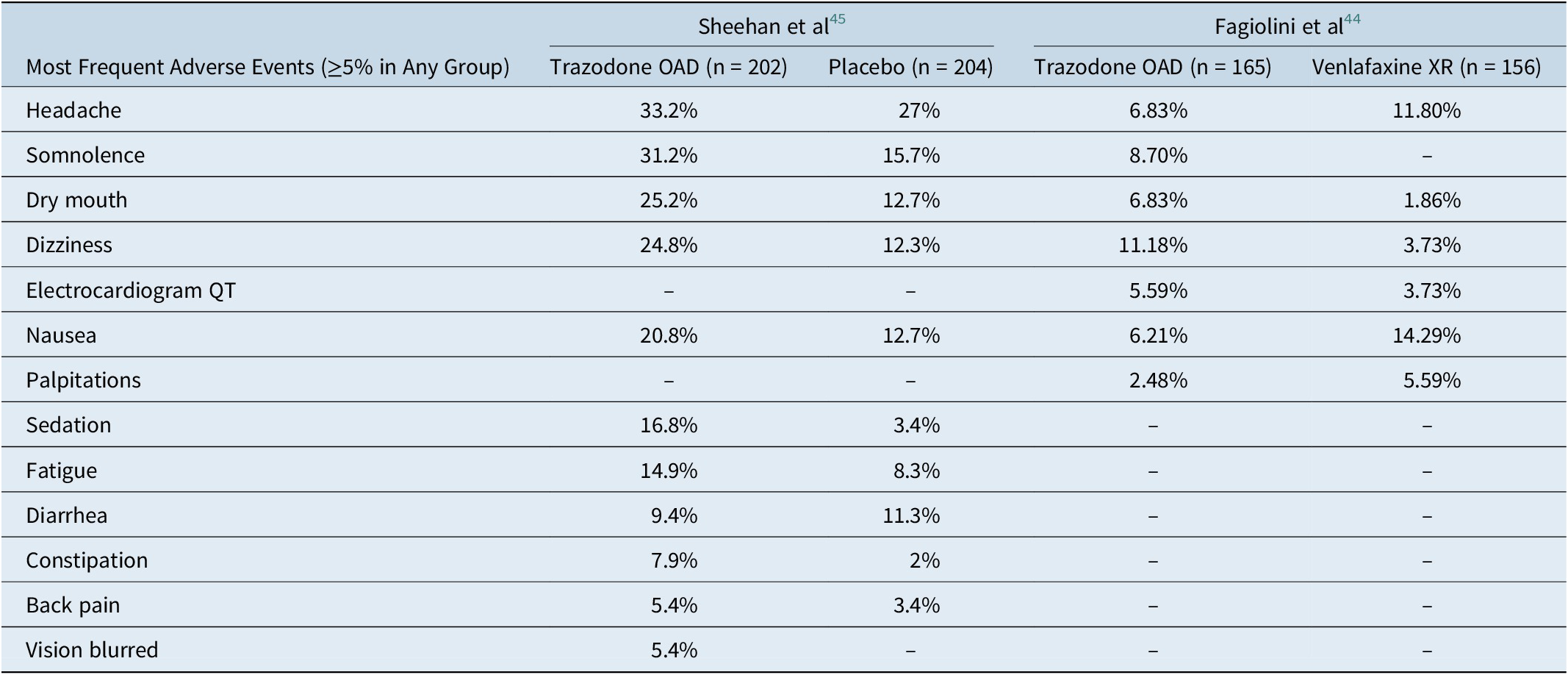
Figure 4. Putative mechanism for the fast onset of antidepressant action of trazodone. (A) Delayed onset of action with serotonin reuptake transporter (SERT) inhibition. (B) Partial agonism at 5-HT1A in combination with SERT inhibition may contribute to faster onset of action.
Trazodone’s early onset of antidepressant action may be attributable to its multifunctional and multimodal pharmacological properties. At a starting dose of 100 to 150 mg/day, trazodone exerts antidepressant action by blocking SERTs.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 - Reference Stahl 26 At these doses, trazodone also acts at other receptors in the range of SERT or for which it has a higher affinity. In fact, according to a published pharmacokinetic simulation study, the binding affinity of trazodone for selected receptors at a dose of 150 mg/day could be sufficient to fully occupy these receptors in steady state conditions. It is possible that trazodone’s activity at these receptors, along with SERT inhibition, has a synergistic effect that accelerates the onset of antidepressant action.Reference Tollefson and Holman 11
Trazodone’s partial agonist actions at the 5-HT1A receptors in combination with SERT inhibition may contribute to its faster onset of antidepressant action.Reference Montalbano, Mlinar and Bonfiglio 63 , Reference Stahl 64 In general, the antidepressant effect of serotonin reuptake inhibition is potentially mediated via presynaptic and postsynaptic 5-HT1A receptors. Following SERT inhibition, the increased serotonin levels inhibit the serotonergic neurons in the raphe nuclei via the stimulation of the presynaptic somatodendritic 5-HT1A receptors, which are believed to cause a delay in the onset of antidepressant effect. Eventually, this increase in the serotonin levels leads to desensitization of these presynaptic 5-HT1A receptors, resulting in enhanced serotonin release at the synapse with subsequent antidepressant actions mediated via the postsynaptic 5-HT1A receptors.Reference Artigas, Bortolozzi and Celada 12 , Reference Stahl 26 Preclinical studies suggest that 5-HT1A partial agonism along with SERT inhibition leads to much faster action at these 5-HT1A receptors, thus resulting in more immediate and robust increase in the serotonin release.Reference Stahl 64 - Reference Page, Cryan and Sullivan 67 It has recently been shown that trazodone partial agonism of 5-HT1A receptors, combined with adrenergic blockade, could help regulate firing of raphe neurons and consequently contribute to serotonin modulation in an early stage of administration.Reference Montalbano, Mlinar and Bonfiglio 63
Trazodone’s most potent binding property, leading to 5-HT2A antagonism, could further complement this rapid onset of antidepressant action.Reference Settimo and Taylor 25 , Reference Stahl 26 , Reference Luparini, Garrone, Pazzagli, Pinza and Pepeu 68 Luparini et alReference Luparini, Garrone, Pazzagli, Pinza and Pepeu 68 demonstrated that trazodone increases serotonin levels through a dual mechanism: at low concentrations by reducing the inhibitory GABAergic tone through the antagonism of 5-HT2A receptors and at high concentrations through SERT inhibition. Therefore, 5-HT2A antagonism along with SERT inhibition may exert an additive effect in terms of serotonin release, which may result in antidepressant activity, possibly through 5-HT1A downregulation.Reference Pazzagli, Giovannini and Pepeu 69 - Reference Subhash, Srinivas and Vinod 71 In addition to an antidepressant effect, antagonism of 5-HT2A and partial agonism of 5-HT1A receptors in combination with histamine H1 receptors and α1 adrenergic receptors potentially contribute to trazodone’s sedative/hypnotic and anxiolytic effects.Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 - Reference Stahl 26
Trazodone also antagonizes 5-HT2C and 5-HT7 receptors, which may further contribute to its fast antidepressant action.Reference Settimo and Taylor 25 , Reference Stahl 26 , Reference Opal, Klenotich and Morais 72 , Reference Mnie-Filali, Faure and Lambas-Senas 73 Trazodone’s affinity for 5-HT2C and 5-HT7 receptors is in the range of SERT.Reference Settimo and Taylor 25 , Reference Stahl 26 , Reference Mangano, Mancini, Durando and Ragni 62 In preclinical in vivo depression models, 5-HT2C antagonists have been shown to induce fast-onset antidepressant action within 5 days of treatment initiation compared to SSRIs.Reference Opal, Klenotich and Morais 72 Evidence from literature suggests that this 5-HT2C antagonism induces rapid onset, possibly through an increase in the mesocortical dopaminergic signaling.Reference Stahl 26 , Reference Opal, Klenotich and Morais 72 , Reference Di Matteo, Di Giovanni, Di Mascio and Esposito 74 , Reference Sotty, Folgering and Brennum 75 Antagonism of 5-HT2C receptors may also contribute to trazodone’s beneficial effect in alleviating symptoms of anxiety. It has been shown that serotonin release from the dorsal raphe nucleus enhances fear and anxiety through activation of 5-HT2C receptors on a subpopulation of corticotropin-releasing factor neurons.Reference Yohn, Gergues and Samuels 76 , Reference Marcinkiewcz, Mazzone and D’Agostino 77 Therefore, antagonism at 5-HT2C receptors along with trazodone’s action at other serotonergic receptors may potentiate its anxiolytic effects.
Pharmacological blockade of 5-HT7 receptors in preclinical studies has also been shown to induce fast antidepressant response within a week of initiating treatment.Reference Mnie-Filali, Faure and Lambas-Senas 73 The firing of raphe serotonergic neurons is under negative 5-HT7 receptor control, possibly through GABAergic neurons.Reference Stahl 24 , Reference Mnie-Filali, Faure and Lambas-Senas 73 Therefore, blocking 5-HT7 receptors increases the release of serotonin, probably by preventing the inhibition of raphe serotonergic neurons by GABA.Reference Stahl 24 , Reference Mnie-Filali, Faure and Lambas-Senas 73 , Reference Yohn, Gergues and Samuels 76 , Reference Bonaventure, Kelly and Aluisio 78 In addition to brainstem raphe nuclei, the 5-HT7 receptors are also localized in the hippocampus, cortex, and thalamus, possibly on GABA interneurons or glutamate terminals. Many preclinical studies suggest that agents with 5-HT7 receptor antagonist property improve cognition, probably through their actions in these brain regions. Therefore, the improvement in the cognitive disturbance HAM-D factor observed in the Sheehan et al study may be attributable to trazodone’s 5-HT7 receptor antagonist activity.Reference Stahl 24 , Reference Stahl 79
Future research to understand the multifunctional properties of trazodone at different doses and treatment times, and a better knowledge of human brain receptor occupancy at different doses, could provide further insight into the mechanism for trazodone’s fast onset of antidepressant action.
The pharmacological profile of trazodone also contributes to its favorable safety profile.Reference Stahl 24 , Reference Stahl 26 The overall severity of the adverse events associated with the treatment of trazodone OAD in both Sheehan et alReference Sheehan, Croft and Gossen 45 and Fagiolini et alReference Fagiolini, Albert and Ferrando 44 studies was mild to moderate. Simultaneous inhibition of SERT and antagonism of 5-HT2A and 5-HT2C with trazodone can lead to an antidepressant effect while avoiding the side effects associated with use of conventional antidepressants (including SSRIs and SNRIs) due to 5-HT2A/2C stimulation, such as sexual dysfunction, anxiety, and insomnia.Reference Stahl 26 In fact, in the placebo-controlled study of trazodone OAD, only 1 patient reported anxiety in the trazodone group compared to 5 patients in the placebo group. Moreover, the incidence of sexual dysfunction was also low (trazodone OAD 4.9% vs placebo 2.5%).Reference Sheehan, Croft and Gossen 45 Additionally, the OAD formulation has a better tolerability profile compared to the IR and PR formulations. Reference Fagiolini, Comandini, Catena Dell’Osso and Kasper 23 , Reference Stahl 24 The antidepressant efficacy, combined with improved tolerability and improvements in sleep disturbance observed with trazodone OAD, may have important clinical implications, like enhanced compliance and decreased use of concomitant medications. In fact, the observational study conducted by Češková et alReference Češková, Šedová, Kellnerová and Starobová 41 showed that adherence with trazodone OAD formulation was high (>70%) after 21 weeks of treatment. The study also reported a significant reduction in the need for concomitant medications, such as anxiolytics, hypnotics, and other psychotropic medications.
One of the main limitations of this systematic review on early response to trazodone OAD in depression is that it is based on results from only 2 RCTs that were designed to evaluate efficacy at the study endpoint and not specifically to examine onset and time course of improvement. Therefore, the evidence supporting our findings about the early onset of antidepressant action with trazodone OAD treatment is limited. Second, infrequent assessments in a typical RCT study design (such as weekly intervals) may have low sensitivity to detect early improvements in depressive symptoms. However, consistent evidence from multiple RCTs for different formulations of trazodone, including the IR and PR formulations suggests that the trazodone may exhibit a fast onset of antidepressant action. However, future clinical studies to evaluate the timing of onset of depressive symptom improvement with trazodone OAD treatment are needed to confirm our analysis. An additional potential limitation is that the mean baseline HAM-D-17 total scores measured between both RCTs ranged from 22.4 to 24.1, which although considered severe or very severe, may be below the threshold for detecting moderate effects sizes of treatments relative to placebo.Reference Fagiolini, Albert and Ferrando 44 , Reference Sheehan, Croft and Gossen 45 , Reference Fournier, DeRubeis and Hollon 80 The efficacy of a treatment over placebo may be more readily detectable in cases of even greater severity where HAM-D-17 total scores meet or exceed 25.Reference Fournier, DeRubeis and Hollon 80 Therefore, it is possible that more robust early treatment effects may have been observed if baseline HAM-D-17 total scores had been higher.
Conclusion
Trazodone is a once-a-day antidepressant with rapid onset and efficacy in treating the symptoms of anxiety and insomnia, while possibly avoiding weight gain, sexual dysfunction, and activating side effects.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1092852921000304.
Acknowledgments
Editorial assistance for the preparation of this manuscript was provided by Arbor Scientia, Inc., which was supported by Angelini Pharma S.p.A. We would like to thank Giorgina Mangano, Giorgio Di Dato, and Giorgio Di Loreto for their contribution to the research studies that have been reviewed in this article.
Funding
This work was funded by Angelini Pharma S.p.A.
Disclosures
Stephen M. Stahl, MD, PhD, Dsc (Hon.) is an Adjunct Professor of Psychiatry at the University of California San Diego, Honorary Visiting Senior Fellow at the University of Cambridge, UK and Director of Psychopharmacology for California Department of State Hospitals. Over the past 36 months (January 2018 to December 2020) Dr. Stahl has served as a consultant to Acadia, Adamas, Alkermes, Allergan, Abbvie, Arbor Pharmaceutcials, AstraZeneca, Avanir, Axovant, Axsome, Biogen, Biomarin, Biopharma, Celgene, Concert, ClearView, DepoMed, EnVivo, EMD Serono, Eisai Pharmaceuticals, Ferring, Forest, Forum, Genomind, Innovative Science Solutions, Impel, Karuna, NeuroPharma, Intra-Cellular Therapies, Ironshore Pharmaceuticals, Janssen, Jazz, Lilly, Lundbeck, Merck, Neos, Novartis, Noveida, Otsuka, Perrigo, Pfizer, Pierre Fabre, Relmada, Reviva, Sage Therapeutics, Servier, Shire, Sprout, Sunovion,, Takeda, Taliaz, Teva, Tonix, Tris Pharma, Trius, Vanda, Vertex and Viforpharma; he has been a board member of RCT Logic and Genomind; he has served on speakers bureaus for Acadia, Forum, Genentech, Janssen, Lundbeck, Merck, Otsuka, Servier, Sunovion, Takeda, and Teva and he has received research and/or grant support from Acadia, Alkermes, AssureX, Astra Zeneca, Arbor Pharmaceuticals, Avanir, Axovant, Biogen, Braeburn Pharmaceuticals, BristolMyer Squibb, Celgene, CeNeRx, Cephalon, Dey, Eli Lilly, EnVivo, Forest, Forum, GenOmind, Glaxo Smith Kline, Intra-Cellular Therapies, ISSWSH, Janssen, JayMac, Jazz, Lundbeck, Merck, Neurocrine, Neuronetics, Novartis, Otsuka, Pfizer, Reviva, Roche, Servier, Shire, Sprout, Sunovion, TMS NeuroHealth Centers, Takeda, Teva, Tonix, and Vanda.
Prof. Umberto Albert received honoraria from Ethos S.p.A on behalf of Angelini Pharma S.p.A.
Pallavi Lamba is an employee of Arbor Scientia, Inc. and has no other disclosures.