Introduction
Forensic entomology is becoming an integral part of death investigations due to the unique information it offers to the unfolding investigation (Benecke Reference Benecke2001; Gennard Reference Gennard2012) As the use of entomology in death investigations becomes more widely applied, many publications have been written on the entomological species, such as colonising dipteran species, present in geographical areas in order to share information among entomologists and track geographical differences (Anderson and VanLaerhoven Reference Anderson and VanLaerhoven1996; Dillon Reference Dillon1997; Watson and Carlton Reference Watson and Carlton2005; Matuszewski et al. Reference Matuszewski, Bajerlein, Konwerski and Szpila2008; Voss et al. Reference Voss, Cook and Dadour2011; Mabika et al. Reference Mabika, Masendu and Mawera2014; Fong Reference Fong2017). Published research on forensic entomology in Canada has been lacking in recent years and, out of the 13 provinces and territories in Canada, only eight have been represented in the published literature. Research on the decomposition of domestic pigs, Sus scrofa domestica (Artiodactyla: Suidae), has been conducted and published in British Columbia (Anderson and VanLaerhoven Reference Anderson and VanLaerhoven1996; Anderson Reference Anderson2001), Alberta (Hobischak et al. Reference Hobischak, VanLaerhoven and Anderson2006; Anderson Reference Anderson2011), Saskatchewan (Sharanowski et al. Reference Sharanowski, Walker and Anderson2008), Manitoba (Gill Reference Gill2005), Québec (Maisonhaute and Forbes Reference Maisonhaute and Forbes2021, Reference Maisonhaute and Forbes2022), New Brunswick (Michaud and Moreau Reference Michaud and Moreau2011), Nova Scotia (LeBlanc and Strongman Reference LeBlanc and Strongman2002; Simpson and Strongman Reference Simpson and Strongman2002), and the Yukon Territory (Bygarski and LeBlanc Reference Bygarski and LeBlanc2012). Published research performed in Ontario, however, has been limited. One study was published that involved the trapping of flies using baited traps in urban and rural areas in Ontario (Langer et al. Reference Langer, Kyle, Illes, Larkin and Beresford2019). Langer et al. (Reference Langer, Kyle, Illes, Larkin and Beresford2019), however, did not include colonisation or succession information.
Blow flies (Diptera: Calliphoridae) are typically the first to colonise deceased remains (Payne Reference Payne1965; Goff Reference Goff2009; Gennard Reference Gennard2012); this can occur quickly, even within minutes of death (Catts and Goff Reference Catts and Goff1992; Barton et al. Reference Barton, Archer, Quaggiotto and Wallman2020). Insects colonise the remains based on many factors, including availability, season, competition, and stage of decay (Campobasso et al. Reference Campobasso, Di Vella and Introna2001; MacInnis and Higley Reference MacInnis and Higley2020). The succession of insect species that colonise remains can be associated with known stages within the decomposition process. Each stage of decomposition provides different nutrients and, therefore, attracts different families of insects (Anderson Reference Anderson2015). Knowing the stage of decomposition can help to understand presence or absence of specific insect species. The stages of decomposition typically used are fresh, bloated, active decay, advanced decay, and dry remains or skeletonisation (Anderson and VanLaerhoven Reference Anderson and VanLaerhoven1996; Goff Reference Goff2009; Gennard Reference Gennard2012). The fresh stage begins at the moment of death and continues until the bloated stage commences (Goff Reference Goff2009). In the fresh stage, the primary insects seen are blow flies because they are immediately attracted to decomposing remains, and flesh flies (Diptera: Sarcophagidae) may also be observed or recorded at this stage (Payne Reference Payne1965; Goff Reference Goff2009; Gennard Reference Gennard2012). The bloated stage is marked when the abdomen of the remains begins to rise due to the accumulation of gasses in the torso (Goff Reference Goff2009; Gennard Reference Gennard2012). The active decay stage begins when the extended abdomen from the bloated stage has deflated due to insect activity (Goff Reference Goff2009; Gennard Reference Gennard2012). It is also during the active decay stage when the Calliphoridae larvae are in greatest abundance and most are in a postfeeding stage, transitioning to the pupal stage (Barton et al. Reference Barton, Archer, Quaggiotto and Wallman2020). The advanced decay stage marks the time when the majority of the Calliphoridae larvae have migrated from the remains. This is also when families such as sepsids (Diptera: Sepsidae) and piophilids (Diptera: Piophilidae) are present in greater abundance and are colonising the remains. In this stage, the majority of flesh has been removed from the carcass, and only bones, skin, tendon, and dehydrated tissues remain (Connor et al. Reference Connor, Baigent and Hansen2019; Goff Reference Goff2009). The final stage, the dry remains stage, occurs when all flesh and tendon are removed, there is little to no insect activity, and decomposition is essentially complete (Goff Reference Goff2009; Gennard Reference Gennard2012).
In order to understand the typical insects that visit and colonise remains in each province, a short literature review of the studies performed in Canada is warranted. Two studies were performed in British Columbia, one in the spring (Anderson and VanLaerhoven Reference Anderson and VanLaerhoven1996) and one in the summer (Anderson Reference Anderson2001). In these two studies, the prominent primary colonisers discussed were Protophormia terraenovae (Robineau-Desvoidy, 1830) (Diptera: Calliphoridae), Lucilia illustris (Meigen, 1826) (Diptera: Calliphoridae), and Phormia regina (Meigen, 1826) (Diptera: Calliphoridae) (Anderson and VanLaerhoven Reference Anderson and VanLaerhoven1996; Anderson Reference Anderson2001). Other families present included muscids (Diptera: Muscidae), Drosophilidae (Diptera), Sarcophagidae (Diptera), Coleoptera families, and Piophilidae (Diptera) and Sepsidae (Diptera) insects (Anderson and VanLaerhoven Reference Anderson and VanLaerhoven1996; Anderson Reference Anderson2001). A study performed in Alberta in the spring identified different primary colonisers such as Calliphora vicina (Robineau-Desvoidy, 1830) (Diptera: Calliphoridae), Lucilia sericata (Meigen, 1826) (Diptera: Calliphoridae), and Lucilia illustris (Anderson Reference Anderson2011). A few beetle species from the Staphylinidae (Coleoptera) and Silphidae (Coleoptera) families were also reported as colonisers (Anderson Reference Anderson2011). The study performed in Saskatchewan took place during spring, summer, and fall, reporting varying colonising blow fly species, including Cynomya cadaverina (Robineau-Desvoidy, 1830) (Diptera: Calliphoridae), Protophormia terraenovae, and Phormia regina (Sharanowski et al. Reference Sharanowski, Walker and Anderson2008). Other Calliphoridae species were observed as adults in the area but did not colonise the remains (Sharanowski et al. Reference Sharanowski, Walker and Anderson2008). A technical report written on the insect succession in spring, summer, and fall in Manitoba reported the presence of some adult blow fly species, such as P. regina, but did not analyse any immature stages of Calliphoridae (Gill Reference Gill2005). Other orders and families in the area were noted as present, including adult piophilids, sphaerocerids (Diptera: Sphaeroceridae), sepsids, muscids, clerids (Coleoptera: Cleridae), histerids (Coleoptera: Histeridae), and scarabs (Coleoptera: Scarabidae) (Gill Reference Gill2005). Studies that were performed in summer in Québec have indicated that primary colonisers include Calliphora livida (Hall, 1948) (Diptera: Calliphoridae), Calliphora vomitoria (Linnaeus, 1758) (Diptera: Calliphoride), P. regina, and L. illustris (Maisonhaute and Forbes Reference Maisonhaute and Forbes2021, Reference Maisonhaute and Forbes2022). Protophormia terraenovae was observed as an adult but was not seen as a coloniser (Maisonhaute and Forbes Reference Maisonhaute and Forbes2021, Reference Maisonhaute and Forbes2022). One publication discusses insects in New Brunswick; however, the focus was on statistical approaches on accumulated degree-days (ADDs) to predict decomposition processes and, as such, entomological results were not included (Michaud and Moreau Reference Michaud and Moreau2011). Insect succession in Nova Scotia has been discussed in two publications: one study took place in the fall and reported the presence of either adult or immature stages of C. vicina, C. vomitoria, Calliphora terraenovae (Macquart, 1851) (Diptera: Calliphoridae), and P. regina (LeBlanc and Strongman Reference LeBlanc and Strongman2002). The other publication reported only one colonising species, Cy. Cadaverina, and collected adult species P. regina, P. terraenovae, and L. illustris during the summer (Simpson and Strongman Reference Simpson and Strongman2002). The final study to be discussed took place during the summer in the Yukon Territories: this publication reports that P. terraenovae, P. regina, L. illustris, C. vicina, C. vomitoria, C. terraenovae, and Cy. cadaverine were collected as adults from decomposing remains; however, only P. terraenovae was seen as a coloniser (Bygarski and LeBlanc Reference Bygarski and LeBlanc2012). The published works demonstrate a research gap when it comes to studies performed in Ontario. As such, the current study explores the insects present, in both immature and adult stages, during the decomposition of domestic pigs in three separate experiments in Oshawa, southern Ontario. Because primary colonisers and blow fly species have been shown to differ across various locations in Canada, a forensic entomological succession study must be published to inform the entomological community of the insects present during decomposition in southern Ontario.
Methods
Experimental design
Three studies investigating the decomposition of domestic pigs were conducted at the Forensic Ecology Research Facility, at Ontario Tech University, Oshawa, Ontario; elevation 107 m (Maplogs.com 2023a). The Forensic Ecology Research Facility is a secure grassy area, approximately 75 m × 20 m in area, surrounded by a 2.5-m-high fence topped with barbed wire. The facility has a slight gradient and some minor drainage and depression areas and is bordered on two sides by a small woodlot that predominantly contains eastern white cedar, Thuja occidentalis (Linnaeus, 1753), maples, Acer spp., and trembling aspen, Populus tremuloides (Michaux) (Watson and Forbes Reference Watson and Forbes2008). The substrate at the facility is a grey–brown podzolic soil of the Darlington series, which has a loamy texture and is free-draining. Soil characteristics (± standard error) include pH (7.3 ± 0.1), total carbon (5.3% ± 0.8), total nitrogen (0.3% ± 0.01), total phosphorus (0.001 μg g–1 soil ± 0.0001), and electrical conductivity (1.33 mS.cm–1 ± 0.07; Watson and Forbes Reference Watson and Forbes2008).
Study 1 took place from 5 July 2016 to 18 August 2016 (summer 2016), when the remains of three domestic pigs were observed. Study 2 was conducted from 8 September 2016 to 2 November 2016 (late summer–fall 2016), when the remains of two domestic pigs were observed. Finally, study 3 was conducted from 9 September 2019 to 7 November 2019 (late summer–fall 2019), when the remains of three domestic pigs were observed.
For each study, domestic pigs were obtained from a local pig farmer in Port Perry, Ontario. These pigs (approximately 45 kg each) were euthanised using a bolt-gun puncture to the head approximately 1 hour before placement in the Forensic Ecology Research Facility. In study 3, pig 1 was slightly smaller in size: approximately 35–40 kg. The pigs were placed 6 m apart directly on the grass in areas with approximately equal sun exposure. The 6-m distance was chosen to conform to our space limitations while still mitigating instances in which larvae could travel from one carcass to another. Despite the precautions taken, intercarcass travel may have occurred. The date and time of placement were documented, and the day of placement was deemed experimental day (ED) 0. Data loggers (Onset, HOBO, Bourne, Massachusetts, United States of America) were used during each study to monitor temperature and relative humidity hourly.
Field collections
Field visits took place daily for the first 10–13 days in order to document changes to the carcasses during the early stages of decomposition. After this, field visits took place every two days for one week and then further reduced to every three days for one week. Finally, field visits were reduced to once per week for one week and then once every two weeks until the end of the study.
During each visit to the site, the weather, decomposition stage, fly activity, and any other entomology notes were recorded. The stages of decomposition were categorised based on the postmortem changes described by Goff (Reference Goff2009). A 30-second fly count was performed by counting the number of adult dipteran flies that can be seen in a 30-second time period. This was performed so that the abundance of attracted flies present near the remains could be compared between carcasses, between stages of decomposition, and to the temperature at the time of the fly count. Samples of insects present on and near the remains were collected to document the flies, eggs, larvae, and beetles present. Egg and larval samples collected from the carcasses were reared to adulthood and identified, whereas fly and beetle samples were frozen for preservation, to be later identified. Pitfall traps were dug in the ground approximately 60 cm from the head, rear, and abdomen of each pig. Cups placed on the ground were filled with soapy water to kill insects that fell into the cups. These pitfall traps permitted the entomological community to be sampled outside of visit times.
Rearing and insect identification
In the laboratory, immature specimens were reared to adulthood using beef liver as a protein source. The rearing took place in an incubator (Caron, model 6021-1, SN012512-6012-1-34; Caron Products, Marietta, Ohio, United States of America) at 23.5 °C. Adult flies that emerged were identified using taxonomic keys.
Adult and immature specimens were observed through a stereo microscope (Leica S8 APO B, SN OT11205; Leica, Wetzlar, Germany). Adult and immature Calliphoridae were identified to the species level (Whitworth Reference Whitworth2006; Szpila Reference Szpila2010a, Reference Szpila2015; Marshall et al. Reference Marshall, Whitworth and Roscoe2011). Some of the L. illustris flies had ambiguous features; Dr. Terry Whitworth (Washington State University, Pullman, Washington, United States of America) confirmed these identifications. Other families of Diptera, any Coleoptera, and all other orders of insects were identified to the family (Scudder and Cannings Reference Scudder and Cannings2005; Szpila Reference Szpila2010b).
Data analysis
Accumulated degree-day values were calculated using a lower threshold of 5 °C. This threshold temperature was used because it led to the best overall fit and percentage of variability models (Michaud and Moreau Reference Michaud and Moreau2013).
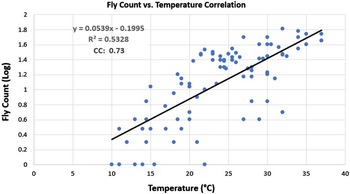
The correlation analysis of fly count versus temperature, including the trendline and R 2 value determinations, was performed using Microsoft Excel 2017 (Microsoft, Redmond, Washington, United States of America). The fly count values were normalised by calculating the log of each value. Any entries in which the fly count was 0 were removed. The correlation coefficient was calculated using the correlation statistical test found in Excel using the Data Analysis package in the Data tab.
Results
Environmental conditions
In study 1 (summer 2016), temperatures were high, ranging from a low of just under 20 °C to a high of over 30 °C. After the initially high average temperature on ED 0, the daily average temperature in study 1 remained consistent between 19 °C and 27 °C during the early stages of decomposition. In study 2, which took place in the fall of 2016, temperatures were cooler, ranging from just over 0 °C (night) to almost 27 °C (day). The daily average temperatures during early decomposition in study 2 were between 14 °C and 27 °C. Temperatures were coolest during study 3 (fall of 2019), with a low of 0 °C and a high of just over 23 °C. Average daily temperatures during the early decomposition period in study 3 ranged from 15 °C to 23 °C.
The percent relative humidity values for studies 2 and 3 were comparable, ranging from about 80% to about 100%, whereas the percent relative humidity value for study 1 was lower, ranging from about 45% to about 90%.
Primary dipteran colonisers and fly counts
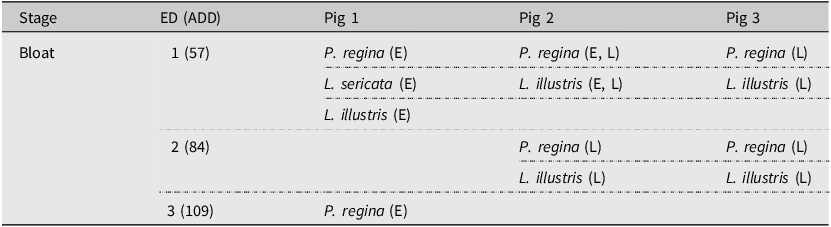
The primary dipteran colonisers that laid eggs on the remains were documented. They included primarily species belonging to the family Calliphoridae (Diptera). Colonisation was determined through the presence of clutches of eggs or first-instar larvae. During study 1, three primary colonising species were noted throughout the decomposition process: P. regina, L. illustris, and L. sericata. Lucilia sericata was observed only on pig 1, whereas L. illustris and P. regina were seen to colonise all three pigs. No delay in colonisation was observed: all three pigs were colonised by ED 1, during the bloat stage; however, some samples were collected as larvae on ED 1, indicating that colonisation would have occurred sometime on ED 0 (Table 1).
Table 1. The primary colonising species for each carcass observed during study 1. The life cycle stage of the sample collected is indicated in parentheses beside the species name: E, eggs and L, first-instar larvae. The accumulated degree-day (ADD) values are given in parentheses beside each experimental day (ED).
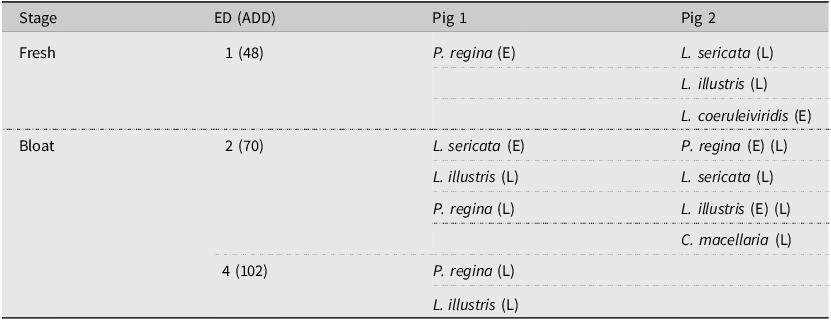
The variety of colonising Calliphoridae species was slightly more diverse during study 2, with four main colonising species noted during the fresh stages of decomposition: P. regina, L. sericata, L. illustris, and Lucilia coeruleiviridis (Macquart, 1855) (Diptera: Calliphoridae). Interestingly, pig 1 was colonised by only one of these four species on ED 1, whereas pig 2 was colonised by three species. As seen in study 1, no delay in colonisation occurred, with colonising eggs collected on ED 1 from both pigs. Lucilia sericata and L. illustris were collected as larvae from pig 2, indicating that colonisation by these species occurred before ED 1. One additional colonising species was collected from pig 2 on ED 2 during the bloat stage – Cochliomya macellaria (Fabricius, 1775) (Diptera: Calliphoridae) (Table 2). Colonisation of pig 2 by C. macellaria would make a total of five colonising species for pig 2, two less than for pig 1. In addition, on ED 1, a sample of eggs was collected from pig 2 that did not emerge as adults, further indicating that colonisation of pig 2 most likely occurred before ED 1.
Table 2. The primary colonising species for each carcass observed during study 2. The life cycle stage of the sample collected is indicated in parentheses beside the species name: E, eggs and L, first-instar larvae. The accumulated degree-day (ADD) values are given in parentheses beside each experimental day (ED).
The variety of colonising species noted during study 3 was identical to those noted in study 2, in that the same four colonising species were observed on ED 1: L. illustris, P. regina, L. sericata, and L. coeruleiviridis. Although the colonising species seen on ED 1 for each pig were slightly different, each pig was colonised by a combination of three of these four main colonising species. As with studies 1 and 2, no delay in colonisation was observed: all three pigs were colonised with eggs by ED 1. Phormia regina larvae were collected from pig 3 on ED 2, indicating that colonisation by this species occurred before ED 2. Pig 3 was further colonised in the late bloat stage by one additional species – P. terraenovae (Table 3).
Table 3. The primary colonising species for each carcass observed during study 3. The life cycle stage of the sample collected is indicated in parentheses beside the species name: E, eggs and L, first-instar larvae. The accumulated degree-day (ADD) values are given in parentheses beside each experimental day (ED).

Fly counts
A regression analysis was performed to determine if a correlation existed between the fly counts collected and the ambient temperatures during collection. The vast majority of the fly counts collected in lower temperatures (<17 °C) were less than 10 flies, which is considered to be low. Much higher fly counts were observed at temperatures above 17 °C, and an upward trend can be seen in the fly counts as the temperature increased (Fig. 1).
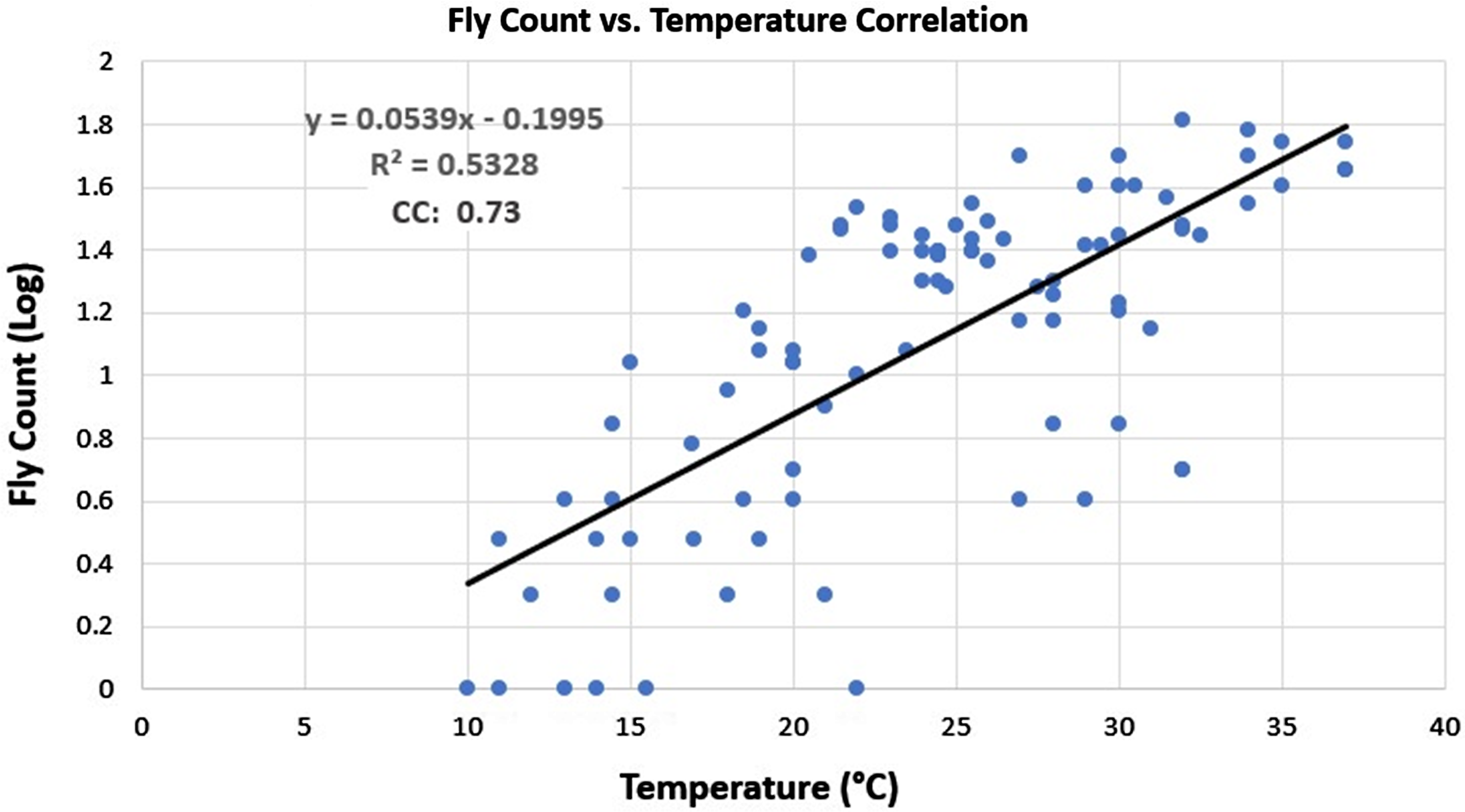
Figure 1. A correlation analysis of the normalised fly counts versus temperature for each collection visit during the fresh to active decay decomposition stages. The vertical axis indicates the fly count log, and the horizontal axis shows the temperature in degrees Celsius. A 0.5328 R 2 value was calculated. The equation of the line of best fit is y = 0.0539x – 0.1995. The correlation coefficient was 0.73.
Insect communities
A representation of the insect community has been generated for each study performed (Tables 4, 5 and 6). These representations show when the insects were collected within the decomposition period, according to both ED and stage of decomposition. In the three tables, it can be seen that adult and immature stages of blow fly species are present in ED 1–16, approximately. Adult specimens of blow flies were seen, but instances of immature stages were few after this period. Adult Coleoptera species began to arrive around ED 2–5, but any immature Coleoptera species observed were seen later during decomposition, towards the advanced decay stage. Many Muscidae species were collected and seen throughout decomposition, but no immature stages were collected. Sepsidae and Piophilidae species began to arrive in small amounts at the beginning of decomposition, but they became more prominent towards the active decay stage. Any immature stages of Piophilidae and Sepsidae observed were seen in the advanced decay stages. For most of the studies performed, hornet and wasp species (Hymenoptera: Vespidae) and ant species (Hymenoptera: Formicidae) were present throughout decomposition; however, they were more abundant in the early stages of decomposition.
Table 4. A representation of the adult (a) and immature (i) insects that were collected or observed during the decomposition of domestic pigs during study 1. The experimental day and the average accumulated degree-day (ADD) value for each day are noted. Stages of decomposition are indicated as follows: F, fresh; B, bloated; AcD, active decay; AdD, advanced decay; and DR, dry remains.
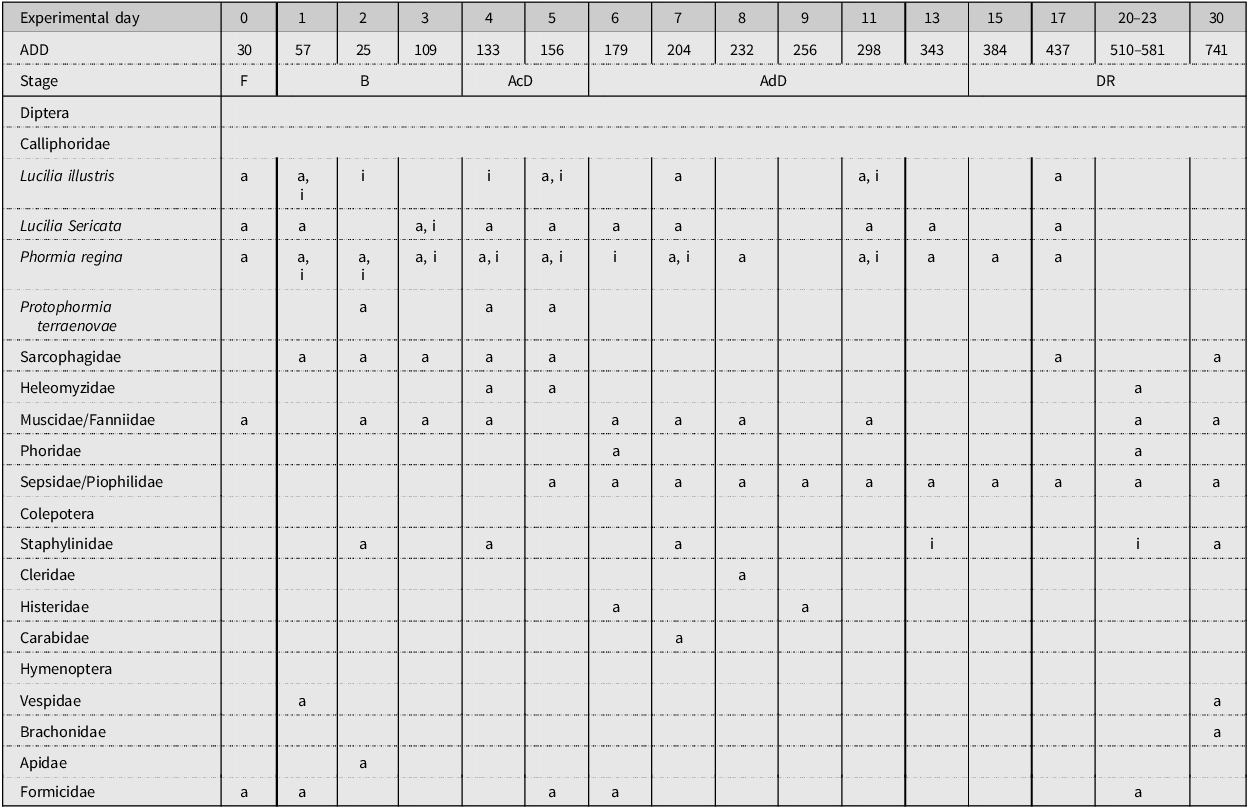
Table 5. A representation of the adult (a) and immature (i) insects that were collected or observed during the decomposition of domestic pigs during study 2. The experimental day and the average accumulated degree-day (ADD) value for each day are noted. Stages of decomposition are indicated as follows: F, fresh; B, bloated; AcD, active decay; AdD, advanced decay; and DR, dry remains.
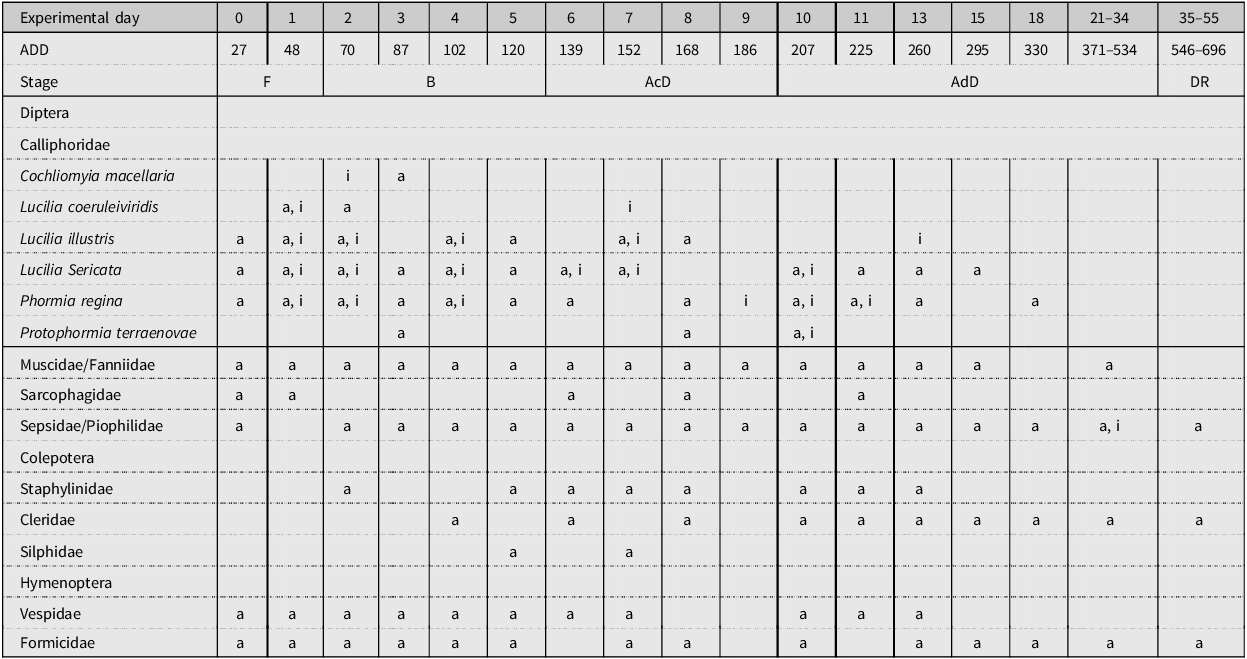
Table 6. A representation of the adult (a) and immature (i) insects that were collected or observed during the decomposition of domestic pigs during study 3. The experimental day and the average accumulated degree-day (ADD) value for each day are noted. Stages of decomposition are indicated as follows: F, fresh; B, bloated; AcD, active decay; AdD, advanced decay; and DR, dry remains.
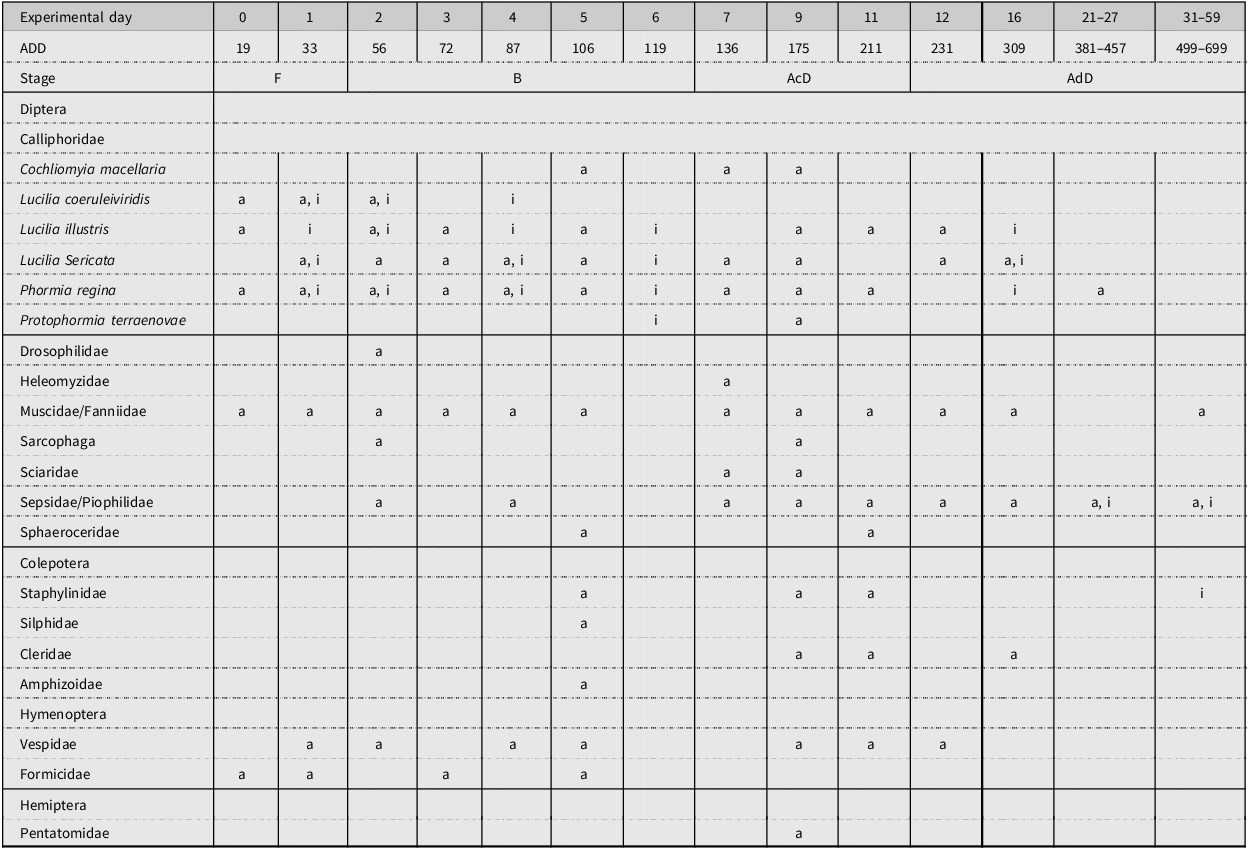
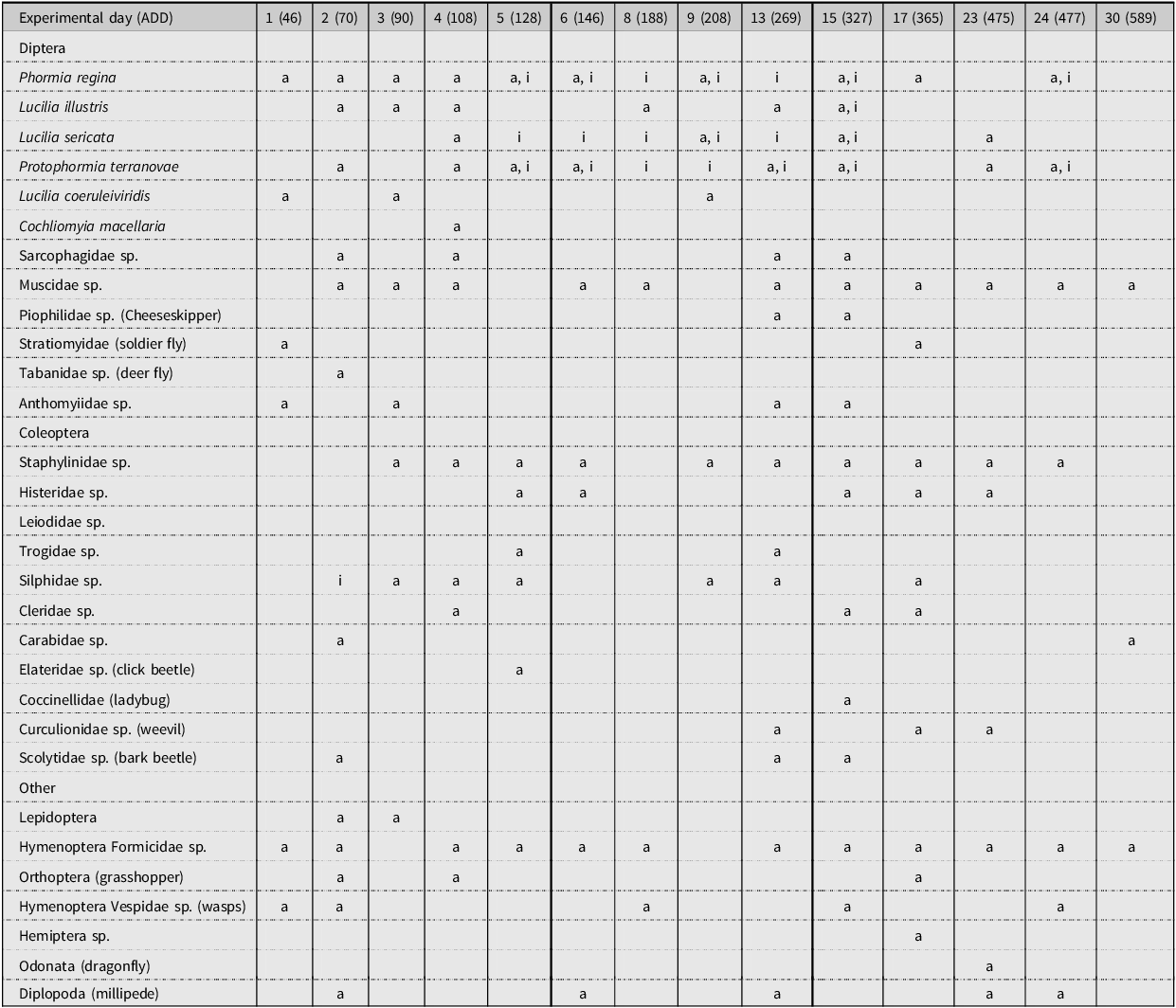
A summary of the pitfall trap data is given in Table 7. Trends in this data occurred; for instance, the presence of adult blow fly species can be seen in the first 4–5 EDs, but beyond this period, the presence of immature stages of blow flies began to take place. Similarly, Coleoptera species were not present in the pitfall traps until at least ED 2–3; however, once they were detected, their presence persisted throughout decomposition. Muscidae and Formicidae species were seen in the pitfall traps throughout decomposition, whereas Vespidae specimens were observed more sporadically during the decomposition process.
Table 7. A representation of the adult (a) and immature (i) insects that were collected from the pitfall traps for studies 1–3. The experimental day of collection and accumulated degree-days are also noted.
Discussion
One major factor that affects a carcass’s decomposition process is insect access to the remains. No obstacles were present that deterred insects’ access to the remains during the present studies, and as such, colonisation of all observed pigs occurred on or before ED 1. This means that the pre-colonisation intervals (Pre-CI) were short and likely did not influence the decomposition rate (Skopyk et al. Reference Skopyk, Forbes and LeBlanc2021).
The reduced variety of colonising blow fly species during study 1 compared to studies 2 and 3 may have been due to the effect of the high temperatures that occurred during the observation period. These high temperatures increased the rate of decay, with the bloat stage already commencing by ED 1. This would, theoretically, leave less time for many species to colonise the remains. This could be due to the rapid loss of humidity that can occur in higher temperatures, as reported by Campobasso et al. (Reference Campobasso, Di Vella and Introna2001). The few species that were able to colonise the remains would have developed at a faster pace than those species’ larvae in the other studies, causing the flesh to be consumed at a faster pace as well. This is supported by the fact that more colonising species were observed during studies 2 and 3 when temperatures were cooler and the rate of decay was slightly slower. A distinct trend can be seen in the fly count regression analysis, where fly counts begin to rise as the temperature increases. The R 2 value for this analysis was 0.5328, indicating the data were moderately variable. The correlation coefficient calculated, however, summarises the relationship between fly count and temperature more efficiently. With a coefficient of 0.73, a strong correlation exists between the two variables. A factor not considered in this regression is volatile organic compound (VOC) production during decomposition. High concentrations of VOCs can both attract and repel flies and would have influenced the fly counts. However, because VOC production can be influenced by temperature (Kasper et al. Reference Kasper, Mumm and Ruther2012; Stefanuto et al. Reference Stefanuto, Perrault, Stadler, Pesesse, Brokl, Forbes and Focant2014), perhaps its importance in this analysis is mitigated.
The colonising blow fly species reported in the current research were comparable between studies and, as such, the similarity in the colonisation of pig remains is evident; however, one should be cautious not to compare the succession of primary colonising species during different seasons because succession patterns can differ between seasons (Sharanowski et al. Reference Sharanowski, Walker and Anderson2008).
Some trends were evident in the successional and pitfall trap data of the three present studies. For instance, when comparing the insect community collected in each study, blow fly species were observed on ED 0–1. This is expected because blow flies are known to be attracted to decomposing remains minutes to hours after death (Amendt et al. Reference Amendt, Richards, Campobasso, Zehner and Hall2011). The presence of various blow fly species was documented into the advanced decay stage, long after the initial blow fly colonisation was completed. Adult blow fly species in the vicinity of the remains during late decomposition could be attributed to the number of newly emerged blow flies that were present in the area. The larvae that colonised the remains during the fresh stage would have emerged from their puparia, completing their life cycle. For example, based on development data by Anderson (Reference Anderson2000), using the average temperature of 23 °C, such as those recorded in study 1, adult P. regina would have emerged sometime between 353.9 and 416.7 ADD, or roughly between 15.4 and 18.1 days (Anderson Reference Anderson2000). Coleoptera species were not observed until approximately ED 3–5. Considering that Coleoptera generally colonise remains during the advanced decay stage (Payne Reference Payne1965; Goff Reference Goff2009; Gennard Reference Gennard2012; Barton et al. Reference Barton, Archer, Quaggiotto and Wallman2020), these Coleoptera adults were likely there for food. Sepsidae and Piophilidae species were present throughout decomposition; however, the observation of immature stages of these species was not noted until the advanced decay stage, when colonisation from these species generally occurs (Payne Reference Payne1965; Gennard Reference Gennard2012).
The most prominent colonising blow fly species in the Oshawa studies are P. regina, L. illustris, and L. sericata. These species colonised remains within the first 2–3 EDs in all three studies. Of these three species, P. regina was the most abundant species present. In the literature, P. regina has been reported in British Columbia, Saskatchewan, Manitoba, Quebec, Nova Scotia, and the Yukon Territories (Anderson and VanLaerhoven Reference Anderson and VanLaerhoven1996; Anderson Reference Anderson2001; Leblanc and Strongman Reference LeBlanc and Strongman2002; Simpson and Strongman Reference Simpson and Strongman2002; Gill Reference Gill2005; Sharanowski et al. Reference Sharanowski, Walker and Anderson2008; Bygarski and LeBlanc Reference Bygarski and LeBlanc2012; Maisonhaute and Forbes Reference Maisonhaute and Forbes2021, Reference Maisonhaute and Forbes2022). In VanLaerhoven’s (Reference VanLaerhoven2008) Ontario study, P. regina was seen as a coloniser in all mock cases studied. With the current studies’ report of P. regina as a prominent coloniser in Ontario, this species clearly is a forensically important primary coloniser in Canada. When comparing the colonising species reported within Canadian provinces, the published literature shows that the most abundant blow fly species in each of the provinces, both present and colonising, overlap but are predominantly different. For example, the literature published on the insect community in Québec dates from only 2021 and 2022, with the most abundant blow fly species reported being C. livida, C. vomitoria, P. regina, and L. illustris (Maisonhaute and Forbes Reference Maisonhaute and Forbes2021, Reference Maisonhaute and Forbes2022). Calliphora livida and C. vomitoria, two of the most prominent species reported in Québec, were neither detected as colonisers nor collected as adults during the present 2016 and 2019 Ontario studies. The elevation of Oshawa is almost double that of Trois-Rivieres, Québec (Maplogs.com 2023a, 2023b); however, the latitudes of Oshawa and Trois-Rivieres are similar, at 43.9 °N and 46.3 °N, respectively (www.gps-coordinates.net). As such, the seasonal average temperatures of both cities are comparable (Maisonhaute and Forbes Reference Maisonhaute and Forbes2021, Reference Maisonhaute and Forbes2022), yet the succession of blow flies differs considerably: the species reported in Quebec are more northerly.
These recorded differences support the need for updated publications on the insect communities during carrion decomposition in all provinces and territories of Canada. Sharing local successional, developmental, and colonisation data with others in the entomology community will only strengthen our knowledge and understanding of insects and how they behave. Rising global temperatures may affect blow fly succession in Canada; for this reason, researchers should continue to document the local fauna associated with decomposition and publish their findings, so they may be compared with those from other geographical areas.
Competing interests
The authors declare that they have no competing interests.