Introduction
Nuclear power, including the production of low-carbon electricity, is responsible at this time for almost one-third of global total energy generation. According to the International Atomic Energy Agency (IAEA), the main reason for this use is the low investment cost relative to the amount of energy produced and the fact that nuclear power plants produce virtually no greenhouse gas emissions or air pollutants during their operation, and very low emissions over their entire life cycle. Therefore, due to these benefits, there are now 447 nuclear power reactors in operation in 30 countries and another 58 reactors are under construction, based on IAEA information. However, the use of nuclear energy remains a cause of concern around the world, due to the devastating effects of accidents caused by core damage in nuclear plants. This concern is based on the long-term consequences of accidents, such as the accidents at Chernobyl (1986) and Fukushima-Daiichi (2011).
As in nuclear power plants, mitochondria exhibit high energy production capacities. However, in situations in which the structure of this organelle is compromised, the potential to release extremely toxic products is also worrying. Such toxic substances may lead to damage of the surrounding cells and other tissues. In fact, several studies have linked mitochondrial dysfunction to some pathological conditions such as neurodegenerative diseases (Lin & Beal, Reference Lin and Beal2006), type 2 diabetes (Lowell & Shulman, Reference Lowell and Shulman2005) and neoplasia (Modica-Napolitano & Singh, Reference Modica-Napolitano and Singh2004).
In regards to the spermatozoa, several studies related mitochondria to be the main source of cell energy, playing an important role in cellular homeostasis and sperm motility (Travis et al., Reference Travis, Foster, Rosenbaum, Visconti, Gerton, Kopf and Moss1998; St John, Reference St John2002). However, for some species, evidence suggests that glycolysis may be also an important source of ATP production for sperm motility, superior to oxidative phosphorylation (Mukai and Okuno, Reference Mukai and Okuno2004; Ford, Reference Ford2006; Nascimento et al., Reference Nascimento, Shi, Tam, Chandsawangbhuwana, Durrant, Botvinick and Berns2008).
Despite the importance of mitochondria to sperm metabolism, during oxidative phosphorylation, metabolites called reactive oxygen species (ROS) are produced and are a trigger for several reproductive physiological mechanisms (de Lamirande et al., Reference de Lamirande, Jiang, Zini, Kodama and Gagnon1997). Nevertheless, an unbalance between ROS production and mechanisms aiming to avoid their powerful oxidative potential (i.e. antioxidants), may be extremely harmful to the spermatozoa (Halliwell, Reference Halliwell1999; Nichi et al., Reference Nichi, Goovaerts, Cortada, Barnabe, De Clercq and Bols2007a).
In this context, mitochondria are highlighted as source of pro-oxidative factors that are crucial in the disruption of oxidative homeostasis (Agarwal et al., Reference Agarwal, Virk, Ong and du Plessis2014). Several studies have demonstrated correlations between impaired mitochondrial activity, oxidative stress and sperm DNA fragmentation, indicating a close relationship between these variables on sperm damage (Barros, Reference Barros2007; Nichi et al., Reference Nichi, Goovaerts, Cortada, Barnabe, De Clercq and Bols2007b; Blumer et al., Reference Blumer, Restelli, Giudice, Soler, Fraietta, Nichi, Bertolla and Cedenho2012).
Since the accident at Chernobyl, several safety improvements have been adopted and, after the Fukushima accidents, new generations of more safe designs for nuclear power stations have been developed. The main concern of nuclear energy specialists and the community, in general, is the approaches made to prevent the destruction and long-term consequences caused by an eventual nuclear disaster. If possible, the deactivation of power plants prior to predictable stressful events would probably avoid most damage. Similarly, mitochondrial therapy is applied in situations in which organelle dysfunction occurs (i.e., sperm cryopreservation) (O’Connell et al., Reference O’Connell, McClure and Lewis2002; Sariozkan et al., Reference Sariozkan, Bucak, Tuncer, Ulutas and Bilgen2009; Thomson et al., Reference Thomson, Fleming, Aitken, De Iuliis, Zieschang and Clark2009), and aimed to improve sperm viability by prevention of pro-oxidative factors release. Actually, some studies have suggested that, for certain cell types, uncouplers of oxidative phosphorylation are capable of reducing oxidative stress (Vincent et al., Reference Vincent, Olzmann, Brownlee, Sivitz and Russell2004; Mailloux & Harper, Reference Mailloux and Harper2011).
This review aimed to compile available data on the role of mitochondria in oxidative homeostasis and sperm functionality as well as suggesting some tools to assess the sperm mitochondrial function.
The mitochondrial paradox: physiological and pathological role on spermatozoa
According to the endosymbiotic theory, millions of years ago the mitochondrion was a prokaryotic unicellular organism. Formerly a free-living bacterium, the mitochondrion was capable of metabolizing oxygen in an environment rich in carbon dioxide. After penetrating a host eukaryotic cell that was incapable of metabolizing oxygen, a symbiotic relationship was established, later evolving into a more complex organism capable of producing energy more efficiently than the previously available glycolysis pathways (Margulis, Reference Margulis1970; Cummins, Reference Cummins1998). In fact, aerobic metabolism is highly dependent on mitochondrial functionality. The aerobic respiration is then, a consequence of the mitochondrial demand for oxygen which, by means of oxidative phosphorylation, is capable of producing approximately 90% of cellular energy (Saraste, Reference Saraste1999; Copeland, Reference Copeland2002).
Role of mitochondria in ATP production and sperm physiology
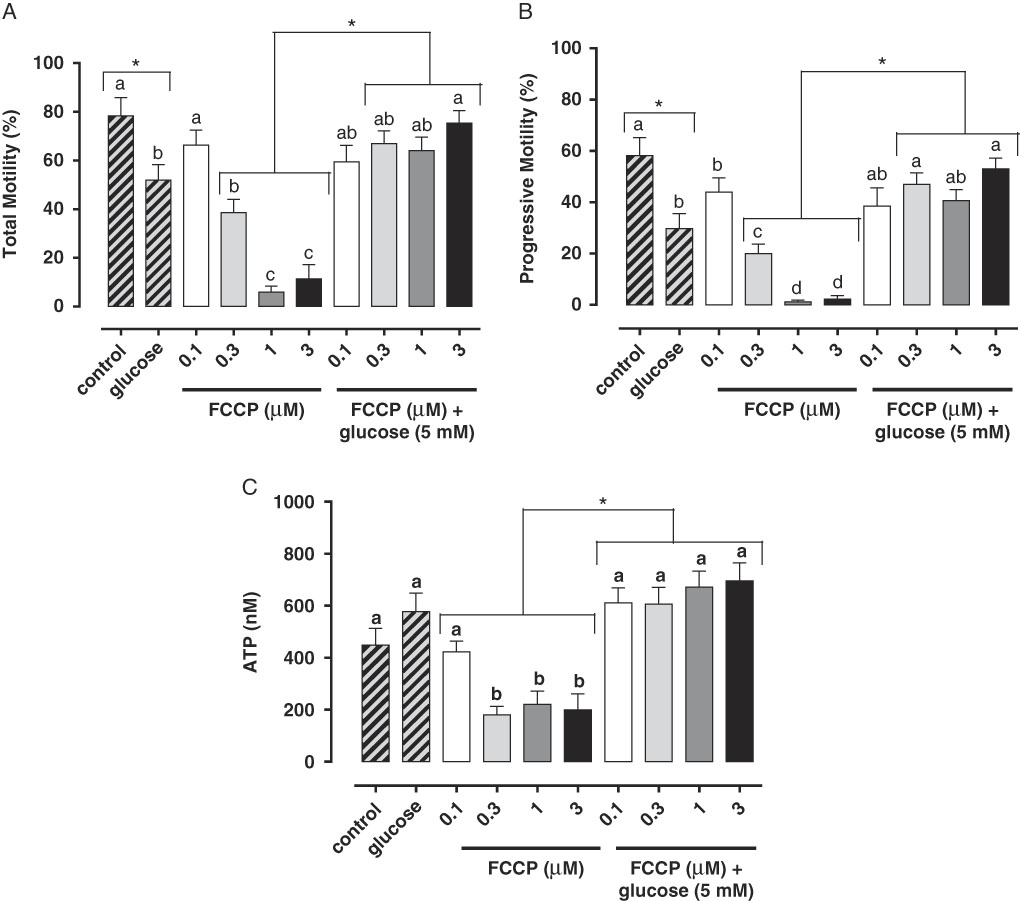
Studies have demonstrated the main role of mitochondria on sperm functionality, referring to this organelle as the main source of ATP for cellular homeostasis and motility (Travis et al., Reference Travis, Foster, Rosenbaum, Visconti, Gerton, Kopf and Moss1998; St John, Reference St John2002). However, its role in sperm metabolism has been a matter of debate. Mukai & Okuno (Reference Mukai and Okuno2004), when inhibiting sperm mitochondrial activity in mice concomitantly to glycolytic pathway supplementation, observed that ATP production and flagella beat remained unaltered. However, when glycolysis was inhibited and oxidative phosphorylation was stimulated, a drastically reduction in flagella beat and ATP production occurred. This finding suggested that glycolysis is more relevant than oxidative phosphorylation in the energetic metabolism of murine sperm. In a recent study conducted by our group, we observed similar results in bovine epididymal spermatozoa subjected to mitochondrial uncoupling and glycolysis stimulation (Losano et al., Reference Losano, Padín, Méndez-López, Angrimani, García and Barnabe2017a; Fig. 1). In addition, Nascimento et al. (Reference Nascimento, Shi, Tam, Chandsawangbhuwana, Durrant, Botvinick and Berns2008) observed similar results in human sperm. These authors suggested that, despite the important contribution of oxidative phosphorylation for ATP production, glycolysis is the primary source of energy in human sperm. Conversely, other studies in humans have described the opposite effect when sperm samples are incubated with inhibitors of the enzymatic electron transport complexes, with a decrease in sperm motility (Ruiz-Pesini et al., Reference Ruiz-Pesini, Lapeña, Díez-Sánchez, Pérez-Martos, Montoya, Alvarez, Díaz, Urriés, Montoro, López-Pérez and Enríquez2000; John et al., Reference John, Jokhi and Barratt2005). Furthermore, we verified that ovine sperm undergoing mitochondrial depolarization that did not alter their total motility. Spermatic kinetic patterns were affected, suggesting that mitochondria are very important in maintaining the quality of ovine spermatozoa movement (Losano et al., Reference Losano, Angrimani, Dalmazzo, Rui, Brito and Mendes2017b; Fig. 2).
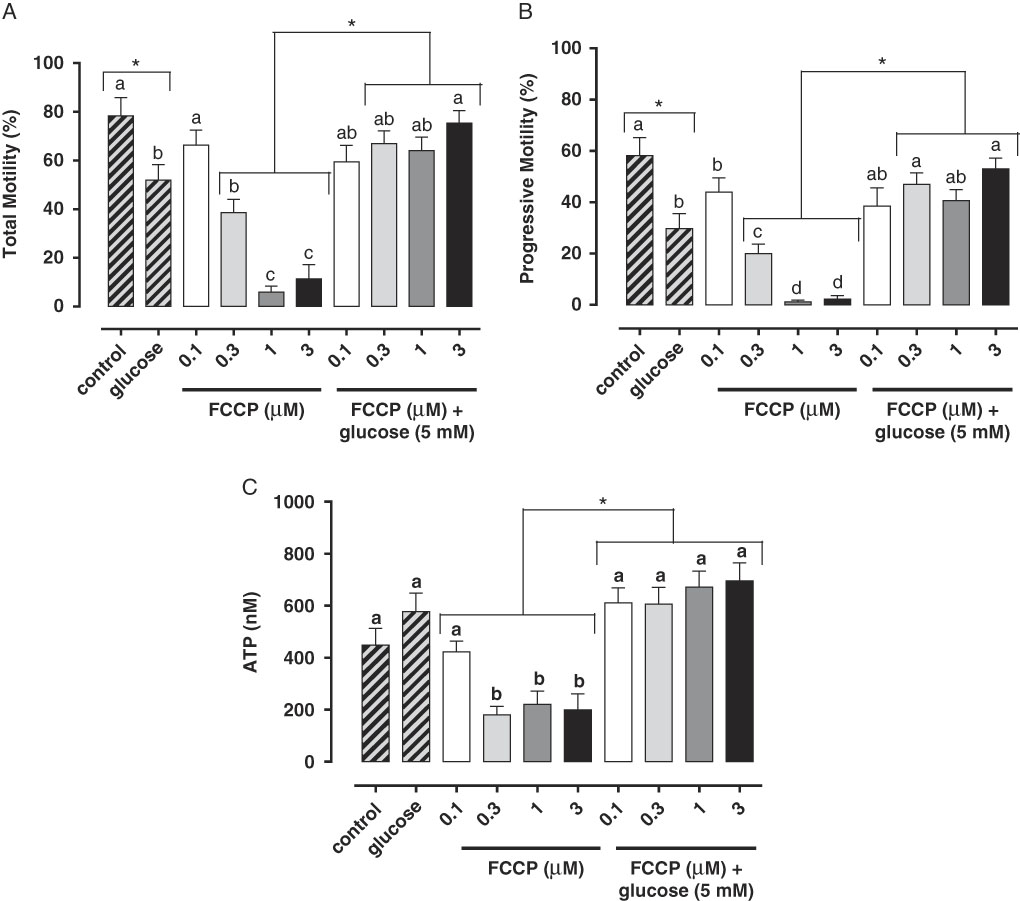
Figure 1 In this study, we verified that the stimulated glycolytic pathway (glucose 5 mM) is able to maintaining total (A) and progressive (B) motilities and ATP levels (C) of bovine epididymal spermatozoa subjected to mitochondrial uncoupling [carbonyl cyanide 4-trifluoromethoxy phenylhydrazone (FCCP); 0.1, 0.3, 1 and 3 µM] (Losano et al., Reference Losano, Padín, Méndez-López, Angrimani, García and Barnabe2017a). a,b,c,dDifferent letters on the bars indicate significant differences between treatments (P<0.05).
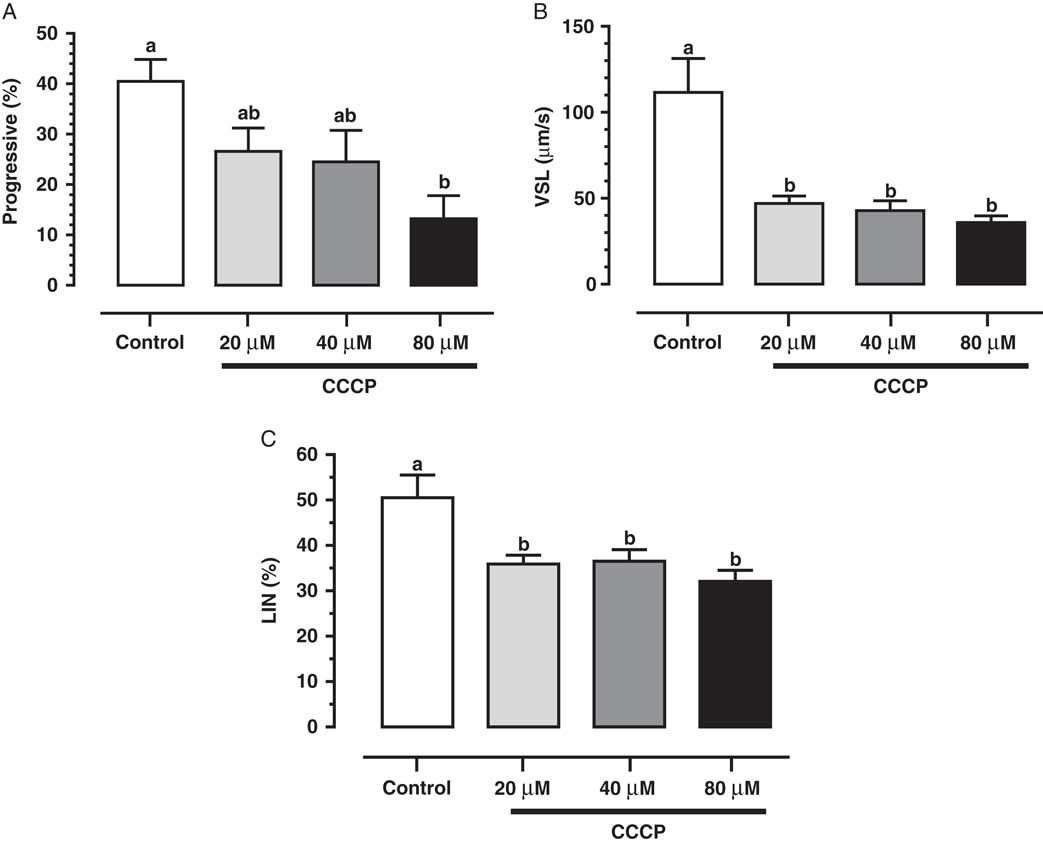
Figure 2 We verified that mitochondrial uncoupling [carbonyl cyanide 3 chlorophenylhydrazone (CCCP); 20, 40 and 80 µM] impairs ovine sperm kinetic patterns such as progressive motility (A), straight-line velocity (VSL; B) and linearity (LIN; C), indicating an essential role of mitochondria to sperm quality movement related to progressivity (Losano et al., Reference Losano, Angrimani, Dalmazzo, Rui, Brito and Mendes2017b). a,bDifferent letters on the bars indicate significant differences between treatments (P<0.05).
Mitochondria are essential to sperm functionality due to the relationship between their functional and fertilizing capacities (Marchetti et al., Reference Marchetti, Obert, Deffosez, Formstecher and Marchetti2002, Reference Marchetti, Jouy, Leroy-Martin, Defossez, Formstecher and Marchetti2004; Gallon et al., Reference Gallon, Marchetti, Jouy and Marchetti2006; St John et al., Reference St John, Bowles and Amaral2006). Nonetheless, it is still not clear how mitochondria can contribute to the energy capacity of sperm. The organelle has distinct contributions to sperm metabolism, dependent on experimental conditions and animal species (Storey, Reference Storey2008; Amaral et al., Reference Amaral, Lourenço, Marques and Ramalho-Santos2013).
The importance of the glycolytic pathway on ATP generation and on sperm function, has been described previously (Mukai & Okuno, Reference Mukai and Okuno2004). Lardy and colleagues (Reference Lardy, Winchester and Phillips1945) first showed that mitochondrial inhibition leads to asthenospermia. However, with glucose supplementation to the samples, sperm motility was re-acquired. In addition, White & Wales (Reference White and Wales1961) observed that ovine sperm maintain their motility through two parallel mechanisms of energy generation, i.e. glycolysis and oxidative phosphorylation. Moreover, Krzyzosiak and colleagues (Reference Krzyzosiak, Molan and Vishwanath1999) also observed that bovine sperm were capable of maintaining similar motility patterns in both aerobic and anaerobic conditions, assuming that glycol sable substrates are available. Furthermore, previous studies have suggested that ATP molecules supplied by oxidative phosphorylation in the sperm midpiece are not efficiently diffused to the more distal regions of the tail, indicating that glycolysis would probably play a key role in flagella beat in this region (Nevo & Rikmenspoel, Reference Nevo and Rikmenspoel1970; Turner, Reference Turner2003).
Role of calcium on mitochondrial function
A hypothesis on the main regulatory mechanisms of oxidative phosphorylation considers ADP and inorganic phosphate as feedback substrates for ATP synthesis through several cellular kinases. Therefore, an interesting analogy can be employed with the economic model of supply and demand, with ATP as the unit price for cellular energy. Evidence to support this theory showed that mitochondria isolated in suspension increased their ATP production when ADP and inorganic phosphate was supplemented in the presence of oxygen. Despite the well known ‘economic model of equilibrium’, recent studies have shown that ATP synthesis rate is not strictly controlled by such mechanisms (Gunter et al., Reference Gunter, Yule, Gunter, Eliseev and Salter2004).
Mitochondrial calcium ([Ca2+]m) has been referred to as the central regulator of oxidative phosphorylation, acting as the primary metabolic mediator for NADH production and activity controller of the enzymatic complexes pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase (McCormack et al., Reference McCormack, Halestrap and Denton1990; McCormack & Denton, Reference McCormack and Denton1993). The [Ca2+]m is also directly involved in ATP production, playing an important role in ADP phosphorylation through the enzyme ATP synthase (Territo et al., Reference Territo, French, Dunleavy, Evans and Balaban2001). Moreover, mitochondrial calcium also participates in the apoptotic mechanism of somatic cells, triggering the release of pro-apoptotic agents by the mitochondria (Szalai et al., Reference Szalai, Krishnamurthy and Hajnóczky1999).
If [Ca2+]m action on physiological processes of somatic cells is established, the precise role of this ion in sperm mitochondria is still under debate (Amaral et al., Reference Amaral, Lourenço, Marques and Ramalho-Santos2013). In a proteomic approach, studies identified sperm mitochondrial calcium uniporter (MCU) proteins that are responsible for controlling mitochondria calcium signalling, metabolism and cellular survival. However, sperm mitochondrial calcium concentration is seemingly unaltered by mitochondrial uncoupling (Machado-Oliveira et al., Reference Machado-Oliveira, Lefièvre, Ford, Herrero, Barratt, Connolly, Nash, Morales-García, Kirkman-Brown and Publicover2008; Wang et al., Reference Wang, Guo, Zhou, Shi, Yu, Yang, Wu, Wang, Liu, Chen, Tu, Zeng, Jiang, Li, Zhang, Zhou, Zheng, Yu, Zhou, Guo and Sha2013). Additionally, in bulls, mitochondrial activity on hyperactivated sperm appears to be unregulated by calcium release. In this context, further studies are vital to establish the real function of calcium in mitochondrial physiology, the reference values for [Ca2+]m, and to correlate such values with sperm function (Irvine and Aitken, Reference Irvine and Aitken1986; Ramalho-Santos et al., Reference Ramalho-Santos, Varum, Amaral, Mota, Sousa and Amaral2009; Amaral et al., Reference Amaral, Lourenço, Marques and Ramalho-Santos2013).
Reactive oxygen species and the spermatozoa
During aerobic cell metabolism, ROS are formed. This event occurs firstly because the mitochondrial environment is rich in oxygen and electrons, and almost all of these electrons participate in the reduction of oxygen directly to water, the final product of oxidative phosphorylation. Physiologically, some of these electrons escape from the oxidative phosphorylation enzymatic complex and bind to molecular oxygen, leading to first ROS, the superoxide anion, generation. From this primary product, a redox reaction cascade occurs leading to the formation of other reactive oxygen species, such as hydrogen peroxide (H2O2) and the hydroxyl radical (OH–) respectively.
ROS produced are involved in many physiological triggers such as sperm hyperactivation (de Lamirande & Cagnon, Reference de Lamirande and Cagnon1993), sperm capacitation (Aitken et al., Reference Aitken, Ryan, Baker and McLaughlin2004), acrosome reaction (de Lamirande et al., Reference de Lamirande, Tsai, Harakat and Gagnon1998), and interaction between spermatozoa and the zona pellucida (Aitken et al., Reference Aitken, Paterson, Fisher, Buckingham and van Duin1995). While ROS are formed by other mechanisms, such as glycolysis, mitochondria are the main ROS source with approximately 2% of consumed oxygen being converted to superoxide anions (Koppers et al., Reference Koppers, De Iuliis, Finnie, McLaughlin and Aitken2008).
Some enzymatic and non-enzymatic antioxidants act synergistically to prevent ROS accumulation, in which each of these metabolites is inactivated by specific antioxidants. Superoxide dismutase (SOD) is considered the primary line of antioxidant defence acting through dismutation of two molecules of superoxide anion (O2 –) forming an oxygen molecule and a hydrogen peroxide molecule (H2O2; Fig. 3) (Alvarez et al., Reference Alvarez, Touchstone, Blasco and Storey1987). Hydrogen peroxide can be destroyed by two antioxidants independent systems, the enzyme catalase and the glutathione peroxidase/reductase systems (Fig. 3; Nordberg & Arnér, Reference Nordberg and Arnér2001). If these two systems fail, the H2O2 will react with an Fe2+ or Cu+ molecule (called the Fenton reaction) and will produce the hydroxyl radical (OH–, Fig. 3). This ROS is considered the most reactive in biological systems, and can be destroyed by non-enzymatic antioxidants such as ascorbic acid and α-tocopherol (Fig. 3; Halliwell & Gutteridge, Reference Halliwell and Gutteridge1985).
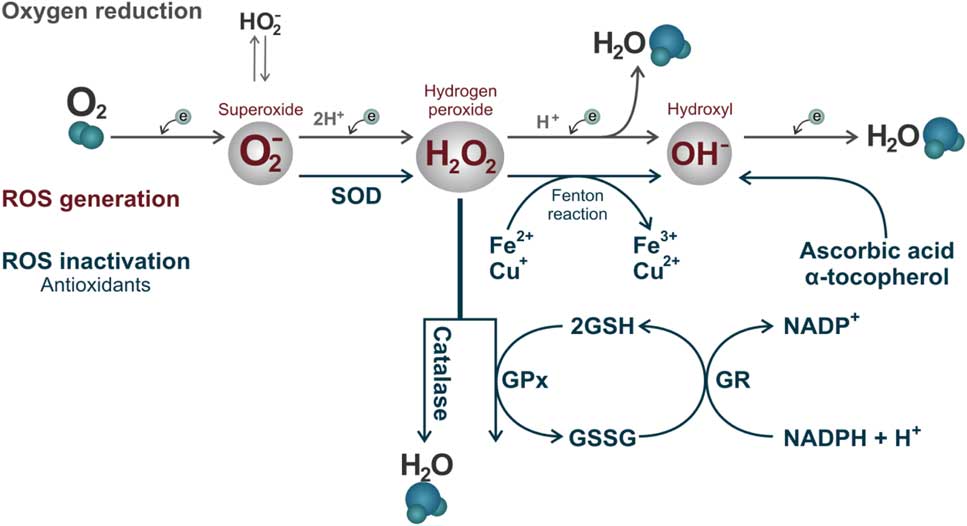
Figure 3 Reactive oxygen species formed by the oxy-reduction process from O2 to H2O and their respective inactivation antioxidant systems. The enzyme superoxide dismutase (SOD) acting through dismutation of two molecules of superoxide anion (O2 –) forming an oxygen molecule and a hydrogen peroxide molecule. Hydrogen peroxide (H2O2) can be destroyed by two antioxidants independent systems, the enzyme catalase and glutathione peroxidase (GPx)/glutathione reductase (GR) system, with the participation of oxidized (GSSG) and reduced (GSH) glutathione. If these two systems fail, H2O2 will react with an iron (Fe2+) or (Cu+) molecule (Fenton reaction) and will form the hydroxyl radical (OH–). This ROS can be destroyed by non-enzymatic antioxidants such as ascorbic acid and α-tocopherol.
Mitochondrial dysfunctions and spermatozoa
Despite the physiological function, any imbalance in ROS production and antioxidant mechanisms can lead to oxidative stress, which may be lethal for sperm cells (Fig. 4; de Lamirande et al., Reference de Lamirande, Jiang, Zini, Kodama and Gagnon1997; Agarwal et al., Reference Agarwal, Nallella, Allamaneni and Said2004). Sperm is particularly susceptible to oxidative stress due to a limited amount of cytoplasm and consequently low antioxidant activity and also a high quantity of polyunsaturated fatty acids which is easily oxidized. Thus, oxidative stress may cause damage to different sperm structures such as in plasma and acrosomal membranes, mitochondria and sperm DNA. Spermatozoa cannot restore damages caused by oxidative stress due to deficiency of cytoplasmic repair enzymes (Vernet et al., Reference Vernet, Aitken and Drevet2004; Nichi et al., Reference Nichi, Goovaerts, Cortada, Barnabe, De Clercq and Bols2007b; Agarwal et al., Reference Agarwal, Virk, Ong and du Plessis2014).
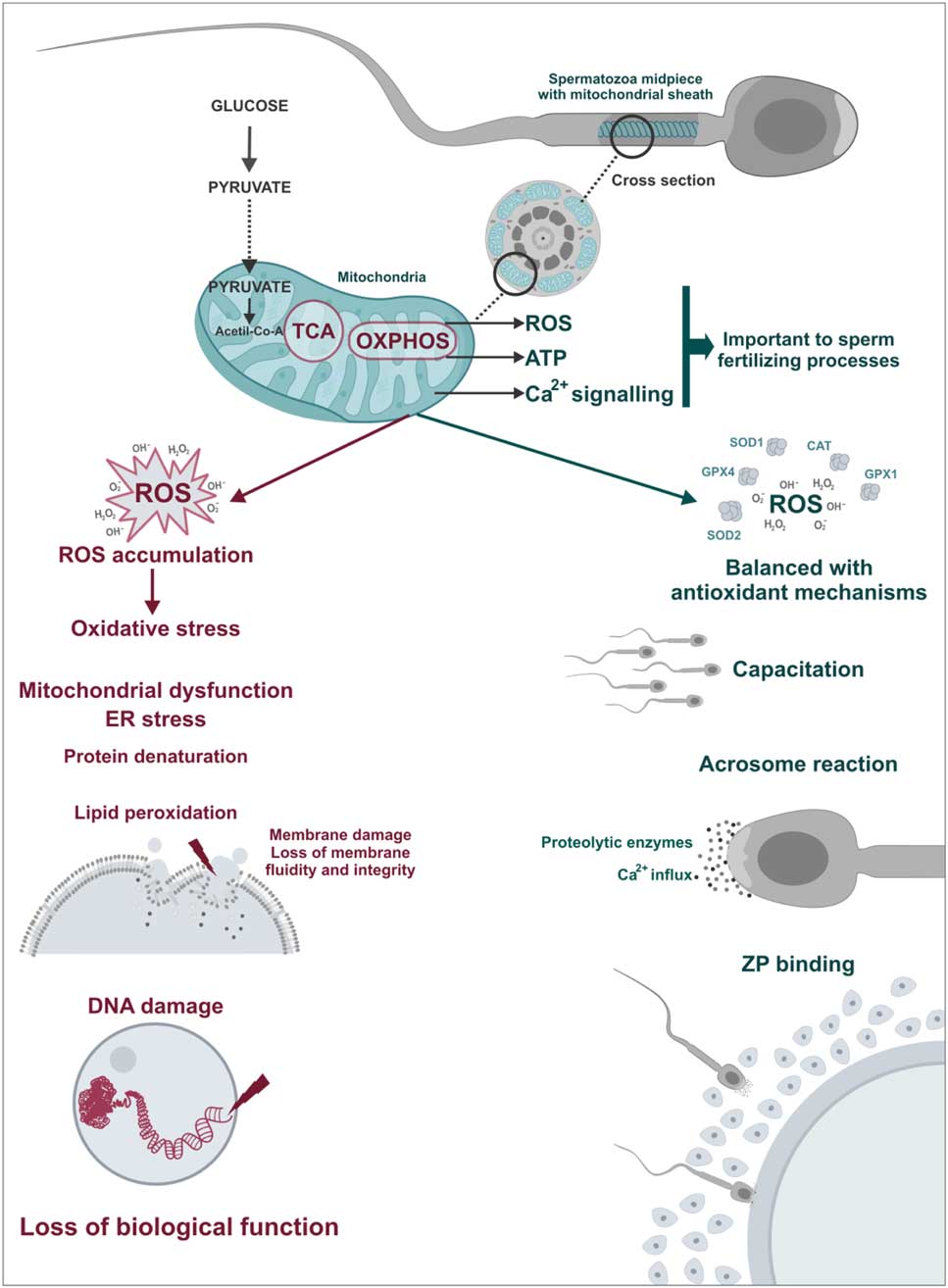
Figure 4 Physiological and pathological role of sperm mitochondria. ROS play a important role in sperm physiology acting as triggers of fertilization processes such as hyperactivation, acrosome reaction and spermatozoa–oocyte binding. However, in cases of mitochondrial dysfunctions, there is an imbalance between ROS production and antioxidant capacity, the oxidative stress. In this case, ROS cause damage to sperm structures including lipid peroxidation of the plasma membrane and DNA damage leading to loss of biological function of spermatozoa.
As mitochondria are the major source of pro-oxidative agents, it is suggested therefore that dysfunction in this organelle would have a fundamental role in the oxidative imbalance affecting sperm function (Agarwal et al., Reference Agarwal, Virk, Ong and du Plessis2014). Wang and colleagues (Reference Wang, Sharma, Gupta, George, Thomas, Falcone and Agarwal2003) identified low mitochondrial membrane potential and high ROS production in sperm from infertile patients, probably as a consequence of such mitochondrial injury, suggesting that mitochondrial function can be a marker of male fertility. In fact, other researchers have observed changes in mitochondrial function in sperm derived from infertile men (Troiano et al., Reference Troiano, Granata, Cossarizza, Kalashnikova, Bianchi, Pini, Tropea, Carani and Franceschi1998; Gallon et al., Reference Gallon, Marchetti, Jouy and Marchetti2006). However, sperm samples with high mitochondrial membrane potential have been identified in fertile patients (Kasai et al., Reference Kasai, Ogawa, Mizuno, Nagai, Uchida, Ohta, Fujie, Suzuki, Hirata and Hoshi2002; Marchetti et al., Reference Marchetti, Obert, Deffosez, Formstecher and Marchetti2002).
Studies performed in different species have shown a negative correlation between both oxidative stress and high mitochondrial activity. The occurrence of this stress and the sperm DNA integrity indicated that these variables are linked and lead a single pathogenic mechanism (Barros, Reference Barros2007; Nichi et al., Reference Nichi, Goovaerts, Cortada, Barnabe, De Clercq and Bols2007b; Blumer et al., Reference Blumer, Restelli, Giudice, Soler, Fraietta, Nichi, Bertolla and Cedenho2012). Correlation was also found between variables in spermatic oxidative stress and lower blastocyst rates, as rates of blastomeres increased with DNA damage, confirming the negative effect of seminal oxidative stress in in vitro embryonic development (Simões et al., Reference Simões, Feitosa, Siqueira, Nichi, Paula-Lopes, Marques, Peres, Barnabe, Visintin and Assumpção2013).
Mitochondrial disorders have multifactorial origins, and some mechanisms have not been totally elucidated (Amaral et al., Reference Amaral, Lourenço, Marques and Ramalho-Santos2013). These changes can be triggered even in the testis during spermatogenesis, for example if the testicular thermoregulatory mechanism is inefficient. Only 50% of the blood supply reaches the testes through the testicular artery, therefore male gonads are subjected to near hypoxic environments (Meijer & Fentener Van Vlissingen, Reference Meijer and Fentener, Van Vlissingen1993). Testis metabolism increased as consequence of pathological conditions that raised testicular temperature and were not compensated by increase in blood flow, causing a testicular hypoxic condition (Paul et al., Reference Paul, Teng and Saunders2009). Beyond these conditions and at the beginning of oxygenation, there is an increase in ROS production that leads to oxidative stress. This mechanism is known as ischaemia-reperfusion injury (Nichi et al., Reference Nichi, Bols, Züge, Barnabe, Goovaerts, Barnabe and Cortada2006; Reyes et al., Reference Reyes, Farias, Henríquez-Olavarrieta, Madrid, Parraga, Zepeda and Moreno2012). Increase in ROS production in this condition is related to mitochondrial dysfunction and the subsequent activation of enzymes that play a role in a ROS generated systems, such as xanthine oxidase (XO). These changes in the mitochondria are related to lack of O2 during ischaemia, which leads to ATP depletion and consequently mitochondrial injury. Moreover, the increase in testicular temperature promotes an influx of calcium and is also related to changes in this organelle (Dorweiler et al., Reference Dorweiler, Pruefer, Andrasi, Maksan, Schmiedt, Neufang and Vahl2007; Reyes et al., Reference Reyes, Farias, Henríquez-Olavarrieta, Madrid, Parraga, Zepeda and Moreno2012).
Sperm cryopreservation is a key process in assisted reproduction techniques (Hammerstedt et al., Reference Hammerstedt, Graham and Nolan1990; Zapzalka et al., Reference Zapzalka, Redmon and Pryor1999; Holt, Reference Holt2000). However this technique promotes a decrease in sperm quality, and also in mitochondrial damage during cryopreservation due to excessive production of pro-oxidative factors that, ultimately, cause post-thaw sperm damage and decrease in motility (O’Connell et al., Reference O’Connell, McClure and Lewis2002; Sariozkan et al., Reference Sariozkan, Bucak, Tuncer, Ulutas and Bilgen2009; Thomson et al., Reference Thomson, Fleming, Aitken, De Iuliis, Zieschang and Clark2009). Additionally, the process promotes a reduction in antioxidant capacity after sperm cryopreservation, a further factor that also predisposes these cells to oxidative stress (Bilodeau et al., Reference Bilodeau, Chatterjee, Sirard and Gagnon2000).
Consequently, several studies have used antioxidant treatment in sperm samples submitted to cryopreservation, aimed at preventing oxidative stress caused by mitochondrial injuries (Askari et al., Reference Askari, Check, Peymer and Bollendorf1994; Bilodeau et al., Reference Bilodeau, Blanchette, Gagnon and Sirard2001; Fernández-Santos et al., Reference Fernández-Santos, Martínez-Pastor, García-Macías, Esteso, Soler, Paz, Anel and Garde2007; Taylor et al., Reference Taylor, Roberts, Sanders and Burton2009). However, the use of a specific mitochondrial shield during cryopreservation also appeared as an option, aimed at improving post-thaw sperm quality (Schober et al., Reference Schober, Aurich, Nohl and Gille2007). A possible alternative would be to reduce mitochondrial activity, which can be induced by uncouplers of oxidative phosphorylation during the cryopreservation process, thus preventing ROS accumulation caused by any mitochondrial dysfunction that can occur during this process. Some uncoupler activities have been identified in the physiological processes of somatic cells, acting even in oxidative stress reduction (Vincent et al., Reference Vincent, Olzmann, Brownlee, Sivitz and Russell2004; Brand & Esteves, Reference Brand and Esteves2005).
Inhibitors and uncouplers of oxidative phosphorylation: action mechanisms and their possible applications
Inhibitors and uncouplers of oxidative phosphorylation are important in the study of mitochondrial physiology, and have been widely used in pharmacology as many chemical compounds can inhibit the specific processes of oxidative phosphorylation. Therefore, it is possible to observe their role by preventing a single process without inhibiting other mechanisms (Nelson & Cox, Reference Nelson and Cox2008).
Inhibitors can act towards complex electron carriers and also in mitochondrial channels. Rotenone (e.g. insecticide class), can block the transfer of electrons from complex I to ubiquinone, inhibiting the overall process of oxidative phosphorylation (Sherer et al., Reference Sherer, Betarbet, Testa, Seo, Richardson, Kim, Miller, Yagi, Matsuno-Yagi and Greenamyre2003). Conversely, antimycin A, an antibiotic produced by the Streptomyces fungus, blocks the transport of electrons from complex III to complex IV (Slater, Reference Slater1973). Cyanide inhibits the electron transport complex IV to oxygen. Furthermore, it is possible to directly inhibit ATP synthesis with oligomycin, which is widely used in this process. This compound acts on the enzyme ATP synthase by blocking the flow of protons through the F0 subunit of this enzyme to the mitochondrial matrix and, consequently, preventing ATP synthesis (Penefsky, Reference Penefsky1985). As well as enzyme complex inhibitors, there are also calcium channel blockers such as RU360, Na+/Ca2+pump inhibitors or CGP 37157 (García-Rivas et al., Reference García-Rivas, de, Carvajal, Correa and Zazueta2006; Thu et al., Reference Thu, Ahn and Woo2006).
In addition to these inhibitors, uncouplers of oxidative phosphorylation were widely used not only as a tool to study cell physiology, but also as a possible therapeutic application (Kasianowicz et al., Reference Kasianowicz, Benz and McLaughlin1984). ATP synthesis occurs through coupling of two reactions, electron transport and phosphorylation, as a result of a proton gradient this class of substances uncouples these two reactions, preventing or decreasing ATP synthesis. However, electron flow activity across the mitochondrial complexes is not inhibited, and even could be increased (Terada, Reference Terada1990). Most of these molecules are hydrophobic and have protonophore activity, depolarization of mitochondrial membranes allows protons to return to the mitochondrial matrix and dissipate the mitochondrial membrane potential and pH difference, inhibiting the driving proton force, essential for ATP synthesis (Chen, Reference Chen1988; Terada, Reference Terada1990). Uncoupling proteins have been identified in some cells and are related to some physiological roles such as in adaptive thermogenesis in adipose tissue.
Moreover, these proteins have been identified in researches related to obesity, diabetes, neurodegenerative disease and ageing in humans (Brand and Esteves, Reference Brand and Esteves2005). These studies emerged as previous researches found out that mitochondrial uncouplers can control mitochondria ROS production and, therefore, prevent oxidative stress, which is related to these diseases. Therefore, the use of these proteins in cell therapy for the treatment of these pathologies is suggested (Brand & Esteves, Reference Brand and Esteves2005; Lowell & Shulman, Reference Lowell and Shulman2005; Lin & Beal, Reference Lin and Beal2006; Mailloux & Harper, Reference Mailloux and Harper2011). Decrease in ROS production promoted by uncouplers is due to an increase in the respiratory rate followed by a decrease in mitochondria intermediate reduced states, capable of donating single electrons to oxygen, thereby preventing the generation of superoxide anions.
The uncoupled process has been applied in an energy study of spermatozoa (Mukai & Okuno, Reference Mukai and Okuno2004), however there is still no evidence that these compounds can control ROS production by sperm mitochondria. However, the use of these substances may bring interesting results for the prevention of oxidative stress in seminal samples in front of possible mitochondrial dysfunction. Thus, the application of this treatment can be attractive, especially for use in reproductive biotechnologies due to the highest susceptibility to oxidative stress.
Tools for assessing sperm mitochondrial function
Sperm mitochondria can be involved in both physiological as pathological processes, therefore the importance of assessing the functionality of this organelle is evident. The use of tools to evaluate sperm mitochondrial function associated with other sperm analysis can be applied for the prediction of fertilizing capacity (Troiano et al., Reference Troiano, Granata, Cossarizza, Kalashnikova, Bianchi, Pini, Tropea, Carani and Franceschi1998; Kasai et al., Reference Kasai, Ogawa, Mizuno, Nagai, Uchida, Ohta, Fujie, Suzuki, Hirata and Hoshi2002; Aitken, Reference Aitken2006). In this context, sperm mitochondria have been studied for some decades (Christen et al., Reference Christen, Schackmann and Shapiro1983; Hrudka, Reference Hrudka1987; Graham et al., Reference Graham, Kunze and Hammerstedt1990) Thus, several tools have been developed for assessment of mitochondrial function (Table 1).
Table 1 Available tools for assessing sperm mitochondrial functionality (mitochondrial activity, mitochondrial membrane potential and calcium levels assessments)
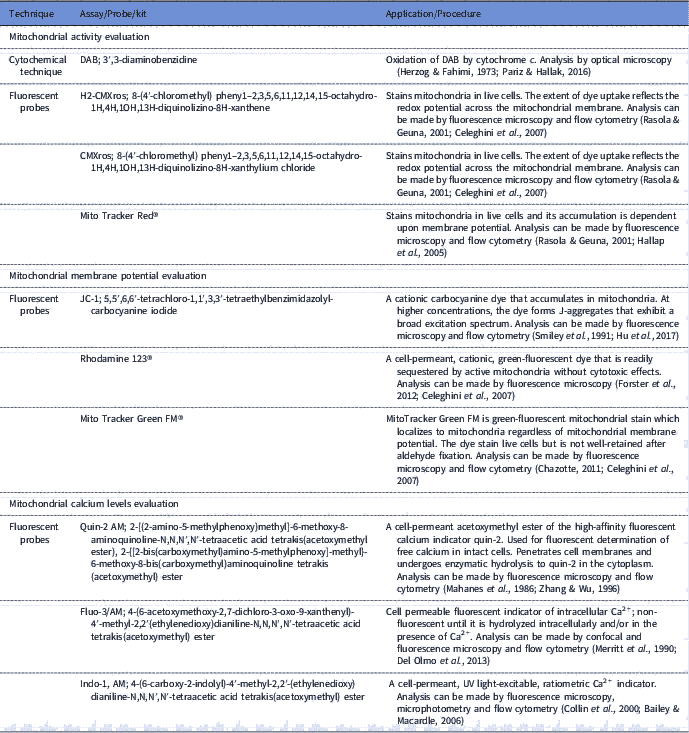
Mitochondria activity evaluation aims to infer the efficiency of electron transport between enzymatic complexes and also in the redox processes involved in oxidative phosphorylation. In classic research, Hrudka (Reference Hrudka1987) developed a cytochemical technique to evaluate mitochondrial activity. This cytochemical assay is based on the oxidation of 3′3-diaminobenzidine (DAB) by cytochromec, an enzyme involved in electron transport between the enzymatic complexes. Subsequently, some fluorescent probes such as H2-CMXros and CMXros, were developed with the same purpose and commercially sold as Mito Tracker Red® (Poot et al., Reference Poot, Zhang, Krämer, Wells, Jones, Hanzel, Lugade, Singer and Haugland1996; Wojcik et al., Reference Wojcik, Sawicki, Marianowski, Benchaib, Czyba and Guerin2000; Celeghini et al., Reference Celeghini, De Arruda, De Andrade, Nascimento and Raphael2007).
Fluorescent probes, such as JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide; Garner et al., Reference Garner, Thomas, Joerg, DeJarnette and Marshall1997), Mito Tracker Green FM® (Gillan et al., Reference Gillan, Evans and Maxwell2005) and Rhodamine 123® (Graham et al., Reference Graham, Kunze and Hammerstedt1990), were also developed to assess mitochondrial membrane potential. These probes diffuse freely through the plasma membrane to the cell cytosol and accumulate electrophoretically in the mitochondrial matrix, determined by proton motive force and acting in accordance with mitochondria ability to pump protons from the matrix to the intermembrane area (Chen, Reference Chen1988; Garner et al., Reference Garner, Thomas, Joerg, DeJarnette and Marshall1997; Piccoli et al., Reference Piccoli, Scrima, D’Aprile, Ripoli, Lecce, Boffoli and Capitanio2006). Membrane potential and mitochondrial activity are indicators of mitochondrial function and are related, however it is important to note that these parameters cannot be confused, as the mitochondria can maintain their redox processes by electron transport even with low membrane potential (Chen, Reference Chen1988; Terada, Reference Terada1990). Therefore, evaluation of these two parameters can be used in a complementary form.
Furthermore, it is possible to measure mitochondria calcium levels, as this mineral is considered to be the central regulator of oxidative phosphorylation (Irvine and Aitken, Reference Irvine and Aitken1986; McCormack & Denton, Reference McCormack and Denton1993). Calcium measurement in spermatozoa has been reported by the use of the fluorescent probes Quin-2 AM (Irvine & Aitken, Reference Irvine and Aitken1986), fluo-3/AM (Giojalas, Reference Giojalas1998; Harrison et al., Reference Harrison, Mairet and Miller1993) and indo-1AM (Brewis et al., Reference Brewis, Morton, Mohammad, Browes and Moore2000). However, it would ideal to measure intramitochondrial calcium, as well as create reference indices, considering that calcium has other functions in the cell such as its role in sperm capacitation (Breitbart, Reference Breitbart2002).
Although these assessments are indicative of mitochondria function, these techniques cannot be applied to quantify energy efficiency of sperm cells. Studies aimed at the evaluation of sperm energy metabolism using measurement of ATP levels are important to complement the mitochondria status assessment (Mukai and Okuno, Reference Mukai and Okuno2004). High-performance liquid chromatography (Samizo et al., Reference Samizo, Ishikawa, Nakamura and Kohama2001) or dosage by commercial kits are among the methods that can be used to measure the ATP and ADP levels (Perchec et al., Reference Perchec, Jeulin, Cosson, Andre and Billard1995). Measurement of ATP and ADP molecules was performed in several species such as mice (Mukai & Okuno, Reference Mukai and Okuno2004), birds (Rowe et al., Reference Rowe, Laskemoen, Johnsen and Lifjeld2013) and humans. However, more studies are necessary to develop indexes between production and ATP consumption, and relate these with sperm function.
Conclusion
In conclusion, there are still several questions covering the real contribution of the mitochondrial metabolism in sperm function in each species, although it is clear that this organelle can affect reproductive processes both positively and negatively (Amaral et al., Reference Amaral, Lourenço, Marques and Ramalho-Santos2013). Moreover, as mitochondria are the main ROS source and sperm are extremely susceptible to oxidative damage (Nichi et al., Reference Nichi, Goovaerts, Cortada, Barnabe, De Clercq and Bols2007b; Vernet et al., Reference Vernet, Aitken and Drevet2004), the development of studies aimed at the prevention of mitochondrial dysfunction in sperm cells is extremely important, such as the regarding improvement of mechanisms to reduce ROS release or inactivation of mechanisms for a better mitochondrial function.
Acknowledgements
The authors thank the Department of Animal Reproduction of University of São Paulo for the knowledge designed to compile the data in this review.
Financial support
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, project: 2017/13090-9), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support for the research mentioned in this review.
Conflicts of interest
None of the authors has any conflict of interest to declare.