Introduction
Neuropsychiatric symptoms of dementia including agitation affect the majority of patients with dementia at some point during their illness and are associated with worse cognition and poor health outcomes (Aronson et al., Reference Aronson, Post and Guastadisegni1993, Kunik et al., Reference Kunik2010). These symptoms are burdensome for the patient, families, and caregivers and are the leading cause of inpatient hospitalizations in people with dementia (Aigbogun et al., Reference Aigbogun, Stellhorn, Hartry, Baker and Fillit2019, Soto et al., Reference Soto2012). Atypical antipsychotic medications are commonly used for patients with severe agitation and aggression in dementia, and have shown modest efficacy (Azermai et al., Reference Azermai, Petrovic, Elseviers, Bourgeois, van Bortel and Vander Stichele2012, Yunusa et al., Reference Yunusa, Alsumali, Garba, Regestein and Eguale2019). However, they are associated with adverse effects including extrapyramidal symptoms, falls, stroke, and an increased risk of death (Maust et al., Reference Maust2015). Further, there have been concerns about inappropriate medication use in these patients which results in polypharmacy and an increased risk of adverse effects (Azermai et al., Reference Azermai, Elseviers, Petrovic, van Bortel and Stichele2011, Gustafsson et al., Reference Gustafsson, Karlsson and Lovheim2013, Maust et al., Reference Maust2021). Although several guidelines recommend minimizing the use of medications when possible, there are challenges in their implementation (Baiardini et al., Reference Baiardini, Braido, Bonini, Compalati and Canonica2009). Decision tree-based approaches have been proposed for treatment of agitation in dementia (Livingston et al., Reference Livingston2020, Salzman et al., Reference Salzman2008) but have not impacted prescribing patterns to date. Thus, the treatment of agitation in dementia often remains suboptimal with a lack of standardization and high rates of polypharmacy (Cioltan et al., Reference Cioltan2017, Health-Quality-Ontario, 2015, Vasudev et al., Reference Vasudev2015).
To address these issues, we developed an integrated care pathway (ICP) for the treatment of agitation and aggression in Alzheimer’s or mixed vascular dementia, comprising protocolized assessments, non-pharmacological interventions, a medication wash-out, and a sequential medication algorithm (Davies et al., Reference Davies2018). This ICP described in more detail below has been implemented by several hospitals including our inpatient geriatric psychiatry unit at the Centre for Addiction and Mental Health (CAMH), Toronto, Canada. The objectives of this pilot study were to analyze the impact of the ICP on clinical outcomes in patients treated according to the ICP and to compare them to a group of similar patients who received “treatment as usual” (TAU) prior to the introduction of the ICP. We hypothesized that the ICP would be effective in treating agitation in dementia and would result in a shorter length of inpatient stay (LOS), lower rate of psychotropic polypharmacy, and a lower rate of falls when compared to TAU. To assess whether potential differences between the ICP and TAU groups could be due to general changes in clinical practice between the two time periods during which these two groups were treated, we also compared the LOS, rates of polypharmacy, and falls in patients without dementia exhibiting agitation or aggression and treated on the same unit contemporaneously to patients in the TAU and ICP groups.
Methods
Overview
This study was conducted at the geriatric psychiatry inpatient unit at CAMH. CAMH research ethics board (REB) approved the study, with a waiver of informed consent, given that the ratings in the ICP group were collected as part of standard care and the other analyzed data were derived from a chart review.
Integrated care pathway (ICP)
As described previously (Davies et al., Reference Davies2018), the ICP consists of a standardized step-wise approach based on best practice guidelines appraised and integrated by a multidisciplinary team. It starts with a thorough medical and psychiatric workup to rule out other causes of agitation or aggression, followed by a “clean-up” phase during which ineffective medications prescribed specifically for agitation are slowly discontinued while individualized non-pharmacological interventions are implemented by an interdisciplinary team. Then, if the patient remains symptomatic, a sequential algorithm of psychotropic medications is started (Davies et al., Reference Davies2018). The algorithm consists of a fixed sequence of medications with dose titrations guided by periodic pre-specified assessment of global clinical status using the “Improvement” item of the Clinical Global Impression Scale (CGI-I) (Busner and Targum, Reference Busner and Targum2007, Davies et al., Reference Davies2018). The eventual decision regarding implementation of a particular recommendation from the algorithm is made by the clinical team in consultation with the patient or their substitute decision makers, allowing some flexibility regarding the choice of agent based on clinical rationale. The burden of neuropsychiatric symptoms including agitation, caregiver burden, and adverse effects related to medications are assessed using standardized assessments. Patients are treated to clinical remission, defined as a CGI-I rating of 1 (“very much improved”) or 2 (“much improved”).
Four patient groups included in the study
Patients in the ICP group had a diagnosis of Alzheimer’s or mixed dementia who were admitted to the inpatient geriatric unit at CAMH for the treatment of agitation (including aggression) and were enrolled into the ICP. As the ICP was phased in starting in the last week of July 2013, patients in the ICP group were treated on the unit between July 2013 and July 2016. We identified 32 such unique patients, and three were excluded from the analysis because the ICP was interrupted by a transfer to a general medical hospital due to physical illnesses unrelated to the ICP, and one patient was excluded because he died due to causes unrelated to the ICP. Thus, the ICP group comprises 28 patients whose data were analyzed.
Patients in the TAU were selected because, like the patients in the ICP group, they had a diagnosis of Alzheimer’s or mixed dementia with agitation and they were admitted to the same inpatient unit for the treatment of agitation (including aggression). However, they were admitted between July 2010 and July 2013, that is, before the introduction of the ICP. Based on these criteria, we identified 28 patients who constitute the TAU group.
To account for the effect of time or “secular trends” when TAU and ICP groups were treated, we included two other groups comprising patients treated on the same unit contemporaneously to the ICP or TAU patients who had a mood disorder but no dementia diagnosis and had agitation (including aggression) on admission or within seven days of admission. Excluding patients with an unclear diagnosis, 17 patients treated contemporaneously to the TAU patients (i.e. from July 2010 to July 2013) constituted the “control group-1,” and 36 patients treated contemporaneously to the ICP patients (i.e. from July 2013 to July 2016) constituted the “control group-2.” None of the patients included in the study were enrolled in any other standardized pathway of care during the study period.
Measures
As part of the ICP, the following assessments were administered by members of the interdisciplinary team to assess symptom burden at entry and exit of the ICP: the Cohen Mansfield Agitation Inventory (CMAI) (Cohen-Mansfield et al., Reference Cohen-Mansfield, Marx and Rosenthal1989) and the Neuropsychiatric Inventory Questionnaire (NPIQ) (Kaufer et al., Reference Kaufer2000). Abnormal Involuntary Movement Scale (AIMS) (Guy, Reference Guy1976), Simpson Angus Scale (SAS) (Simpson and Angus, Reference Simpson and Angus1970), and Barnes Akathisia Rating Scale (BAS) (Barnes, Reference Barnes1989) were completed to assess medication related adverse effects. As the patients in the TAU and two control groups did not have their care guided by the ICP, we did not have the standardized assessments measures at the beginning and end of their treatment as these are not typically performed under usual care conditions.
For all four groups, charts were reviewed; admission and discharge dates were used to calculate the LOS. List of medications at admission and discharge were used to identify psychotropic polypharmacy, defined as receiving two or more psychotropic medications; for the TAU and ICP groups, we considered only psychotropic medications prescribed to treat agitation; for the two control groups, we counted all psychotropic medications. Finally, falls documented in the charts were identified.
Statistical Analyses
First, we examined the variables for their distribution using histograms and used log transformation or non-parametric tests as needed (if the distribution was not normal). We compared the demographic and clinical characteristics of the four groups using independent sample t tests, chi-square tests, or the Mann-Whitney U test, as applicable. Further, to answer our research questions we conducted the following analyses.
Effectiveness of the ICP: We compared the CMAI and NPIQ scores within this group between entry and exit from the ICP using paired t tests. We also compared the measures of adverse effects (AIMS, SAS, and BAS) within the group between entry and exit from the ICP using Wilcoxon signed rank test. Cohen’s d statistic was used to calculate effect sizes as applicable. To calculate effect size for paired samples, we used pooled standard deviation from baseline and endpoint (G*Power, 17 March 2020 – Release 3.1.9.7).
Comparison of ICP and TAU groups: We compared the LOS using linear regression of log-transformed LOS as dependent variable, group as independent variable, and age at admission, gender, and number of psychotropic medications at admission as covariates. We examined the distribution of residuals to assess the assumption of normality of residuals for regression, which was found to be satisfactory. We used pooled standard deviation to calculate Cohen’s d statistic for effect size of between group differences. Further, we conducted a Cox proportional hazards regression analysis, with LOS as dependent variable, discharge from hospital as “event,” and study group, age at admission, gender, and psychotropic medications at admission as variables of interest. We examined log-log plots to assess the proportional hazards assumption and found it to be satisfactory. Finally, we compared the proportions of patients receiving psychotropic polypharmacy or experiencing falls between groups using logistic regression while controlling for age, gender, and number of psychotropic medications at admission.
We similarly compared the LOS and rates of polypharmacy and falls between the two control groups.
All statistical tests were two-tailed with significance level set at alpha = 0.05 and were performed using SPSS software (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY).
Results
Patient characteristics
Table 1 presents the patient characteristics at admission for all four groups. Between both the ICP and TAU groups of patients with dementia, and between the two control groups, there were significant differences in terms of proportions of males vs females.
Table 1. Demographic and clinical characteristics of the patients on admission and discharge
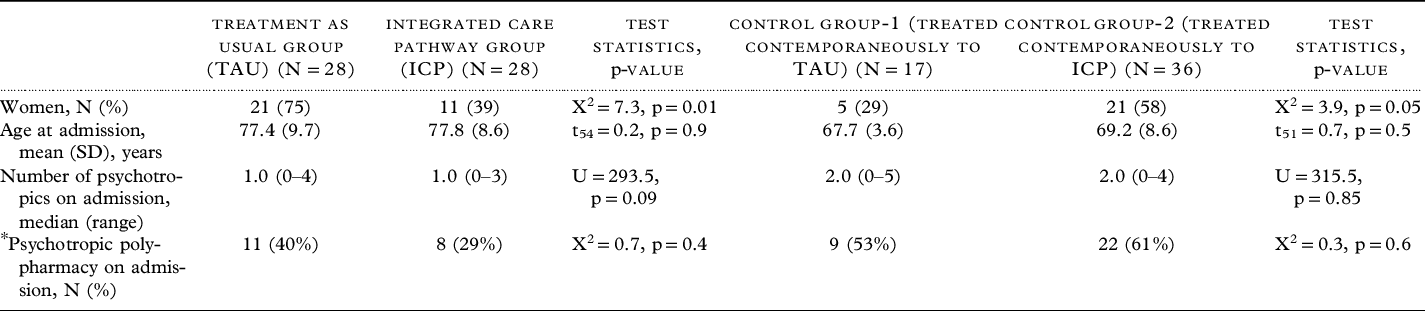
Abbreviations: SD – standard Deviation, X2 – Chi-square statistic, t – independent sample t test, U – Mann-Whitney U test statistic, p – statistical significance.
* For TAU and ICP groups “psychotropics” refer to psychotropic drugs used to treat agitation/aggression only. For control groups, “psychotropics” refer to all psychotropic drugs only.
Effectiveness of the ICP
Table 2 and Figure 1 present the clinical measures within the ICP group at time of entry and exit from the ICP. As expected, the level of agitation, the burden of neuropsychiatric symptoms, and the caregiver distress related to neuropsychiatric symptoms were high at baseline. From ICP entry to exit, the corresponding scores decreased significantly, with moderate to large effect sizes (CMAI frequency total scores: Cohen’s d = 0.9, CMAI distress scores: Cohen’s d = 1.0, NPIQ: Cohen’s d = 0.7, NPIQ caregiver distress score: Cohen’s d = 0.8). There were no significant changes in MoCA, AIMS, SAS, or BAS scores between ICP entry and exit.
Comparisons between the ICP and TAU groups
In the linear regression model controlling for age, gender, and psychotropic medications on admission, the ICP group showed a trend towards shorter mean LOS as compared to the TAU group (F1,56 = 3.65, p = 0.085, Cohen’s d = 0.45). In the Cox regression model with the same covariates as above, ICP group had a higher chance of an earlier discharge from hospital as compared to the TAU group (adjusted hazard ratio = 1.85, 95% confidence interval [CI] = 1.0–3.4, p = 0.05) (Figure 2). ICP group had a median time to discharge of 57 days as compared to 82 days in the TAU group.
Using logistic regression, while controlling for the same covariates as above, the odds of psychotropic polypharmacy at discharge was lower in the ICP vs. the TAU group (adjusted OR = 0.17; 95% CI = 0.029–0.97; p = 0.046). Similarly, the odds of experiencing a fall were significantly lower in the ICP group (adjusted OR = 0.08; 95% CI = 0.014–0.45; p = 0.004).
Comparisons between the two control groups
There was no difference between the two control groups in LOS, rates of psychotropic polypharmacy on discharge, or proportion of patients experiencing a fall (Figure 2 and Table 3).
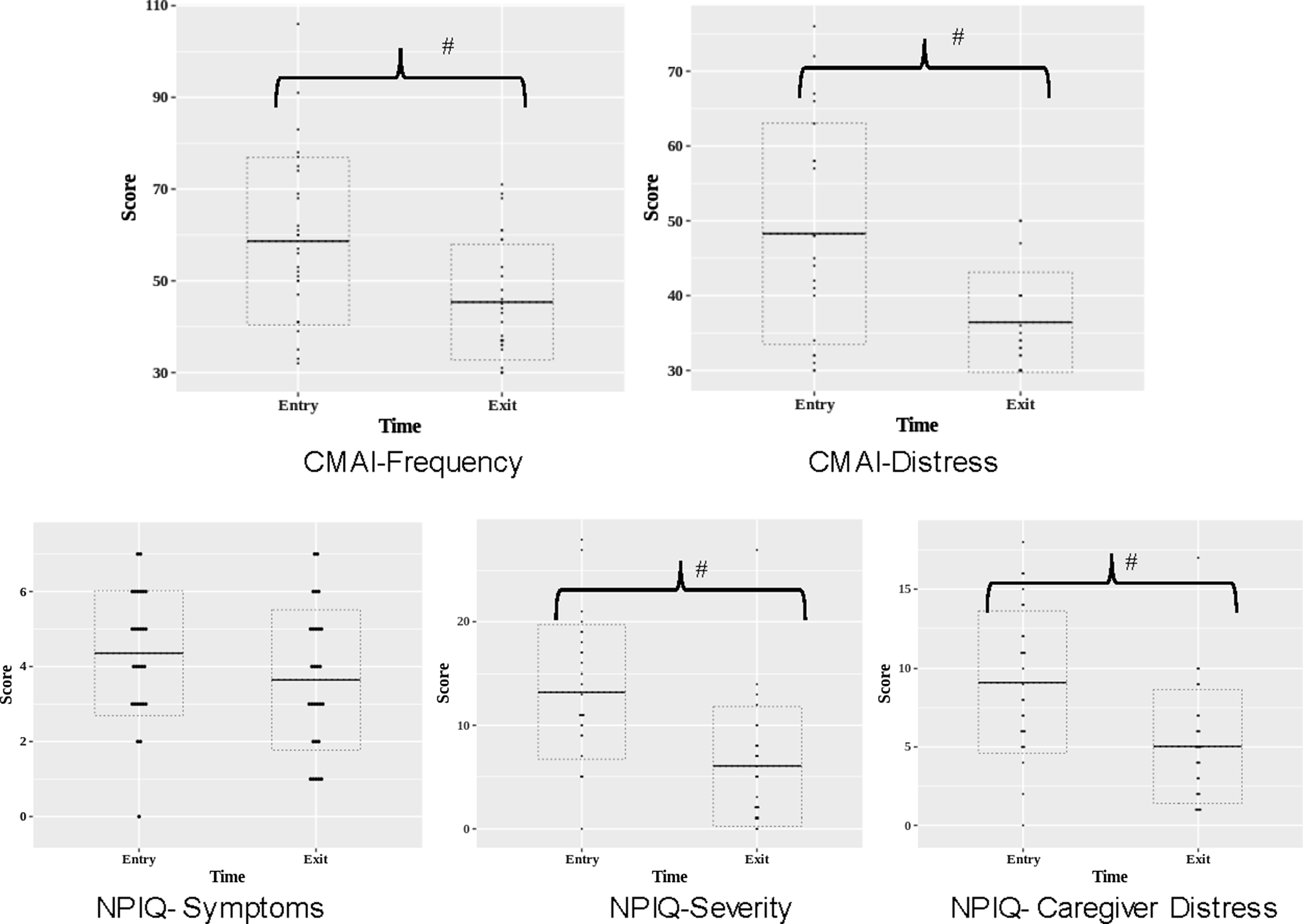
Figure 1. Clinical outcomes at entry and exit from the Integrated Care Pathway group. CMAI: Cohen Mansfield Agitation Inventory; NPIQ: Neuropsychiatric Inventory Questionnaire, # indicates statistically significant group differences at p < 0.05.
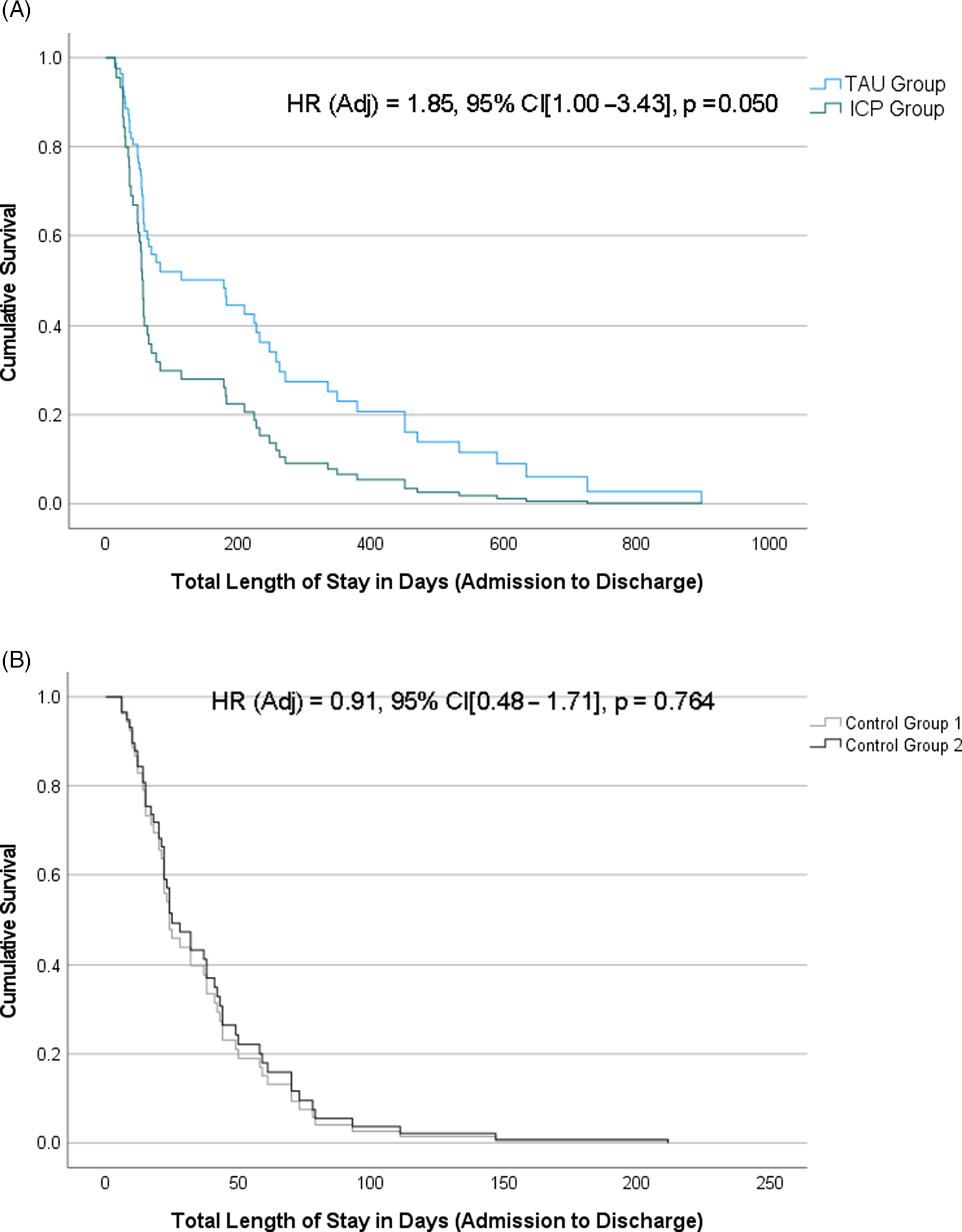
Figure 2. Kaplan-Meier survival curves for time from admission to discharge in days between (A) ICP vs. TAU; and (B) Control Group 1 vs. Control Group 2. Cox proportional-hazards regression estimates (hazard ratio [HR], 95% confidence intervals [CI], and p-values) are provided for each model, adjusting for age at admission, gender, and psychotropic medications at admission.
Table 2. Clinical measures in the integrated care pathway (ICP) group (n = 28) at entry and exit from the ICP
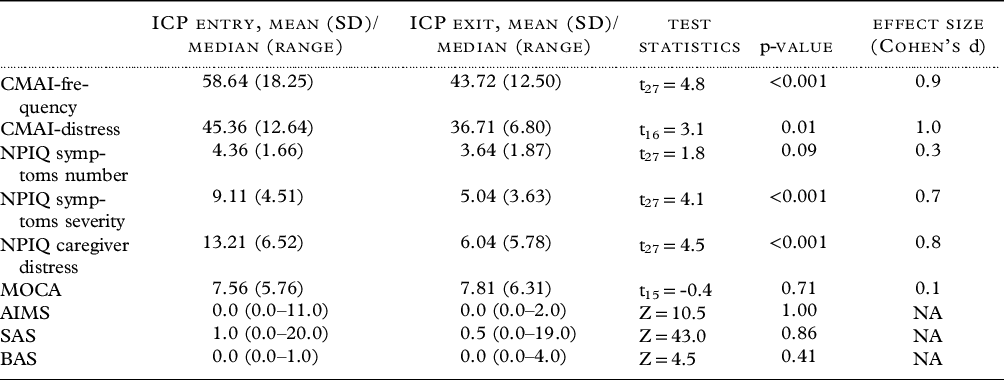
Abbreviations: AIMS – Abnormal Involuntary Movement Scale; BAS – Barnes Akathisia rating Scale; CMAI – Cohen Mansfield Agitation Inventory; MOCA – Montreal Cognitive Assessment; NPIQ – Neuropsychiatric Inventory Questionnaire; SAS – Simpson Angus Scale; t – Paired t test; Z – Wilcoxon Signed Rank Test Statistic, NA – not applicable.
Table 3. Comparisons of outcome measures between integrated care pathway and treatment-as-usual groups, and between the two control groups
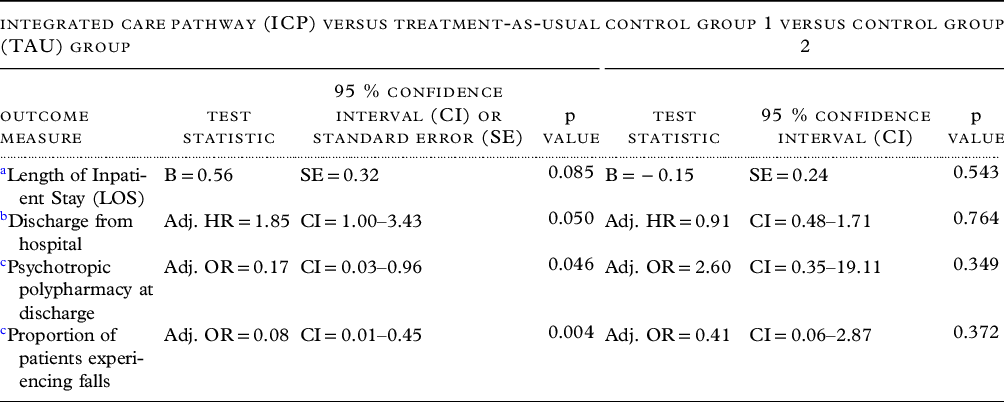
a Linear regression of log-transformed LOS as dependent variable, group as independent variable, and age at admission, gender, and number of psychotropic medications at admission as covariates. B – Parameter estimate.
b Cox proportional-hazards regression, with LOS as dependent variable, discharge from hospital as “event,” and study group, age at admission, gender, and psychotropic medications at admission as covariates. Adj. HR – adjusted hazard ratio.
c Logistic regression with psychotropic polypharmacy or proportion of patients experiencing falls as dependent variable, and the same covariates as above. Adj. OR – adjusted odds ratio.
Discussion
This pilot study analyzed the impact of an ICP including a medication algorithm for the treatment of agitation (including aggression) associated with dementia on an inpatient geriatric psychiatry unit. The study shows several promising results. First, the ICP was successful in treating agitation in most patients as evidenced by decreased burden of symptoms and caregiver distress. Second, the ICP was well-tolerated, without adverse cognitive or extrapyramidal effects. Third, while both ICP and TAU resulted in successful clinical treatment, the ICP resulted in a higher likelihood of earlier discharge, lower rates of psychotropic polypharmacy, and lower chances of experiencing a fall during hospital admission. Notably, these changes in LOS, polypharmacy, and falls were not observed in comparisons of the two control groups treated contemporaneously to the TAU and ICP groups, suggesting that the changes in LOS or polypharmacy associated with the ICP were not likely to be due to overarching changes in clinical practice on the unit over time.
Taken together, these preliminary results support the feasibility of treating agitation in dementia using the ICP in patients admitted to a geriatric psychiatry inpatient unit in a tertiary care hospital. These patients have a significant illness burden, and they are likely to have failed to respond to several treatments in community setting (Vasudev et al., Reference Vasudev2015). The prevalence of inappropriate medication use and lack of adherence to guidelines are known to be problematic in this population (Gallagher et al., Reference Gallagher2016, Goga et al., Reference Goga2017, Haw et al., Reference Haw, Stubbs and Yorston2008). Recent investigations into systematic approaches to treat agitation have shown varying results (Lichtwarck et al., Reference Lichtwarck2018, Livingston et al., Reference Livingston2019, Rapp et al., Reference Rapp2013). Some of these interventions focused on staff education and training while others used active strategies to enhance non-pharmacological interventions (Lichtwarck et al., Reference Lichtwarck2018, Livingston et al., Reference Livingston2019, Rapp et al., Reference Rapp2013). These interventions were tried in long-term care homes rather than on geriatric psychiatry inpatient units. Also, while these interventions emphasize principles of care, they did not implement a medication algorithm as in our ICP (Davies et al., Reference Davies2018).
Another important aspect of our study is that in parallel to the comparisons of the ICP with TAU, we accounted for “secular trends” by the use of non-dementia control groups with agitation from the same inpatient unit treated contemporaneously to the TAU and ICP groups. While there were differences in the outcomes between the ICP and TAU groups, no such differences were observed between the two control groups. This increases our confidence that the differences between the ICP and TAU groups were not merely attributable to general changes in practice or hospital conditions between the two time periods – that is, a “cohort effect.”
We believe that the putative success of the ICP in terms of enhanced likelihood of earlier discharge may be due to several inherent factors. First, the implementation of an interdisciplinary treatment plan defines roles of team members in carrying out assessments and interventions at specified times and helps to move the care plan forward towards the best patient outcome. Further, with clear treatment steps and decision points based on global clinical impression, the clinical team adopts measurement-based care while maximizing efficiency as recommended by guidelines and expert panels (Azermai et al., Reference Azermai, Petrovic, Elseviers, Bourgeois, van Bortel and Vander Stichele2012, Soto et al., Reference Soto2015). If replicated in larger prospective controlled studies, decreasing the LOS may help to reduce distress to the patients and caregivers and lead to cost savings (Maust et al., Reference Maust, Kales, Mccammon, Blow, Leggett and Langa2017). Standardization of care in institutional settings and in the community may help to meet increased demands for care for patients with dementia and agitation during the pandemic (Brown et al., Reference Brown, Kumar, Rajji, Pollock and Mulsant2020, Keng et al., Reference Keng2020).
Finally, compared to TAU, the ICP was associated with decreased use of psychotropic polypharmacy and lower chances a fall during admission. Studies have reported polypharmacy rates of 50% to 70% for general and psychotropic medications in patients with dementia in the community and for those in intuitional settings (Alpert, Reference Alpert2017, Blass et al., Reference Blass2008, Maust et al., Reference Maust2021). In turn, psychotropic polypharmacy has been associated with adverse outcomes such as falls, increased rates of hospitalizations, and increased mortality (Hanlon et al., Reference Hanlon, Semla and Schmader2014, Maher et al., Reference Maher, Hanlon and Hajjar2014, Mizokami et al., Reference Mizokami, Koide, Noro and Furuta2012). Thus, it is possible that decreased chances of fall in the ICP group were related to lower rate of polypharmacy among other factors. Previous studies have shown the success of standardized algorithmic treatment compared to TAU for treating geriatric depression and success with pharmacist-led interventions for reducing antipsychotic use in patients with dementia (Goga et al., Reference Goga2017, Mulsant et al., Reference Mulsant, Blumberger, Ismail, Rabheru and Rapoport2014, Trivedi et al., Reference Trivedi2004). Our study extends this finding to treatment of agitation in dementia in inpatient geriatric psychiatry units, which has implications for the possibility of standardizing care and reducing polypharmacy for people with dementia and agitation in other settings.
Our study has several limitations; first, this study was not a randomized comparison of the ICP and TAU. We combined observational data and data obtained by a retrospective chart review. However, the ICP and TAU groups were selected from the same inpatient unit and our analyses were controlled for age, gender, and admission medications, and for the possible effect of the different time periods during which the TAU and ICP groups were treated. Second, we could only perform pre-post comparisons in the ICP group since the detailed data on outcomes and adverse effects that were routinely collected as part of the ICP were not collected as part of TAU. However, we were able to compare the ICP and TAU groups only for several outcomes that could be ascertained retrospectively in both groups and detected some meaningful and significant differences. Third, since the TAU and ICP groups were not treated contemporaneously, we assessed the possible effects of time such as changes in hospital or inpatient unit practices in two control groups treated contemporaneously to the TAU or the ICP group. This approach has its own limitations; for example, while the patients in the control groups were inpatients on the same geriatric units as the patients from the ICP and TAU groups, their mean ages were markedly lower. Fourth, the ICP was implemented by the team that created it (Davies et al., Reference Davies2018), which may have increased the chances of success because of the interest and investment of clinical and administrative teams into the ICP. Finally, introduction of the ICP and associated measurements could have resulted in change in clinical teams’ behavior due to the effect of increased observation (Hawthorne effect).
To conclude, this study provides preliminary evidence that a standardized ICP using an algorithmic care model can successfully treat agitation in dementia in a “real-world setting” of a hospital inpatient unit. Further, compared with TAU, the ICP was associated with greater chance of earlier discharge, a lower rate of psychotropic polypharmacy, and a lower rate of falls during hospital stay. If replicated in larger studies, this model can be used to standardize clinical care and provide a platform for studies that evaluates treatment of agitation in dementia. Future studies involving multiple sites using a randomized blinded design are needed to confirm our results.
Disclosures/Conflict of interest
Authors report no Conflicts of interest in relation to this manuscript.
Dr. Davies receives grant support from the Centre for Addiction and Mental Health (CAMH)/University of Toronto, National Institute for Health Research (NIHR) (UK), Canadian Centre for Aging and Brain Health Innovation, Canadian Consortium for Neurodegeneration in Aging (CCNA) and Medical-Psychiatry Alliance.
Dr. Graff receives support from Canadian Institutes of Health Research, Ontario AHSC AFP Innovation Fund, American Foundation for Suicide Prevention, Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness, Northern Ontario Academic Medicine Association, CAMH Discovery Fund, Labatt Family Network for Research on the Biology of Depression, PSI Foundation and Brain Canada Foundation. Society for Nuclear Medicine & Molecular Imaging 2018 Mitzi & William Blahd, MD
Dr. Mulsant holds and receives support from the Labatt Family Chair in Biology of Depression in Late-Life Adults at the University of Toronto. He currently receives research support from Brain Canada, the Canadian Institutes of Health Research, the CAMH Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the US National Institute of Health (NIH), Capital Solution Design LLC (software used in a study founded by CAMH Foundation), and HAPPYneuron (software used in a study founded by Brain Canada). Within the past 5 years, he has also received research support from Eli Lilly (medications for a NIH-funded clinical trial) and Pfizer (medications for a NIH-funded clinical trial). He has been an unpaid consultant to Myriad Neuroscience.
Dr. Kumar has received research support from the Brain and Behavior Foundation, US National Institute on Aging, BrightFocus Foundation, Brain Canada, Canadian Institutes of Health Research, Canadian Consortium on Neurodegeneration in Aging, Centre for Aging and Brain Health Innovation, Centre for Addiction and Mental Health, and an Academic Scholars Award from the Department of Psychiatry, Temerty Faculty of Medicine, University of Toronto. He has also received equipment support from Soterix Medical.
Dr. Pollock holds and receives support from the Peter and Shelagh Godsoe Endowed Chair in Late-Life Mental Health, Centre for Addiction and Mental Health. Dr Pollock’s research is supported by National Institute of Aging, Brain Canada, the Canadian Institutes of Health Research, the Alzheimer’s Drug Discovery Foundation, the Ontario Brain Institute, the Centre for Aging and Brain Health Innovation, Alzheimer’s Society of Canada, the W. Garfield Weston Foundation, the Weston Brain Institute, the Canadian Consortium on Neurodegeneration in Aging and Genome Canada. Dr Pollock receives honoraria from the American Geriatrics Society and holds United States Provisional Patent No. 16/490,680 and Canadian Provisional Patent No. 3,054,093 for a cell-based assay and kits for assessing serum anticholinergic activity.
Dr. Rajji has received research support from Brain Canada, Brain and Behavior Research Foundation, BrightFocus Foundation, Canada Foundation for Innovation, Canada Research Chair, Canadian Institutes of Health Research, Centre for Aging and Brain Health Innovation, National Institutes of Health, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, and the Weston Brain Institute. He also received in-kind equipment support for an investigator-initiated study from Magstim, and in-kind research accounts from Scientific Brain Training Pro. He participated in 2021 in an advisory board for Biogen Canada Inc.
Dr. Burhan served on advisory board for Janssen Canada, Eisai Pharmaceuticals, Avanir Pharmaceuticals, provided expert opinion to Atheneum Partners and HEALTHTECH Connex unrelated to this work. Acknowledges funding support from the National Institute of Aging in the US, Brain Canada, Canadian Institute of Health Research, Canadian Consortium on Neurodegeneration in Aging, Centre of Aging and Brain Health Innovation, Ontario Shores Foundation and Centre for Mental Health Sciences, Saint Joseph’s Health Care Foundation and Centre, London and Schulich School of Medicine at Western University.
Description of authors’ roles
SK, SJCD, and TKR led the design of the study. SK and AS led the acquisition, analysis, and interpretation of data. SK, CM, and SJCD led the statistical analyses and their interpretation. SK, SJCD, TKR, and BHM led the writing of the manuscript. All authors participated in the conception and design, made substantial contributions to the interpretation of the data, revised critically the manuscript, approved its final version; and agree to be accountable for all aspects of the work and ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
We thank the members of the Behavioral and Psychological Symptoms of Dementia – Integrated Care Pathway Group at the Centre for Addiction and Mental Health (CAMH), Toronto, Ontario, Canada, which included Saima Awan, Joydip Banerjee, Amer M Burhan, Charlotte Carlson, Tiffany Chow, Sarah Colman, Simon Davies, Rita Desai, Amy Gowling, Ariel Graff-Guerrero, Philip Gerretsen, Samantha Holowacha, Holly Ito, Sawsan Kalache, Shivali Kapila, Donna Kim, Sanjeev Kumar, Carrie Lawford, Vivien Luk, Kwame McKenzie, Rola Moghabghab, Benoit H. Mulsant, Bruce G. Pollock, Tarek K. Rajji, Efrem Rone, Pushpinder Saini, Ivan Silver, Jyll Simmons, Jonathan Syms, Rong Ting, Michael Tseng, Celine Teo, Christopher Uranis, Vincent L. Woo and Camilo Yang.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1041610222000321







